
- •Preface
- •Textbook Layout and Design
- •Preliminaries
- •See, Do, Teach
- •Other Conditions for Learning
- •Your Brain and Learning
- •The Method of Three Passes
- •Mathematics
- •Summary
- •Homework for Week 0
- •Summary
- •1.1: Introduction: A Bit of History and Philosophy
- •1.2: Dynamics
- •1.3: Coordinates
- •1.5: Forces
- •1.5.1: The Forces of Nature
- •1.5.2: Force Rules
- •Example 1.6.1: Spring and Mass in Static Force Equilibrium
- •1.7: Simple Motion in One Dimension
- •Example 1.7.1: A Mass Falling from Height H
- •Example 1.7.2: A Constant Force in One Dimension
- •1.7.1: Solving Problems with More Than One Object
- •Example 1.7.4: Braking for Bikes, or Just Breaking Bikes?
- •1.8: Motion in Two Dimensions
- •Example 1.8.1: Trajectory of a Cannonball
- •1.8.2: The Inclined Plane
- •Example 1.8.2: The Inclined Plane
- •1.9: Circular Motion
- •1.9.1: Tangential Velocity
- •1.9.2: Centripetal Acceleration
- •Example 1.9.1: Ball on a String
- •Example 1.9.2: Tether Ball/Conic Pendulum
- •1.9.3: Tangential Acceleration
- •Homework for Week 1
- •Summary
- •2.1: Friction
- •Example 2.1.1: Inclined Plane of Length L with Friction
- •Example 2.1.3: Find The Minimum No-Skid Braking Distance for a Car
- •Example 2.1.4: Car Rounding a Banked Curve with Friction
- •2.2: Drag Forces
- •2.2.1: Stokes, or Laminar Drag
- •2.2.2: Rayleigh, or Turbulent Drag
- •2.2.3: Terminal velocity
- •Example 2.2.1: Falling From a Plane and Surviving
- •2.2.4: Advanced: Solution to Equations of Motion for Turbulent Drag
- •Example 2.2.3: Dropping the Ram
- •2.3.1: Time
- •2.3.2: Space
- •2.4.1: Identifying Inertial Frames
- •Example 2.4.1: Weight in an Elevator
- •Example 2.4.2: Pendulum in a Boxcar
- •2.4.2: Advanced: General Relativity and Accelerating Frames
- •2.5: Just For Fun: Hurricanes
- •Homework for Week 2
- •Week 3: Work and Energy
- •Summary
- •3.1: Work and Kinetic Energy
- •3.1.1: Units of Work and Energy
- •3.1.2: Kinetic Energy
- •3.2: The Work-Kinetic Energy Theorem
- •3.2.1: Derivation I: Rectangle Approximation Summation
- •3.2.2: Derivation II: Calculus-y (Chain Rule) Derivation
- •Example 3.2.1: Pulling a Block
- •Example 3.2.2: Range of a Spring Gun
- •3.3: Conservative Forces: Potential Energy
- •3.3.1: Force from Potential Energy
- •3.3.2: Potential Energy Function for Near-Earth Gravity
- •3.3.3: Springs
- •3.4: Conservation of Mechanical Energy
- •3.4.1: Force, Potential Energy, and Total Mechanical Energy
- •Example 3.4.1: Falling Ball Reprise
- •Example 3.4.2: Block Sliding Down Frictionless Incline Reprise
- •Example 3.4.3: A Simple Pendulum
- •Example 3.4.4: Looping the Loop
- •3.5: Generalized Work-Mechanical Energy Theorem
- •Example 3.5.1: Block Sliding Down a Rough Incline
- •Example 3.5.2: A Spring and Rough Incline
- •3.5.1: Heat and Conservation of Energy
- •3.6: Power
- •Example 3.6.1: Rocket Power
- •3.7: Equilibrium
- •3.7.1: Energy Diagrams: Turning Points and Forbidden Regions
- •Homework for Week 3
- •Summary
- •4.1: Systems of Particles
- •Example 4.1.1: Center of Mass of a Few Discrete Particles
- •4.1.2: Coarse Graining: Continuous Mass Distributions
- •Example 4.1.2: Center of Mass of a Continuous Rod
- •Example 4.1.3: Center of mass of a circular wedge
- •4.2: Momentum
- •4.2.1: The Law of Conservation of Momentum
- •4.3: Impulse
- •Example 4.3.1: Average Force Driving a Golf Ball
- •Example 4.3.2: Force, Impulse and Momentum for Windshield and Bug
- •4.3.1: The Impulse Approximation
- •4.3.2: Impulse, Fluids, and Pressure
- •4.4: Center of Mass Reference Frame
- •4.5: Collisions
- •4.5.1: Momentum Conservation in the Impulse Approximation
- •4.5.2: Elastic Collisions
- •4.5.3: Fully Inelastic Collisions
- •4.5.4: Partially Inelastic Collisions
- •4.6: 1-D Elastic Collisions
- •4.6.1: The Relative Velocity Approach
- •4.6.2: 1D Elastic Collision in the Center of Mass Frame
- •4.7: Elastic Collisions in 2-3 Dimensions
- •4.8: Inelastic Collisions
- •Example 4.8.1: One-dimensional Fully Inelastic Collision (only)
- •Example 4.8.2: Ballistic Pendulum
- •Example 4.8.3: Partially Inelastic Collision
- •4.9: Kinetic Energy in the CM Frame
- •Homework for Week 4
- •Summary
- •5.1: Rotational Coordinates in One Dimension
- •5.2.1: The r-dependence of Torque
- •5.2.2: Summing the Moment of Inertia
- •5.3: The Moment of Inertia
- •Example 5.3.1: The Moment of Inertia of a Rod Pivoted at One End
- •5.3.1: Moment of Inertia of a General Rigid Body
- •Example 5.3.2: Moment of Inertia of a Ring
- •Example 5.3.3: Moment of Inertia of a Disk
- •5.3.2: Table of Useful Moments of Inertia
- •5.4: Torque as a Cross Product
- •Example 5.4.1: Rolling the Spool
- •5.5: Torque and the Center of Gravity
- •Example 5.5.1: The Angular Acceleration of a Hanging Rod
- •Example 5.6.1: A Disk Rolling Down an Incline
- •5.7: Rotational Work and Energy
- •5.7.1: Work Done on a Rigid Object
- •5.7.2: The Rolling Constraint and Work
- •Example 5.7.2: Unrolling Spool
- •Example 5.7.3: A Rolling Ball Loops-the-Loop
- •5.8: The Parallel Axis Theorem
- •Example 5.8.1: Moon Around Earth, Earth Around Sun
- •Example 5.8.2: Moment of Inertia of a Hoop Pivoted on One Side
- •5.9: Perpendicular Axis Theorem
- •Example 5.9.1: Moment of Inertia of Hoop for Planar Axis
- •Homework for Week 5
- •Summary
- •6.1: Vector Torque
- •6.2: Total Torque
- •6.2.1: The Law of Conservation of Angular Momentum
- •Example 6.3.1: Angular Momentum of a Point Mass Moving in a Circle
- •Example 6.3.2: Angular Momentum of a Rod Swinging in a Circle
- •Example 6.3.3: Angular Momentum of a Rotating Disk
- •Example 6.3.4: Angular Momentum of Rod Sweeping out Cone
- •6.4: Angular Momentum Conservation
- •Example 6.4.1: The Spinning Professor
- •6.4.1: Radial Forces and Angular Momentum Conservation
- •Example 6.4.2: Mass Orbits On a String
- •6.5: Collisions
- •Example 6.5.1: Fully Inelastic Collision of Ball of Putty with a Free Rod
- •Example 6.5.2: Fully Inelastic Collision of Ball of Putty with Pivoted Rod
- •6.5.1: More General Collisions
- •Example 6.6.1: Rotating Your Tires
- •6.7: Precession of a Top
- •Homework for Week 6
- •Week 7: Statics
- •Statics Summary
- •7.1: Conditions for Static Equilibrium
- •7.2: Static Equilibrium Problems
- •Example 7.2.1: Balancing a See-Saw
- •Example 7.2.2: Two Saw Horses
- •Example 7.2.3: Hanging a Tavern Sign
- •7.2.1: Equilibrium with a Vector Torque
- •Example 7.2.4: Building a Deck
- •7.3: Tipping
- •Example 7.3.1: Tipping Versus Slipping
- •Example 7.3.2: Tipping While Pushing
- •7.4: Force Couples
- •Example 7.4.1: Rolling the Cylinder Over a Step
- •Homework for Week 7
- •Week 8: Fluids
- •Fluids Summary
- •8.1: General Fluid Properties
- •8.1.1: Pressure
- •8.1.2: Density
- •8.1.3: Compressibility
- •8.1.5: Properties Summary
- •Static Fluids
- •8.1.8: Variation of Pressure in Incompressible Fluids
- •Example 8.1.1: Barometers
- •Example 8.1.2: Variation of Oceanic Pressure with Depth
- •8.1.9: Variation of Pressure in Compressible Fluids
- •Example 8.1.3: Variation of Atmospheric Pressure with Height
- •Example 8.2.1: A Hydraulic Lift
- •8.3: Fluid Displacement and Buoyancy
- •Example 8.3.1: Testing the Crown I
- •Example 8.3.2: Testing the Crown II
- •8.4: Fluid Flow
- •8.4.1: Conservation of Flow
- •Example 8.4.1: Emptying the Iced Tea
- •8.4.3: Fluid Viscosity and Resistance
- •8.4.4: A Brief Note on Turbulence
- •8.5: The Human Circulatory System
- •Example 8.5.1: Atherosclerotic Plaque Partially Occludes a Blood Vessel
- •Example 8.5.2: Aneurisms
- •Homework for Week 8
- •Week 9: Oscillations
- •Oscillation Summary
- •9.1: The Simple Harmonic Oscillator
- •9.1.1: The Archetypical Simple Harmonic Oscillator: A Mass on a Spring
- •9.1.2: The Simple Harmonic Oscillator Solution
- •9.1.3: Plotting the Solution: Relations Involving
- •9.1.4: The Energy of a Mass on a Spring
- •9.2: The Pendulum
- •9.2.1: The Physical Pendulum
- •9.3: Damped Oscillation
- •9.3.1: Properties of the Damped Oscillator
- •Example 9.3.1: Car Shock Absorbers
- •9.4: Damped, Driven Oscillation: Resonance
- •9.4.1: Harmonic Driving Forces
- •9.4.2: Solution to Damped, Driven, Simple Harmonic Oscillator
- •9.5: Elastic Properties of Materials
- •9.5.1: Simple Models for Molecular Bonds
- •9.5.2: The Force Constant
- •9.5.3: A Microscopic Picture of a Solid
- •9.5.4: Shear Forces and the Shear Modulus
- •9.5.5: Deformation and Fracture
- •9.6: Human Bone
- •Example 9.6.1: Scaling of Bones with Animal Size
- •Homework for Week 9
- •Week 10: The Wave Equation
- •Wave Summary
- •10.1: Waves
- •10.2: Waves on a String
- •10.3: Solutions to the Wave Equation
- •10.3.1: An Important Property of Waves: Superposition
- •10.3.2: Arbitrary Waveforms Propagating to the Left or Right
- •10.3.3: Harmonic Waveforms Propagating to the Left or Right
- •10.3.4: Stationary Waves
- •10.5: Energy
- •Homework for Week 10
- •Week 11: Sound
- •Sound Summary
- •11.1: Sound Waves in a Fluid
- •11.2: Sound Wave Solutions
- •11.3: Sound Wave Intensity
- •11.3.1: Sound Displacement and Intensity In Terms of Pressure
- •11.3.2: Sound Pressure and Decibels
- •11.4: Doppler Shift
- •11.4.1: Moving Source
- •11.4.2: Moving Receiver
- •11.4.3: Moving Source and Moving Receiver
- •11.5: Standing Waves in Pipes
- •11.5.1: Pipe Closed at Both Ends
- •11.5.2: Pipe Closed at One End
- •11.5.3: Pipe Open at Both Ends
- •11.6: Beats
- •11.7: Interference and Sound Waves
- •Homework for Week 11
- •Week 12: Gravity
- •Gravity Summary
- •12.1: Cosmological Models
- •12.2.1: Ellipses and Conic Sections
- •12.4: The Gravitational Field
- •12.4.1: Spheres, Shells, General Mass Distributions
- •12.5: Gravitational Potential Energy
- •12.6: Energy Diagrams and Orbits
- •12.7: Escape Velocity, Escape Energy
- •Example 12.7.1: How to Cause an Extinction Event
- •Homework for Week 12
418 |
Week 9: Oscillations |
transform a force or potential energy associated with a particle near equilibrium into the form that reveals the simple harmonic oscillator within, so to speak.
At this point, however, we wish to put this idea to a di erent use – to help us bridge the gap between microscopic forces that hold a “rigid” object together and that object’s response to forces applied to it. After all, we know that there is no such thing as a truly rigid object. Steel is pretty hard, but with enough force we can bend “solid” steel, we can stretch or compress it, we can turn it into a spring, we can fracture it. Bone is also pretty hard and it does exactly the same thing: bend, stretch, compress, fracture. The physics of bending, stretching, compressing, and fracturing a solid object is associated with the application of stress to the object. Let’s see how.
9.5.3: A Microscopic Picture of a Solid
Let us consider a solid piece of some simple material. If we look at pure elemental solids – for example metals – we often find that when they form a solid the atoms arrange themselves into a regular lattice that is “close packed” – arranged so that the atoms more or less touch each other with a minimum of wasted volume. There are a number of kinds of lattices that appear (determined by the subtleties of the quantum mechanical interactions between the atoms and hence beyond the scope of this course) but in many cases the lattice is a variant of the cubic lattice where there are atoms on the corners of a regular cartesian grid in three dimensions (and sometimes additional atoms in the center of the cubes or on the faces of the cubes).
Not all materials are so regular. Materials made out of a mixture of atoms, out of molecules made of a mixture of atoms, out of a mixture of molecules, or even out of living cells made out of a mixture of molecules. The resulting materials can be ordered, structured (not exactly the same thing as ordered, especially in the case of solids formed by life processes such as bone or coral), or disordered (amorphous).
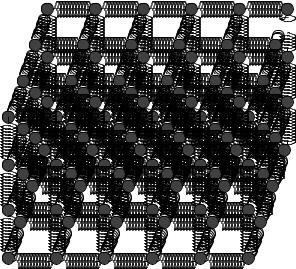
a
a a
a
Figure 127: An idealized simple cubic lattice of atoms separated by the “springs” of interatomic forces that hold them in equilibrium positions. The equilibrium separation of the atoms as a.
As usual, we will deal with all of this complexity by ignoring most of it for now and considering an “ideal” case where a single kind of atoms lined up in a regular simple cubic lattice is su cient to help us understand properties that will hold, with di erent values of course, even for amorphous or structured solids. This is illustrated in figure 127, which is basically a mental cartoon model for a generic solid – lots of atoms in a regular cubic lattice with a cube side a, where the interactomic
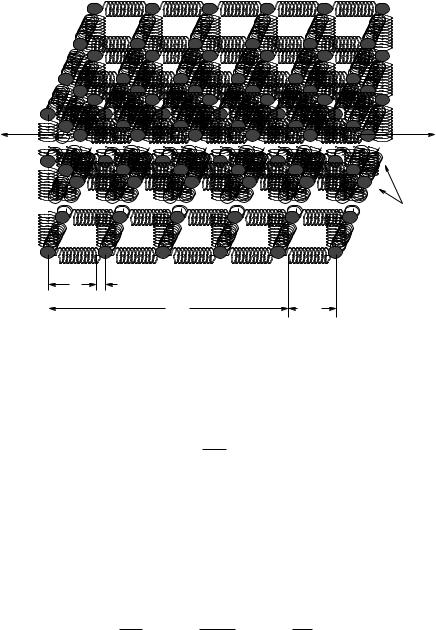
Week 9: Oscillations |
419 |
forces that hold each atom in position is represented by a spring, a concept that is valid as long as we don’t compress or stretch these interatomic “bonds” by too much.
We need to quantify the numbers of atoms and bonds in a way that helps us understand how stretching or compressing forces are distributed among all of the bonds. Suppose we have Nx atoms in the x-direction, so that the length of the solid is L = Nxa. Suppose also that we have Ny and Nz atoms in the y- and z-directions respectively, so that the cross-sectional area A = Ny Nz a2.
Now let us imagine applying a force (magnitude F ) uniformly to all of the atoms on the left and right ends that stretches all of these bonds by a small amount x, presumed to be “small” in precisely the sense that leaves the interatomic bonds still behaving like springs. The force F has to be distributed equally among all of the atoms on the end areas on both sides, so that the force applied to each chain of atoms in the x-direction end to end is:
|
|
|
|
|
|
Fchain = |
|
|
F |
= |
|
F a2 |
(879) |
||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
Ny Nz |
|
|
A |
|||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F













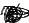
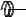


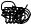

 F
F


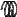
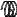

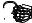
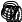
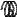




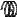
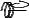



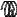

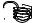
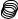
 A
A
a |
|
x |
|
L |
L |
Figure 128: The same lattice stretched by an amount |
L as a force F is applied to both ends, |
spread out uniformly across the cross-sectional area of the faces A.
This situation is portrayed in figure ??.
Each spring in the chain is stretched by this force between the atoms on the ends, so that:
Fchain = − |
F a2 |
|
A = −ke x |
(880) |
The negative sign just means that the springs are trying to go back to their equilibrium length and hence oppose the applied force.
We multiply this by one in the form Nx : |
|
|
|
||
|
Nx |
|
|
|
|
F a2 |
Nx |
|
|||
|
|
|
= −ke |
|
x |
|
A |
Nx |
|||
We multiply this by one in the form L=aNx : |
|
|
|
||
L=aNx |
|
|
|
||
F a2 = −ke a Nx x = −ke a
A Nxa
(881)
L
L
(882)

420 |
Week 9: Oscillations |
where we used the fact that |
L = Nx x just as L = Nxa. |
Finally, we rearrange this by putting all of the extra terms together as follows:
F |
|
ke |
|
L |
L |
|
|||
|
|
= − |
|
|
|
= −Y |
|
|
(883) |
|
A |
a |
L |
L |
|||||
In words, we define F/A to be the Stress, |
L/L to be the Strain, and |
|
|||||||
|
|
|
Y = ke/a |
|
|
(884) |
|||
to be Young’s Modulus. With these definitions, the equation above states that:
Compressive or extensive Stress applied to a solid equals Young’s Modulus times the Strain.
Note well that we can rearrange this equation into “Hooke’s Law” as follows:
Fx = − |
Y A |
x = −K x |
(885) |
L |
which basically states that any given chunk of material behaves like a spring when forces are applied to stretch or compress it, with a spring constant K that is proportional to the cross sectional area A and inversely proportional to the length L! Young’s modulus is the material-dependent contribution from the actual geometry and interaction potential energy that holds the material together, but the rest of the dependence is generic, and applies to all materials.
This scaling behavior of the response of solid (or liquid, or gaseous) matter to applied forces is the important take-home conceptual lesson of this section. Substances like bone or steel or wood or nearly anything are stronger – respond less to an applied force – as they get thicker, and are weaker – respond more to an applied force – as they get longer. This intuitive understanding can be made quantitatively precise to be sure (and for e.g. engineers or orthopedic surgeons will have to be quantitatively precise as they craft buildings that won’t fall down or artificial hips or bones that mimic natural ones) but is su cient in and of itself for people to see the world through new eyes, to understand why there are building codes for houses and decks governing the lengths and cross-sectional areas of support beams and joists and so on. We’ll explore a few of these applications later, but first let us relatively quickly extend the linear extension/compression result to sideways forces and understand shear.
9.5.4: Shear Forces and the Shear Modulus
If we imagine taking our chunk of model solid pictured in figure 127 above and pushing sideways on the top to the right while the bottom is locked to a table by e.g. static friction on the bottom, we are applying a shear force – one that bends the material sideways instead of stretching or compressing it. The layers of the material all shift sideways by an amount that distributes the shear force between all of the layers as shown and the material deforms at an angle θ as shown.
A fully microscopic derivation of the response is still quite possible, but not quite as simple as that for Young’s modulus above. For that reason we will content ourselves with a simple heuristic extension of the scaling ideas we learned in the previous section.
The displacement layer to layer required to oppose a given shear force is going to be inversely proportional to the thickness of the material L, because the longer the material is, the more layers there are to split the restoring force among. So we expect Fs to be proportional to 1/L. The work we do as the material bends comes primarily from the stretching of the vertical bonds. The number of vertical bonds is proportional to the cross-sectional area A of the material at the shear surface on the top and bottom. More bonds, a stronger force for a given displacement. We expect Fs to

Week 9: Oscillations |
421 |
be proportional to A. As before, we expect the response to depend on the microscopic properties of the material and its structure in some way – we wrap all our ignorance of these properties into a modulus that we can more easily measure in the lab than compute from first principles. We then assemble these scaling results into:
Fs = − |
MsA |
x |
(886) |
L |
where Ms is the shear modulus – the equivalent of Young’s modulus for shear forces. Once again
we can put this in a form involving shear stress Fs/A and shear strain |
x/L: |
||||
|
Fs |
= −Ms |
x |
(887) |
|
|
A |
L |
|
||
In words:
Shear Stress applied to a solid equals the Shear Modulus times the Strain.
where note well the minus sign that as always means that the reaction force opposes the applied shear force, trying to make x smaller in magnitude no matter what direction the material is being bent.
Shear stress is how springs really work! A “spring” is just a very long piece of material wrapped around into a coil so that when the ends are pulled, it produces shear stress spread out across the entire coil! So it is not an accident that we discover Hooke’s Law buried within this result – this is a (slighly handwaving) microscopic derivation of Hooke’s Law:
Fx = − |
MsA |
x = −k x |
(888) |
|
L |
||||
where: |
|
MsA |
|
|
|
k = |
(889) |
||
|
L |
|||
|
|
|
||
From this, we can predict how the strength of springs will vary according to the length of the wire in the coil L and its thickness A. Pretty cool!
9.5.5: Deformation and Fracture
What happens when one stretches or compresses or shears a material by an amount (per bond) that is not small? Here is a verbal description of what we might expect based on the microscopic model above and our own experience.
For a range of (relatively small) stresses, the strain remains a linear response – proportional to the stress, acting in a direction opposed to the stress. As one increases the stress, then, the first thing that happens is that the response becomes non-linear. Each additional increment of stress produces more than a linear increment of strain stretching, and quite possible less than a linear increment compressing, as one can understand from the graph of a typical interatomic or intermolecular force above. Materials tend to resist compression better than extension because one can pull bonded atoms apart but one cannot cause two bonded atoms to interpenetrate with any reasonable force – they are very strongly repulsive when their electron clouds start to overlap but only weakly attractive as the electron clouds are pulled apart.
At some point, one adds enough energy to the bonds (doing work as one e.g. stretches the material) that atoms or molecules start to dislocate – leave their normal place in the lattice or structure and migrate someplace else. This leaves behind a defect – a missing atom or molecule in the structure – and correspondingly weakens the entire structure. This migration tends to be permanent – even if one releases the stress on the material, it does not return to its original state but

422 |
Week 9: Oscillations |
remains stretched, compressed, or bent out of place. This region is one of permanent deformation of the material, as when one bends a paper clip.
Finally, if one continues to ratchet up the stress, at some point the number of defects introduced reaches a critical point and each new defect produced weakens the material enough that another defect is produced without further increase in stress, as the number of bonds over which the stress is distributed decreases with each new defect. The number of defects “explodes”, creating a fracture plane and the material breaks.
|F/A| |
|
|
fracture |
|
|
|
|
|
B |
C |
|
|
|
|
|
|
|
permanent deformation |
|
A |
nonlinear response |
||
|
|||
linear response |
|
|
|
|
|
| |
L/L | |
Figure 129: The slope of the linear response part of the curve is Young’s Modulus (note well the absolute magnitude signs). A marks the point where nonlinear response is first apparent. B marks the boundary where dislocations occur and permanent deformation of the material begins. C marks the point where there is a chain reaction – each defect produced produces on average at least one more defect at the same stress, so that the material “instantly” fractures.
These behaviors – and the critical points where the behavior changes – are summarized on the graph in figure 129.
Materials are not just characterized by their moduli. Moduli describe only the simple linear response regime. Furthermore, the moduli themselves depend on how far the atoms or molecules of the material are spending part of their time away from equilibrium without stress of any sort, because the material has a temperature (basically, a mechanical energy per atom that is larger than the minimum associated with sitting at the equilibrium point, at rest). In thermal equilibrium, each atom is already oscillating back and forth around its equilibrium position and temperature increases alone are su cient to drive the system through the same series of states – linear and nonlinear response where it can cool back to the original structure, nonlinear response that introduces defects so that the material “starts to melt” and doesn’t come back to its original state, followed by melting instead of fracture when the energy per bond no longer keeps atoms localized to a lattice or structure at all. When one mixes heating and stress, one can get many di erent ranges of physical behavior.
One last qualitative measure of interest is brittleness or toughness – a measure of how likely a material is to bend versus fracture when stresses are applied. Some materials, such as steel, are very tough and not very brittle – they do not easily deform or fracture. Others, like diamond, can be very hard indeed but are easy to fracture – they are brittle.
Human bone varies tremendously in the range of its brittleness with the lifetime of the human involved, with genetic factors, with dietary factors, and with the history of the person involved. Young people (on average) have bones that bend easily but aren’t very brittle. Old people have bones that become progressively more brittle as they decalcify with age or disease. Normal adults tend to fall in between – bones that are not as flexible but that are also not particularly brittle.
