
- •Contents
- •Contributors
- •Brain Tumor Imaging
- •1 Introduction
- •1.1 Overview
- •2 Clinical Management
- •3 Glial Tumors
- •3.1 Focal Glial and Glioneuronal Tumors Versus Diffuse Gliomas
- •3.3 Astrocytomas Versus Oligodendroglial Tumors
- •3.4.1 Diffuse Astrocytoma (WHO Grade II)
- •3.5 Anaplastic Glioma (WHO Grade III)
- •3.5.1 Anaplastic Astrocytoma (WHO Grade III)
- •3.5.3 Gliomatosis Cerebri
- •3.6 Glioblastoma (WHO Grade IV)
- •4 Primary CNS Lymphomas
- •5 Metastatic Tumors of the CNS
- •References
- •MR Imaging of Brain Tumors
- •1 Introduction
- •2 Brain Tumors in Adults
- •2.1 Questions to the Radiologist
- •2.2 Tumor Localization
- •2.3 Tumor Malignancy
- •2.4 Tumor Monitoring
- •2.5 Imaging Protocol
- •Computer Tomography
- •2.6 Case Illustrations
- •3 Pediatric Brain Tumors
- •3.1 Standard MRI
- •3.2 Differential Diagnosis of Common Pediatric Brain Tumors
- •3.3 Early Postoperative Imaging
- •3.4 Meningeal Dissemination
- •References
- •MR Spectroscopic Imaging
- •1 Methods
- •1.1 Introduction to MRS
- •1.2 Summary of Spectroscopic Imaging Techniques Applied in Tumor Diagnostics
- •1.3 Partial Volume Effects Due to Low Resolution
- •1.4 Evaluation of Metabolite Concentrations
- •1.5 Artifacts in Metabolite Maps
- •2 Tumor Metabolism
- •3 Tumor Grading and Heterogeneity
- •3.1 Some Aspects of Differential Diagnosis
- •4 Prognostic Markers
- •5 Treatment Monitoring
- •References
- •MR Perfusion Imaging
- •1 Key Points
- •2 Methods
- •2.1 Exogenous Tracer Methods
- •2.1.1 Dynamic Susceptibility Contrast MRI
- •2.1.2 Dynamic Contrast-Enhanced MRI
- •3 Clinical Application
- •3.1 General Aspects
- •3.3 Differential Diagnosis of Tumors
- •3.4 Tumor Grading and Prognosis
- •3.5 Guidance for Biopsy and Radiation Therapy Planning
- •3.6 Treatment Monitoring
- •References
- •Diffusion-Weighted Methods
- •1 Methods
- •2 Microstructural Changes
- •4 Prognostic Marker
- •5 Treatment Monitoring
- •Conclusion
- •References
- •1 MR Relaxometry Techniques
- •2 Transverse Relaxation Time T2
- •4 Longitudinal Relaxation Time T1
- •6 Cest Method
- •7 CEST Imaging in Brain Tumors
- •References
- •PET Imaging of Brain Tumors
- •1 Introduction
- •2 Methods
- •2.1 18F-2-Fluoro-2-Deoxy-d-Glucose
- •2.2 Radiolabeled Amino Acids
- •2.3 Radiolabeled Nucleoside Analogs
- •2.4 Imaging of Hypoxia
- •2.5 Imaging Angiogenesis
- •2.6 Somatostatin Receptors
- •2.7 Radiolabeled Choline
- •3 Delineation of Tumor Extent, Biopsy Guidance, and Treatment Planning
- •4 Tumor Grading and Prognosis
- •5 Treatment Monitoring
- •7 PET in Patients with Brain Metastasis
- •8 Imaging of Brain Tumors in Children
- •9 Perspectives
- •References
- •1 Treatment of Gliomas and Radiation Therapy Techniques
- •2 Modern Methods and Strategies
- •2.2 3D Conformal Radiation Therapy
- •2.4 Stereotactic Radiosurgery (SRS) and Radiotherapy
- •2.5 Interstitial Brachytherapy
- •2.6 Dose Prescription
- •2.7 Particle Radiation Therapy
- •3 Role of Imaging and Treatment Planning
- •3.1 Computed Tomography (CT)
- •3.2 Magnetic Resonance Imaging (MRI)
- •3.3 Positron Emission Tomography (PET)
- •4 Prognosis
- •Conclusion
- •References
- •1 Why Is Advanced Imaging Indispensable for Modern Glioma Surgery?
- •2 Preoperative Imaging Strategies
- •2.4 Preoperative Imaging of Function and Functional Anatomy
- •2.4.1 Imaging of Functional Cortex
- •2.4.2 Imaging of Subcortical Tracts
- •3 Intraoperative Allocation of Relevant Anatomy
- •Conclusions
- •References
- •Future Methods in Tumor Imaging
- •1 Special Editing Methods in 1H MRS
- •1.1 Measuring Glycine
- •2 Other Nuclei
- •2.1.1 Spatial Resolution
- •2.1.2 Measuring pH
- •2.1.3 Measuring Lipid Metabolism
- •2.1.4 Energy Metabolism
- •References

Advanced Imaging Modalities and Treatment of Gliomas: Neurosurgery |
145 |
|
|
in LGG, these newer studies suggest that even the removal of small residual contrast-enhancing tumor volumes is a decisive element and should be strived for (Stummer et al. 2012; Kreth et al. 2013), as was the case in earlier studies conducted prior to the dawn of concomitant radiochemotherapy for glioblastomas (Stummer et al. 2008; Lacroix et al. 2001). In these studies surgery was combined with radiotherapy only, and small areas of contrast-enhancing tumor remnants significantly worsened prognosis as well.
On the other hand, it is widely accepted that major neurological deficits will have an adverse influence on prognosis (McGirt et al. 2009a, b; Stummer et al. 2012). The reasons for this have not been clarified. Possibly, patients with prolonged and marked postoperative neurological impairments are less likely to undergo intensive second-line therapies in the case of tumor progression; additionally, they might be prone to immobility-related complications.
Altogether, neurosurgeons are faced with conflicting goals, i.e., to achieve maximal resection during removal of diffuse neoplasms of the brain while maintaining neurological integrity, both for lowand high-grade gliomas. In this context, modern preand intraoperative imaging modalities are indispensable for state-of-the-art surgical planning and should be directly integrated into surgical decision making. Imaging, be it preoperative or intraoperative, has greatly helped the surgeon in achieving these opposing aims. Postoperative imaging is equally essential. Not only will it allow the surgeon to assess the quality of his work and whether his predetermined resection aims were achieved, but it will also allow for more differentiated decisions on adjuvant therapies. Residual tumor on postoperative imaging is closely related to prognosis in patients with lowand highgrade gliomas alike.
This chapter gives an overview on how imaging strategies currently assist in surgical decision making and how they can be used intraoperatively for improving surgical management of gliomas.
2Preoperative Imaging Strategies
The decision of the surgeon on whether to perform debulking surgery, maximal cytoreduction, or only a biopsy, and the appropriate counseling of patients and families, depends on preoperative risk assessment. This assessment in turn relies on preoperative imaging, which gives information on the anatomical extent of tumor deemed appropriate for resection, on its potential dignity, and on the limits of resection, the latter being defined by functionally important brain structures, i.e., the cortex or deep white matter tracts that border on the tumor. Thus, preoperative imaging may be subdivided into imaging of surgical tumor morphology and imaging of functional brain anatomy.
2.1What Is the Surgical Target in Low-Grade Gliomas?
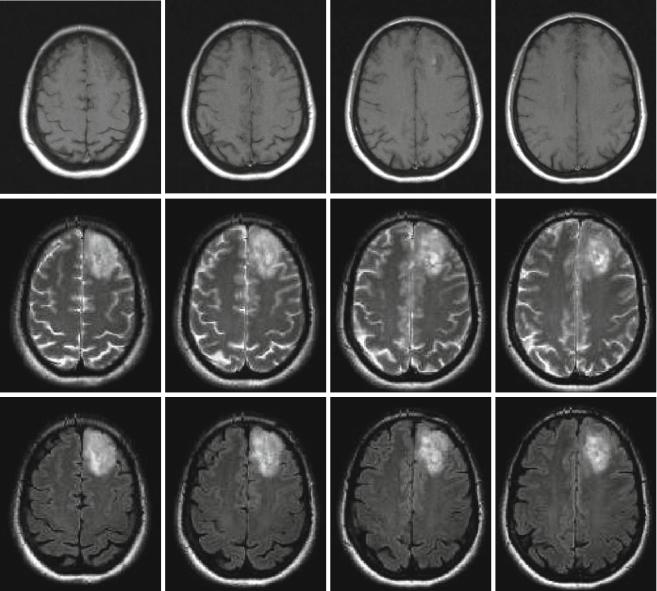
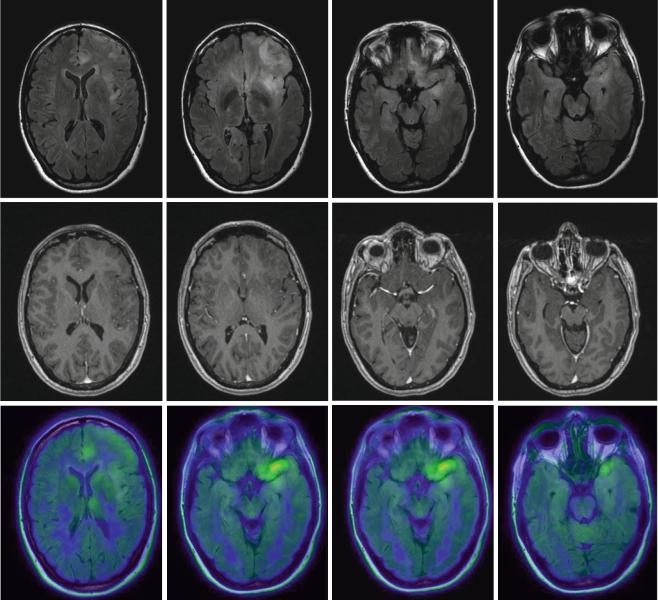
LGGs are diffuse lesions which extend further than to be assumed from the usual MRI sequences. Nevertheless, for the sake of defining resectability1 before, the estimated extent of resection during, and the surgical result after an operative procedure, standard MRI sequences will have to be relied upon as the modality giving the best morphological information. Among the available sequences, fluid attenuated inversion recovery (FLAIR) is the one most often used. To this end, three large studies which are frequently cited in conjunction with the value of resection in low-grade gliomas (Smith et al. 2008; McGirt et al. 2008; De Witt Hamer et al. 2012) rely on FLAIR images to define the extent of tumor and resection (Fig. 2). Apart from FLAIR images, additional sequences are considered necessary (see Conventional MR imaging), especially T2-weighted images (possibly in two orientations) and T1-weighted images before and after intravenous application of Gd-containing contrast agent, the latter intended to identify areas with possible malignant transformation. Identification of such hot spots is crucial since the surgeon has to ensure that these areas are specifically included into the histopathological analysis to avoid undergrading of tumors. Hot spots may also be found in LGG presenting as gliomatosis (Fig. 3). It has not yet been addressed in specific studies whether focal resection and treatment of such hot spots in an otherwise unresectable diffuse LGG influence prognosis.
Due to the diffuse nature of LGG with cells extending beyond what can generally be imaged by standard MRI (Sahm et al. 2012), “supratotal” resection strategies have been proposed (Duffau 2013; Yordanova et al. 2011), which consider functional rather than morphological borders of surgery. In such surgery, resection would not just be limited to the extent of the tumor as defined by the FLAIR image, but extend further up to cortex areas or deep white matter tracts which are considered functionally relevant. Such approaches are justified according to the value of maximal cytoreduction, provided they are safe. However, it remains to be established whether such surgery offers additional benefit.
1 “Resectability” in glioma surgery is a complex concept which is determined by the surgeon’s perception of possible neurological deficits related to surgical removal, which in turn is influenced by tumor location, tumor extension, and morphology, including vessels traversing or deep white matter tracts bordering to the tumor. Resectability is further influenced by the intended use of intraoperative monitoring and mapping techniques for minimizing risk while maximizing resection. Certain neurological functions are considered indispensable, such as language or motor function, while others might be considered amenable to limited sacrifice, such as visual field defects, or are simply not prioritized and therefore not monitored in the context of glioma treatment, such as elements of neurocognitive function.
146 |
J. Wölfer and W. Stummer |
|
|
Fig. 2 Left frontal low-grade glioma in different MR sequences; female, 51 years. – native T1 (top), T2 (middle), and FLAIR (bottom) sequences (Courtesy of the Institute of Clinical Radiology, Münster)
2.2The Role of Modern Imaging
in Indicating Surgery in Low-Grade Gliomas
The EORTC study (Pignatti et al. 2002) has helped to define LGG patients with poor prognosis as opposed to patients with a longer survival, based on morphological criteria, histology, patient age, and neurological deficits. Higher age (>40 years), diffuse astrocytic pathology rather than oligoastrocytoma and oligodendroglioma, and neurological deficits contribute to the risk. Large tumors (>6 cm) and tumors crossing the midline were independent risk factors as well.
Due to the restricted prognosis of high-risk patients, it might appear justified to treat these more aggressively, including surgical debulking or cytoreduction. Others have observed that the speed of growth, as determined from MR imaging, will also help to discern those tumors which carry a bad prognosis and possibly require a more aggressive surgical approach. Pallud et al. (2006) have demonstrated that if LGGs grow more than 8 mm per year, their prognosis is considerably worse than that of slower-growing tumors.
Amino acid positron emission tomography (PET) is a modern tool with increasing availability and acceptance for guiding the decision whether to perform surgery in appar-
Advanced Imaging Modalities and Treatment of Gliomas: Neurosurgery |
147 |
|
|
Fig. 3 Low-grade gliomatosis with metabolic hot spots demonstrated by PET; female, 45 years. – FLAIR (top), contrast-enhanced T1 (middle), and 18FET-PET (bottom) (Courtesy of the Institute of Clinical Radiology and Clinic for Nuclear Medicine, Münster)
ently low-grade gliomas and in which areas to specifically collect biopsies. Hot spots in diffusely infiltrating tumors do not only delineate malignant tumor regions (Kunz et al. 2012; Ewelt et al. 2011) but they will also help to avoid undergrading if areas of malignant degeneration are missed (Fig. 3). It is estimated that high-grade astrocytomas are frequently undergraded if they are only biopsied instead of being resected after craniotomy, the latter with a higher likelihood of finding the anaplastic focus in up to 30 % of cases (Glantz et al. 1991; Woodworth et al. 2005; Jackson et al. 2001; Muragaki et al. 2008). Even in histologically proven LGG, increased amino acid uptake appears to signify a worse prognosis than in LGG without enhanced
uptake (Floeth et al. 2007). Consequently, if a presumed LGG shows enhanced amino acid uptake, cytoreductive therapy should be considered more strongly, which can be based on the available evidence from the surgical treatment of HGG and the probability that such tumors might actually represent malignant gliomas which require adjuvant therapies.
PET (Chap. 7) and MR modalities (Chaps. 3, 4, 5, and 6) are currently being introduced into routine use, supplementing the information to be derived from standard sequences and possibly helping to identify low-grade tumors at risk for rapid progression (Guillevin et al. 2012; Geer et al. 2012; Sahin et al. 2013).
