
- •Contents
- •Contributors
- •Brain Tumor Imaging
- •1 Introduction
- •1.1 Overview
- •2 Clinical Management
- •3 Glial Tumors
- •3.1 Focal Glial and Glioneuronal Tumors Versus Diffuse Gliomas
- •3.3 Astrocytomas Versus Oligodendroglial Tumors
- •3.4.1 Diffuse Astrocytoma (WHO Grade II)
- •3.5 Anaplastic Glioma (WHO Grade III)
- •3.5.1 Anaplastic Astrocytoma (WHO Grade III)
- •3.5.3 Gliomatosis Cerebri
- •3.6 Glioblastoma (WHO Grade IV)
- •4 Primary CNS Lymphomas
- •5 Metastatic Tumors of the CNS
- •References
- •MR Imaging of Brain Tumors
- •1 Introduction
- •2 Brain Tumors in Adults
- •2.1 Questions to the Radiologist
- •2.2 Tumor Localization
- •2.3 Tumor Malignancy
- •2.4 Tumor Monitoring
- •2.5 Imaging Protocol
- •Computer Tomography
- •2.6 Case Illustrations
- •3 Pediatric Brain Tumors
- •3.1 Standard MRI
- •3.2 Differential Diagnosis of Common Pediatric Brain Tumors
- •3.3 Early Postoperative Imaging
- •3.4 Meningeal Dissemination
- •References
- •MR Spectroscopic Imaging
- •1 Methods
- •1.1 Introduction to MRS
- •1.2 Summary of Spectroscopic Imaging Techniques Applied in Tumor Diagnostics
- •1.3 Partial Volume Effects Due to Low Resolution
- •1.4 Evaluation of Metabolite Concentrations
- •1.5 Artifacts in Metabolite Maps
- •2 Tumor Metabolism
- •3 Tumor Grading and Heterogeneity
- •3.1 Some Aspects of Differential Diagnosis
- •4 Prognostic Markers
- •5 Treatment Monitoring
- •References
- •MR Perfusion Imaging
- •1 Key Points
- •2 Methods
- •2.1 Exogenous Tracer Methods
- •2.1.1 Dynamic Susceptibility Contrast MRI
- •2.1.2 Dynamic Contrast-Enhanced MRI
- •3 Clinical Application
- •3.1 General Aspects
- •3.3 Differential Diagnosis of Tumors
- •3.4 Tumor Grading and Prognosis
- •3.5 Guidance for Biopsy and Radiation Therapy Planning
- •3.6 Treatment Monitoring
- •References
- •Diffusion-Weighted Methods
- •1 Methods
- •2 Microstructural Changes
- •4 Prognostic Marker
- •5 Treatment Monitoring
- •Conclusion
- •References
- •1 MR Relaxometry Techniques
- •2 Transverse Relaxation Time T2
- •4 Longitudinal Relaxation Time T1
- •6 Cest Method
- •7 CEST Imaging in Brain Tumors
- •References
- •PET Imaging of Brain Tumors
- •1 Introduction
- •2 Methods
- •2.1 18F-2-Fluoro-2-Deoxy-d-Glucose
- •2.2 Radiolabeled Amino Acids
- •2.3 Radiolabeled Nucleoside Analogs
- •2.4 Imaging of Hypoxia
- •2.5 Imaging Angiogenesis
- •2.6 Somatostatin Receptors
- •2.7 Radiolabeled Choline
- •3 Delineation of Tumor Extent, Biopsy Guidance, and Treatment Planning
- •4 Tumor Grading and Prognosis
- •5 Treatment Monitoring
- •7 PET in Patients with Brain Metastasis
- •8 Imaging of Brain Tumors in Children
- •9 Perspectives
- •References
- •1 Treatment of Gliomas and Radiation Therapy Techniques
- •2 Modern Methods and Strategies
- •2.2 3D Conformal Radiation Therapy
- •2.4 Stereotactic Radiosurgery (SRS) and Radiotherapy
- •2.5 Interstitial Brachytherapy
- •2.6 Dose Prescription
- •2.7 Particle Radiation Therapy
- •3 Role of Imaging and Treatment Planning
- •3.1 Computed Tomography (CT)
- •3.2 Magnetic Resonance Imaging (MRI)
- •3.3 Positron Emission Tomography (PET)
- •4 Prognosis
- •Conclusion
- •References
- •1 Why Is Advanced Imaging Indispensable for Modern Glioma Surgery?
- •2 Preoperative Imaging Strategies
- •2.4 Preoperative Imaging of Function and Functional Anatomy
- •2.4.1 Imaging of Functional Cortex
- •2.4.2 Imaging of Subcortical Tracts
- •3 Intraoperative Allocation of Relevant Anatomy
- •Conclusions
- •References
- •Future Methods in Tumor Imaging
- •1 Special Editing Methods in 1H MRS
- •1.1 Measuring Glycine
- •2 Other Nuclei
- •2.1.1 Spatial Resolution
- •2.1.2 Measuring pH
- •2.1.3 Measuring Lipid Metabolism
- •2.1.4 Energy Metabolism
- •References
Advanced Imaging Modalities and Treatment of Gliomas: Radiation Therapy |
141 |
|
|
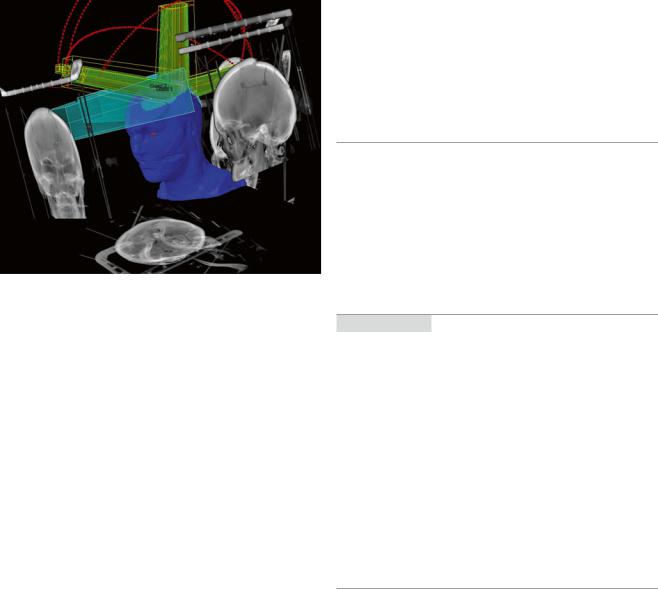
Fig. 3 Image-guided radiation therapy (IGRT). Patient positioning is verified by X-ray images taken directly before treatment. Treatment images are matched to reconstructed images from the planning CT (shown in gray) by using sophisticated software to adjust the treatment table in 6 rotation axes. This guarantees a high precision in dose application. The blue figure represents a patient’s head in a stereotactic RT mask. The yellow and green beams represent the stereotactic photon beams
linear accelerator is monitored by X-rays using formerly with radiographic films and currently electronic portal imaging. Features of IGRT include daily online imaging, X-rays, cone beam CT, and megavolt CT to four-dimensional (4D) target localization. For brain tumors the combination of gantry and a planar kV imaging system leads to high-resolution diagnostic quality images of the patient in treatment position. The target tracking is by orthogonal X-ray images matched using bone or implanted markers (Fig. 3). The exposure to radiation is considerably less than using electronic portal imaging device (EPID).
IGRT has taken on greater importance with the introduction of IMRT including prolonged treatment time and the presence of steep dose gradients. The use of high fraction size in stereotactic radiation therapy requires an optimal target definition patient positioning to limit complication risk.
A cone beam CT can be made before and even during the radiation treatment in the treatment position to check the position of the patient (Jaffray et al. 2002).
3.5Recurrence and Re-irradiation
Diagnostic challenge arises in cases of recurrence in highgrade brain tumors. Often, there are post-therapeutic changes visible on MRI and CT that can develop over a long time span. In cases of contrast enhancement, it is often impossible
to differentiate between post-therapeutic changes and true tumor recurrence.
During follow-up 18F-FET PET contributes important additional information for clinical management over and above the information obtained by MRI response assessment based on RANO criteria. More accuracy is needed in cases of re-irradiation, because of the exposure of organs at risk.
4Prognosis
For the future we will pay more attention to tumor heterogeneity. We know that tumor stem cells are radioresistant while differentiated cells are more radiosensitive. It is worthwhile to learn more about these stem cells in order to optimize the treatment, for example, by modifying the fractionation scheme (Leder et al. 2014).
Conclusion
Radiation therapy plays an important role in the treatment of gliomas and prolongs the survival of the patients. The most effective and widely used technique is LINAC percutaneous radiation therapy with photons.
Radiation techniques have improved during the past decades, starting with opposed fields for whole brain irradiation leading to sophisticated intensity-modulated radiotherapy using more than 150 fields with modified fluency. By upgrading the precision of the beam, imaging modalities became increasingly important in treatment planning and in treatment application. Advanced imaging techniques will stay irreplaceable in the further progress of radiation therapy.
References
Andersen AP (1978) Postoperative irradiation of glioblastomas: results in a randomized series. Acta Oncol 17(6):475–484
Brandsma D, van den Bent MJ (2009) Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 22(6): 633–638
Castro JR, Phillips TL, Prados M et al (1997) Neon heavy charged particle radiotherapy of glioblastoma of the brain. Int J Radiat Oncol Biol Phys 38(2):257–261
Chan JL, Lee SW, Fraass BA et al (2002) Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol 20(6):1635–1642
Chang CH, Horton J, Schoenfeld D et al (1983) Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A Joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer 52(6):997–1007
Clarke JL, Chang S (2009) Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep 9(3):241–246
142 |
I. Goetz and A.-L. Grosu |
|
|
Coffey RJ, Lunsford LD, Taylor FH (1998) Survival after stereotactic biopsy of malignant gliomas. Neurosurgery 22(3):465
Colli B, Al-Mefty O (2001) Chordomas of the craniocervical junction: follow-up review and prognostic factors. J Neurosurg 95(6): 933–943
Combs SE, Kieser M, Rieken S et al (2010) Randomized phase II study evaluating a carbon ion boost applied after combined radiochemotherapy with temozolomide versus a proton boost after radiochemotherapy with temozolomide in patients with primary glioblastoma: the CLEOPATRA trial. BMC Cancer 10:478
Corn BW, Yousem DM, Scott CB et al (1994) White matter changes are correlated significantly with radiation dose. Observations from a randomized dose-escalation trial for malignant glioma (Radiation Therapy Oncology Group 83–02). Cancer 74(10):2828–2835
Fitzek MM, Thornton AF, Rabinov JD et al (1999) Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg 91(2):251–260
Gaedicke S, Braun F, Prasad S et al (2014) Noninvasive positron emission tomography and fluorescence imaging of CD133+ tumor stem cells. Proc Natl Acad Sci U S A 111(6):E692–E701
Galldiks N, Langen KJ, Holy R et al (2012) Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)- L-tyrosine PET in comparison to MRI. J Nucl Med 53(7): 1048–1057
Giglio P, Gilbert MR (2003) Cerebral radiation necrosis. Neurologist 9(4):180–188
Glatstein E, Lichter AS, Fraass BA et al (1985) The imaging revolution and radiation oncology: use of CT, ultrasound, and NMR for localization, treatment planning and treatment delivery. Int J Radiat Oncol Biol Phys 11(2):299–314
Götz I, Grosu AL (2013) [(18)F]FET-PET imaging for treatment and response monitoring of radiation therapy in malignant glioma patients – a review. Front Oncol 3:104
Götz L, Spehl TS, Weber WA, Grosu AL (2012) PET and SPECT for radiation treatment planning. Q J Nucl Med Mol Imaging 56(2): 163–172, Review
Grosu AL, Feldmann HJ, Albrecht C et al (1998) 3-Dimensional irradiation planning in brain tumors. The advantages of the method and the clinical results. Strahlenther Onkol 174(1):7–13, German
Grosu AL, Weber WA, Riedel E et al (2005a) L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys 63(1):64–74
Grosu AL, Weber WA, Franz M et al (2005b) Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys 63(2):511–519
Grosu AL, Astner ST, Riedel E et al (2011) An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl- 11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys 81(4):1049–1058
Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA (2002) Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys 53(5):1337–1349
Langen KJ, Muhlensiepen H, Holschbach M et al (2000) Transport mechanisms of 3-[123I]iodo-alpha-methyl-L-tyrosine in a human glioma cell line: comparison with [3H]methyl]-L-methionine. J Nucl Med 41(7):1250–1255
Leder K, Pitter K, Laplant Q et al (2014) Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell 156(3):603–616
Minniti G, Amelio D, Amichetti M et al (2010) Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol 97(3):377–381
Narayana A, Yamada J, Berry S et al (2006) Intensity-modulated radiotherapy in high-grade gliomas: clinical and dosimetric results. Int J Radiat Oncol Biol Phys 64(3):892–897
Norden AD, Young GS, Setayesh K et al (2008) Bevacizumab for recurrent malignant gliomas Efficacy, toxicity, and patterns of recurrence. Neurology 70(10):779–787
Pauleit D, Floeth F, Hamacher K et al (2005) O-(2-[18F] fluoroethyl)- L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128(Pt 3):678–687
Piroth MD, Pinkawa M, Holy R et al (2012) Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas. Strahlenther Onkol 188(4):334–339
Pöpperl G, Kreth FW, Mehrkens JH et al (2007) FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging 34(12):1933–1942
Rieken S, Habermehl D, Giesel FL et al (2013) Analysis of FET-PET imaging for target volume definition in patients with gliomas treated with conformal radiotherapy. Radiother Oncol 109(3):487–492
Schulz-Ertner D, Tsujii H (2007) Particle radiation therapy using proton and heavier ion beams. J Clin Oncol 25(8):953–964, Review Selker RG, Shapiro WR, Burger P et al (2002) The Brain Tumor
Cooperative Group NIH Trial 87–01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery 51(2):343–355
Shapiro WR (1986) Therapy of adult malignant brain tumors: what have the clinical trials taught us? Semin Oncol 13(1):38–45
Shaw E, Scott C, Souhami L et al (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys 47(2):291–298
Shepherd SF, Laing RW, Cosgrove VP et al (1997) Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol Biol Phys 37(2):393–398
Souhami L, Seiferheld W, Brachman D et al (2004) Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93–05 protocol. Int J Radiat Oncol Biol Phys 60(3):853–860
Stupp R, Mason WP (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10): 987–996
Taal W, Brandsma D, de Bruin HG et al (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113(2):405–410 Tsien C, Moughan J, Michalski JM et al (2009) Phase I threedimensional conformal radiation dose escalation study in newly diagnosed glioblastoma: Radiation Therapy Oncology Group Trial
98–03. Int J Radiat Oncol Biol Phys 73(3):699–708
Walker MD, Alexander E Jr, Hunt WE et al (1978) Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: a cooperative clinical trial. J Neurosurg 49(3):333–343
Walker MD, Green SB, Byar DP et al (1980) Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 303(23):1323–1329
Wallner KE, Galicich JH, Krol G et al (1989) Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys 16(6):1405–1409
Weber WA, Wester HJ, Grosu AL et al (2000) O-(2-[18F]fluoroethyl)- L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med 27(5):542–549 Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in
neuro-oncology working group. J Clin Oncol 28(11):1963–1972

Advanced Imaging Modalities
and Treatment of Gliomas:
Neurosurgery
Johannes Wölfer and Walter Stummer
Contents |
|
|
1 |
Why Is Advanced Imaging Indispensable |
|
|
for Modern Glioma Surgery?........................................... |
143 |
2 |
Preoperative Imaging Strategies...................................... |
145 |
2.1What Is the Surgical Target
in Low-Grade Gliomas?...................................................... |
145 |
2.2The Role of Modern Imaging in Indicating Surgery
|
in Low-Grade Gliomas........................................................ |
146 |
2.3 |
What Is the Surgical Target in High-Grade Gliomas? ........ |
148 |
2.4 |
Preoperative Imaging of Function |
|
|
and Functional Anatomy..................................................... |
148 |
3 |
Intraoperative Allocation of Relevant Anatomy ............ |
150 |
Conclusions.................................................................................. |
151 |
|
References .................................................................................... |
152 |
|
Abstract
Current data warrant cytoreductive surgical approaches in low as well as high grade gliomas. Surgical aims vary depending on histology – speed of growth and impending malignant transformation being major aspects in low grades, while surgical improvement of preconditions for adjuvant therapy gains prognostic relevance in malignant glioma. A delicate balance between the extent of resection and functional integrity has to be kept in all of these procedures. Their planning and realization thus require reliable conceptions of tumor extension with reference to functional anatomy. Radiology and nuclear medicine provide preoperative and, to a certain extent also intraoperative insights. Additional intraoperative assistance is provided by electrophysiology and – in malignant glioma – by direct tumor visualization, which uses pharmacologic agents together with specialized optics. Emerging surgical concepts like functionally guided tissue removal or so-called supramarginal resection are still waiting for their clinical validation and for new techniques which might be able to morphologically substantiate the respective rationale.
J. Wölfer • W. Stummer (*)
Neurochirurgische Klinik, Universitätsklinikum Münster, Albert-Schweitzer-Campus 1, Geb. A1, Münster 48149, Germany e-mail: woelfer@uni-muenster.de; walter.stummer@ukmuenster.de
1Why Is Advanced Imaging Indispensable for Modern Glioma Surgery?
During the last two decades, the understanding of the value of glioma resection has undergone a change. Despite a paucity of randomized studies, a number of prospective cohort studies have provided acceptable evidence that maximal cytoreduction is a meaningful treatment option which serves to improve prognosis in patients suffering from highand low-grade gliomas alike. Nowadays, many surgeons are adapting this strategy.
Low-grade glioma (LGG) patients are frequently young and oligosymptomatic, usually presenting with focal seizures which can be easily managed by appropriate medication. In such patients the neurosurgeon’s responsibility is particularly great since the purpose of surgery is not primarily
E. Hattingen, U. Pilatus (eds.), Brain Tumor Imaging, |
143 |
Med Radiol Diagn Imaging (2016)
DOI 10.1007/174_2016_1023, © Springer International Publishing Switzerland
144 |
J. Wölfer and W. Stummer |
|
|
the amelioration of symptoms. Neurosurgeons must keep this in mind before deciding on whether to operate and to what extent this should be done. On the other hand, recent studies have demonstrated that all LGGs will grow. The rate of growth has been calculated as about 4 mm/year (95 % CI: 3.8–4.4 mm, Mandonnet et al. 2003). This observation certainly questions whether a “wait-and-see” strategy is appropriate for the management of these patients if cytoreductive surgery may be considered an option. To the least, this observation cautions against just comparing one MRI with the previous one but rather suggests to use the earliest available MRI to decide whether a tumor is more or less stable or whether it is growing or changing, especially in the light of the quoad vitam prognosis of these patients. About 75 % of LGG patients die within 5–10 years after initial diagnosis (Keles et al. 2001; McGirt et al. 2008), and the prognosis of a low-grade glioma is similar to many non-glial cancers. Overall, such considerations justify active approaches in low-grade glioma therapy including cytoreductive surgery. Even though prospectively randomized studies which would unequivocally clarify the role of cytoreductive surgery in LGG patients are difficult to envision, a number of large and prospective cohort studies afford useful data on the value of cytoreductive surgery (McGirt et al. 2008; Smith et al. 2008). One such study from Norway provided additional interesting data (Jakola et al. 2013). In Norway basically two neurosurgical departments are involved in glioma care. One department favors biopsies, the other craniotomies. Patients treated in the hospital with the more aggressive approach survived longer. Of note: Even though controlled trials are still miss-
ing (Veeravagu et al. 2013), several large cohort studies indicate that resection should extend as far as possible because only complete or nearly complete resections, as measured by MRI as the best available imaging instrument, will have an impact on prognosis (McGirt et al. 2008; Smith et al. 2008).
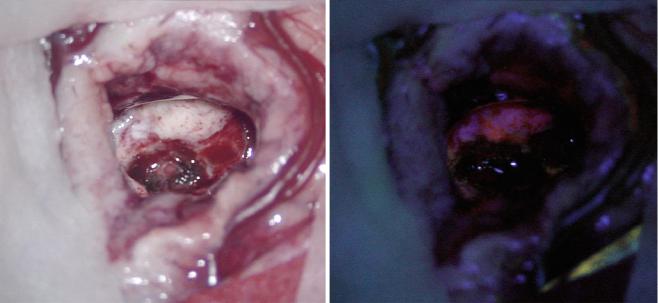
Similarly, data are available which underline the value of resection in high-grade gliomas (HGG; Laws et al. 2003). One small randomized study compared biopsies with resections in elderly (>65 years of age) HGG patients (Vuorinen et al. 2003). Patients treated by craniotomy and tumor resection survived significantly longer. Another prospectively randomized study on 5-ALA for fluorescence-guided resections (Fig. 1) was able to demonstrate that patients had prolonged progression-free survival when the extent of the resection was improved by the use of 5-ALA (Stummer et al. 2006). Similar effects were observed in a study with patients being randomized into surgery with or without intraoperative MRI (Senft et al. 2011). Authors suggest that progression-free survival was improved by the use of intraoperative MRI, albeit not significantly due to the small number of patients in this study. However, most of these studies combined resection with adjuvant radiotherapy alone, and it might be questioned whether surgery in conjunction with adjuvant radiochemotherapy according to the EORTC regime still requires maximal cytoreduction. To this end, newer studies confirm that the prognostic effect of maximum possible resection of con- trast-enhancing tumor remains unmitigated or is even boosted when patients are treated by the current standard regime, concomitant radiochemotherapy, rather than radiotherapy alone (Stummer et al. 2012; Kreth et al. 2013). Similar to the results
Fig. 1 View through the microscope, the left image shows the native surgical site of a small glioblastoma. After exogenous 5-ALA application, tumor cells will become fluorescent under ultraviolet light
(red tumor on the right image). This feature identifies the tumor cells intraoperatively and facilitates complete resection
