
- •Contents
- •Contributors
- •Brain Tumor Imaging
- •1 Introduction
- •1.1 Overview
- •2 Clinical Management
- •3 Glial Tumors
- •3.1 Focal Glial and Glioneuronal Tumors Versus Diffuse Gliomas
- •3.3 Astrocytomas Versus Oligodendroglial Tumors
- •3.4.1 Diffuse Astrocytoma (WHO Grade II)
- •3.5 Anaplastic Glioma (WHO Grade III)
- •3.5.1 Anaplastic Astrocytoma (WHO Grade III)
- •3.5.3 Gliomatosis Cerebri
- •3.6 Glioblastoma (WHO Grade IV)
- •4 Primary CNS Lymphomas
- •5 Metastatic Tumors of the CNS
- •References
- •MR Imaging of Brain Tumors
- •1 Introduction
- •2 Brain Tumors in Adults
- •2.1 Questions to the Radiologist
- •2.2 Tumor Localization
- •2.3 Tumor Malignancy
- •2.4 Tumor Monitoring
- •2.5 Imaging Protocol
- •Computer Tomography
- •2.6 Case Illustrations
- •3 Pediatric Brain Tumors
- •3.1 Standard MRI
- •3.2 Differential Diagnosis of Common Pediatric Brain Tumors
- •3.3 Early Postoperative Imaging
- •3.4 Meningeal Dissemination
- •References
- •MR Spectroscopic Imaging
- •1 Methods
- •1.1 Introduction to MRS
- •1.2 Summary of Spectroscopic Imaging Techniques Applied in Tumor Diagnostics
- •1.3 Partial Volume Effects Due to Low Resolution
- •1.4 Evaluation of Metabolite Concentrations
- •1.5 Artifacts in Metabolite Maps
- •2 Tumor Metabolism
- •3 Tumor Grading and Heterogeneity
- •3.1 Some Aspects of Differential Diagnosis
- •4 Prognostic Markers
- •5 Treatment Monitoring
- •References
- •MR Perfusion Imaging
- •1 Key Points
- •2 Methods
- •2.1 Exogenous Tracer Methods
- •2.1.1 Dynamic Susceptibility Contrast MRI
- •2.1.2 Dynamic Contrast-Enhanced MRI
- •3 Clinical Application
- •3.1 General Aspects
- •3.3 Differential Diagnosis of Tumors
- •3.4 Tumor Grading and Prognosis
- •3.5 Guidance for Biopsy and Radiation Therapy Planning
- •3.6 Treatment Monitoring
- •References
- •Diffusion-Weighted Methods
- •1 Methods
- •2 Microstructural Changes
- •4 Prognostic Marker
- •5 Treatment Monitoring
- •Conclusion
- •References
- •1 MR Relaxometry Techniques
- •2 Transverse Relaxation Time T2
- •4 Longitudinal Relaxation Time T1
- •6 Cest Method
- •7 CEST Imaging in Brain Tumors
- •References
- •PET Imaging of Brain Tumors
- •1 Introduction
- •2 Methods
- •2.1 18F-2-Fluoro-2-Deoxy-d-Glucose
- •2.2 Radiolabeled Amino Acids
- •2.3 Radiolabeled Nucleoside Analogs
- •2.4 Imaging of Hypoxia
- •2.5 Imaging Angiogenesis
- •2.6 Somatostatin Receptors
- •2.7 Radiolabeled Choline
- •3 Delineation of Tumor Extent, Biopsy Guidance, and Treatment Planning
- •4 Tumor Grading and Prognosis
- •5 Treatment Monitoring
- •7 PET in Patients with Brain Metastasis
- •8 Imaging of Brain Tumors in Children
- •9 Perspectives
- •References
- •1 Treatment of Gliomas and Radiation Therapy Techniques
- •2 Modern Methods and Strategies
- •2.2 3D Conformal Radiation Therapy
- •2.4 Stereotactic Radiosurgery (SRS) and Radiotherapy
- •2.5 Interstitial Brachytherapy
- •2.6 Dose Prescription
- •2.7 Particle Radiation Therapy
- •3 Role of Imaging and Treatment Planning
- •3.1 Computed Tomography (CT)
- •3.2 Magnetic Resonance Imaging (MRI)
- •3.3 Positron Emission Tomography (PET)
- •4 Prognosis
- •Conclusion
- •References
- •1 Why Is Advanced Imaging Indispensable for Modern Glioma Surgery?
- •2 Preoperative Imaging Strategies
- •2.4 Preoperative Imaging of Function and Functional Anatomy
- •2.4.1 Imaging of Functional Cortex
- •2.4.2 Imaging of Subcortical Tracts
- •3 Intraoperative Allocation of Relevant Anatomy
- •Conclusions
- •References
- •Future Methods in Tumor Imaging
- •1 Special Editing Methods in 1H MRS
- •1.1 Measuring Glycine
- •2 Other Nuclei
- •2.1.1 Spatial Resolution
- •2.1.2 Measuring pH
- •2.1.3 Measuring Lipid Metabolism
- •2.1.4 Energy Metabolism
- •References
148 |
J. Wölfer and W. Stummer |
|
|
2.3What Is the Surgical Target in HighGrade Gliomas?
In HGG a prognostic value of resection has always been associated with the removal of the contrast-enhancing parts of the tumor, as visualized by the contrast-enhanced
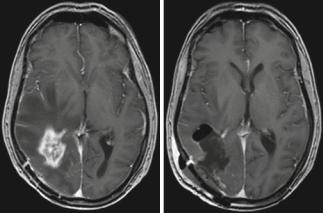
Fig. 4 Glioblastoma; male, 61 years. – contrast-enhanced T1 sequence. Left: Edema encasing the region of contrast enhancement. Right: Postoperative image after “supramarginal” resection guided by 5-ALA fluorescence (Courtesy of the Institute of Clinical Radiology, Münster)
T1-weighted sequence, although it is well known that malignant glioma cells infiltrate many centimeters beyond the contrast-enhancing tumor margins or even beyond the zone of edema (Sahm et al. 2012). This has been observed for both the anaplastic astrocytomas (Keles et al. 2006) and, with several large cohort studies and one randomized study, in glioblastoma (McGirt et al. 2009a, b; LaCroix et al. 2001; Stummer et al. 2008; Stummer et al. 2012; Kreth et al. 2013). This observation may be related to the biological characteristics of contrast-enhancing tumor. Contrastenhancing areas are highly replete with dysfunctional vessels from uncontrolled angiogenesis, which are inefficient in supplying the tumor with oxygen, resulting in tumor hypoxia. This hypoxia, among other factors, renders the tumor resistant to radiotherapy (Stummer et al. 2011a, b).
Recent evidence, however, suggests that resections extending beyond the area of contrast enhancement might further improve prognosis in patients with glioblastomas as demonstrated by Aldave et al. (2013). This group operated glioblastoma patients guided by 5-ALA fluorescence. The zone of fluorescing tissue, which the surgeon can observe intraoperatively, is now known to extend well beyond the area of contrast enhancement as visualized by the MRI or even the 18FET-PET (Fig. 4) (Schucht et al. 2014; Roessler et al. 2012). Aldave et al. (2013) analyzed all their patients without contrast-enhancing residual tumor on early postoperative MRI and stratified by whether all fluorescing tissue
had been resected or not. They demonstrated that patients in whom all fluorescing tissue had been removed survived significantly longer. The MRI equivalent to this region of fluorescence extending beyond contrast enhancement is not known. Whether MR spectroscopy (see Chap. 3), diffusionweighted imaging (Chap. 5), or MR perfusion methods (Chap. 4) will play a future role in helping to predict the extension of fluorescence beyond the region of contrast enhancement cannot yet be foreseen. In a malignant glioma, contrast enhancement will typically be encased by the zone of edema (Fig. 4).
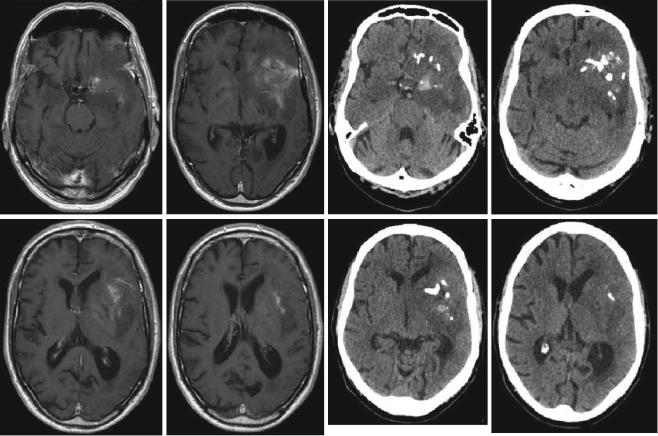
The CT scan may also play a role in defining surgical strategies for oligodendrogliomas or oligoastrocytomas. These tumors frequently show calcifications on CT scans which are sometimes difficult to confidently diagnose on the MRI. Recognizing a possible “oligo” component on preoperative imaging will influence the surgeon’s strategy regarding cytoreduction when neurological function is at stake (Fig. 5). High-grade oligodendrogliomas and oligoastrocytomas have a better prognosis in the face of cytotoxic therapies than anaplastic astrocytomas (Wick et al. 2009), and thus for such patients jeopardizing neurological function for the sake of radical tumor removal need not be an option.
2.4Preoperative Imaging of Function and Functional Anatomy
During glioma surgery preservation of neurological function is paramount compared to radicalness, but safely achievable utmost radicalness must always remain the goal of surgery, or else the risks of surgery would not be justified. Intraoperatively, low-grade or infiltrating high-grade tumor tissue outside the region of gross necrosis is not easily distinguishable using microscopic visualization techniques, nor is function. Thus, preoperative knowledge of the anatomical localization of non-dispensable functions relative to tumor tissue aimed for resection is crucial. This knowledge will influence the surgeon’s judgment of resectability in the individual case and will guide his decision on the surgical approach, the selection of technological aid for surgery (neuronavigation, fluorescence, intraoperative mapping or monitoring), and his counseling of patients and families regarding the risks and gains of surgery.
2.4.1Imaging of Functional Cortex
Intraoperatively, apart from gross anatomical landmarks for indication of functionally important cortex and tracts, the brain offers few clues to individual functional representation on the one hand, or to the extent of resectable tumor on the other (see Conventional MR imaging).
With the exception of the primary motor cortex (Farrel et al. 2007; Shinoura et al. 2009), the classical anatomi-
Advanced Imaging Modalities and Treatment of Gliomas: Neurosurgery |
149 |
|
|
Fig. 5 Anaplastic oligoastrocytoma with calcifications; male, 72 years. – contrast-enhanced T1 (left), and native CT (right; courtesy of the Institute of Clinical Radiology, Münster)
cal representation of functions on the cortical surface may show considerable interindividual variability, especially with regard to the areas of language representation. The so-called “Broca” area in the inferior precentral frontal gyrus and the so-called “Wernicke” region located in the posterior third of the superior temporal gyrus are more or less statistical concentrations of cortical locations relevant for speech phonology, syntax, and semantics (Vigneau et al. 2006). Cortical regions with significant language functions may be found outside the classical regions so that simply respecting anatomical topography will not give the necessary safety for resecting tumors close to the so-called eloquent brain regions (McGirt et al. 2009a, b; Ojemann et al. 2008). Such knowledge has been derived from mapping during brain surgery under local anesthesia. Furthermore, brain tumors sometimes lead to considerable distortion of normal topography, a factor in itself obscuring efforts to maintain safety during resection close to eloquent brain regions. Further, functional rearrangement over time as an indicator of brain plasticity has been demonstrated as a response to tumor invading eloquent brain (Desmurget et al. 2007), which further confounds attempts to localize individual functions based on anatomy alone. Thus, preoperative imaging of the individual
representation of function would be of utmost benefit. To this end functional MRI (fMRI) is the most commonly employed modality (see Conventional MR imaging) (Belliveau et al. 1991). Appropriate computer algorithms serve to convert this information into overlays on conventional MR imagery for the representation of “eloquent” brain regions (Fig. 6).
This information can be imported into the image base used for neuronavigation, which can then be used for locating functionally important cortex intraoperatively (Nimsky et al. 2011). However, the reliability of fMRI in precisely predicting the location of cortical locations critical for language functions is not unanimously accepted (Giussani et al. 2010). Furthermore, fMRI information suffers from brain shift, as does neuronavigation, further losing reliability during the course of surgery. Nevertheless, fMRI gives sufficient preoperative information to determine which hemisphere is dominant for language, thus allowing to determine preoperatively whether advanced neurophysiological monitoring or mapping techniques are necessary and helping to approximately predict at which locations the surgeon should test for function during surgery.
A newer development for the expansion of the potential of MRI and neuronavigation is transcranial magnetic stimula-
