
Книги по МРТ КТ на английском языке / Magnetic Resonance Imaging in Ischemic Stroke - K Sartor R 252 diger von Kummer Tobias Back
.pdf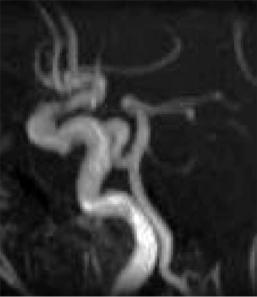
86 |
D. Petersen and S. Gottschalk |
as M2, the opercular vessels M3 and the peripheral cortical branches M4.
Intracranial MR angiography should be sensitive enough to depict stenosis at least in the M1 and M2 segments, even if exact quantification is not reliable. Small vessel diameters and signal loss due to spin saturation do not allow the diagnosis in peripheral MCA branches and lenticulostriatal vessels are normally not depicted. Nevertheless, an asymmetric signal loss within the peripheral MCA branches on MRA can be taken as an indicator for a high grade proximal M1 stenosis (Fujita et al. 1994). Relevant variants of the MCA are rare, differential problems can arise from duplications or fenestrations. The anterior cerebral artery (ACA) is much thinner than the MCA. The A1 segment reaches from the intracranial carotid bifurcation (carotid T) to the anterior communicating artery, the A2 segment up to the corpus callosum, where it divides into the pericallosal artery and the callosomarginal artery, also named as A3 segments. In most cases, a depiction of the ACA is sufficiently possible including the A2 segment, but a definitive evaluation of pathologic vessel wall lesions by means of MRA is not given in the majority of cases.
The vertebral artery normally originates from the subclavian artery. The first extraosseous segment is called V1, the intraforaminal section up to the first cervical vertebral body V2. V3 reaches from the exit of the first vertebral body to the dural passage, the V4 segment runs intradurally through the Foramen magnum and forms the vertebral junction with the contralateral vertebral to the basilar artery. The dural passage is occasionally marked by a small constriction of the vessel, which should not be taken as a stenosis.
Vertebral arteries vary in size and symmetry, where mostly the left vertebral artery is the dominant one. The coincidence of vertebral fenestrations and aneurysms is well known. The anterior spinal artery receives tiny vessels from the vertebral arteries which are physiologically not depicted by MRA due to their size. The posterior inferior cerebellar artery (PICA) as the largest, most important and most variable cerebellar artery; however, under normal conditions it is constantly depictable with TOF and CE-MRA.
The size of the basilar artery varies markedly. Given an embryonic type of PCA, the basilar artery can be extremely small and sometimes it appears to end at the level of the superior cerebellar arteries.
Persisting arterial anastomoses between the ICA and basilar artery occur as the primitive trigeminal
Fig. 5.12. Primitive trigeminal artery connecting the ICA and basilar artery (arrow)
artery (Fig. 5.12), rarely as the primitive acoustic artery, the primitive hypoglossal artery or as the proatlantal artery. They can easily be detected by MRA as well as the clinically relevant fenestrations of the basilar artery, which are seen atopically in more than 1%.
The anterior cerebellar artery (AICA) is normally the thinnest cerebellar artery and in MR angiographies is often insufficiently depicted. The superior cerebellar artery on the other hand can almost constantly be identified and anomalies such as duplications are mostly recognized on MR angiographies (Uchino et al. 2003). For the numerous anomalies and potential collateral circulations see specific literature (Osborn and Anderson 1977).
5.3
Clinical Application of MRA in Stenoocclusive Arterial Diseases
Quality standards for diagnosis of stenosis are set by catheter DSA, and noninvasive methods have to be compared with these. In the majority of cerebrovascular diseases, however, invasive methods are no longer indicated and are replaced by MRA or CT angiography, respectively. To assure an optimal therapy of stroke, vascular imaging has to provide answers to a number of clinical questions:
Vascular Anatomy and Pathology
•Is the sensitivity sufficient for detection or exclusion of a stenosis?
•Can stenoses be quantified by a standardized measure?
•Is it safe to differentiate between stenosis and occlusion?
•Is the underlying disease of a stenosis recognizable and are even mild lesions of the vessel wall visible?
•Can intravasal thrombosis be identified?
•Can collateral circulation be analyzed?
•Which technical limitations hamper the evaluation?
5.3.1
Atherosclerotic Diseases
Atherosclerosis mainly affects largeand mediumsized arteries. Extracranial manifestations at the carotid bifurcation statistically dominate the intracranial arteries. Besides typical manifestations at the carotid siphon or the vertebrobasilar junction, atherosclerosis is occasionally also found in peripheral intracranial vessel segments. Typical sequelae of atherosclerosis are stenosing plaque formations, ulcerations, dilatations or the evolution of fusiform aneurysms, which can be accompanied by extensive formation of thrombus.
5.3.1.1 Plaques
Superior to DSA, MRI can depict arterial flow or lumen as well as the vessel walls. Analysis of plaque morphology was improved in recent years by the development of specific MRI techniques. Plaques can now be characterized in vivo with regard to fibroid or lipid content or hemorrhagic lesions (Fig. 5.8). It can be expected that, in the future, plaque examination will influence stroke treatment protocols if the risk of plaque rupture can be reliably estimated (Cappendijk et al. 2005; Hayes et al. 1996; Trivedi et al. 2004; Yuan et al. 2001). Plaque examinations, however, are not yet clinical routine. Source images of TOF-MRA allow only limited assessment of vascular walls.
87
5.3.1.2
Extracranial Stenoses
The goal of optimized standard imaging protocols in stroke workup is to quantify carotid stenosis, recognize tandem stenosis or multifocal vessel pathology and possibly assess collateral circulation. Quality of flow-dependent MRA techniques for the evaluation of atherosclerotic stenosis of the carotid bifurcation was analyzed in a number of studies. In flow-dependent techniques, turbulent flow patterns and the high variance of flow velocities impair the quality of depiction as the main physiologic phenomena. PC-MRA showed an inferior sensitivity and diagnostic accuracy in the evaluation of stenosis compared with TOF techniques (Benjamin et al. 1997; Scarabino et al. 1998). With high resolution 3D TOF provided a better estimation of stenoses. The classification into <30%, <50%, 50%–70% and >70% proved to be reliable (Carriero et al. 1998; Rasanen et al. 1999). In stenoses higher than 85%, however, flow voids were seen (Anderson et al. 1994) even in transverse source images. A typical problem of 3D TOF-MRA is a mild overestimation of stenoses regarding their grade and length. The signal void in these cases is mainly induced by turbulence.
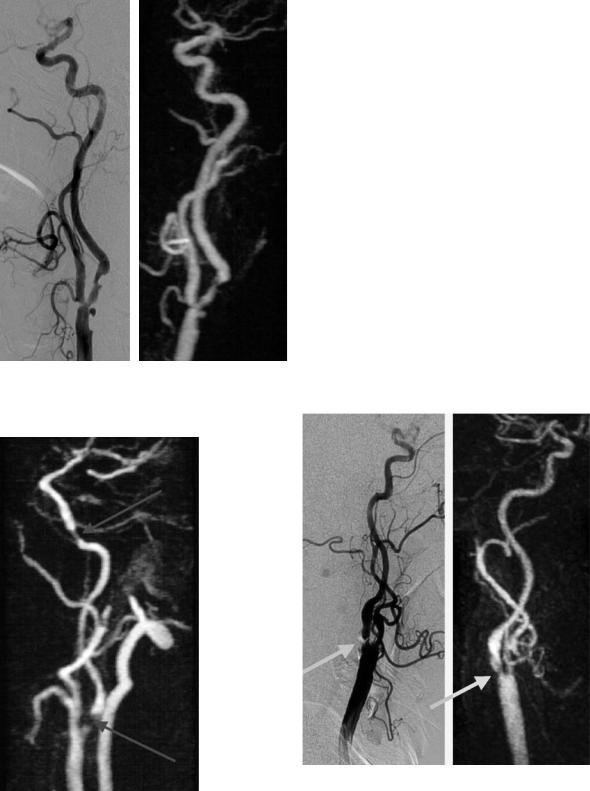
The main disadvantage of TOF angiography is the limited examination volumes. Even using multi-slab techniques it is problematic and extremely time consuming to depict the entire vessel tree from the aortic arch to the circle of Willis. Studies comparing TOF and CE-MRA proved the superiority of CE-MRA (Wetzel et al. 2001; Willig et al. 1998). CE-MRA provides a high sensitivity and no false positive findings in contrast to TOF-MRA, since turbulence and velocity variations do not remarkably influence the images (Stehling et al. 1997). To date, however, definitive standards for the technical performance of CE-MRA have not been established (Sundgren et al. 2002). While some examiners prefer sequences with very short acquisition times to avoid venous contrast overlap, a much better signal-to-noise ratio and spatial resolution are provided by longer data acquisitions in conjunction with a very exact contrast bolus timing. High spatial resolution techniques are necessary to depict short and highgrade stenoses with very complex flow patterns (Fig. 5.13) and to avoid unwanted signal disturbances in physiologically narrow vessel bends. Tandem stenoses of the ICA can thus also be reliably diagnosed (Fig. 5.14).
88 |
D. Petersen and S. Gottschalk |
a |
b |
Fig. 5.13a,b. Detailed depiction of a rather complex carotid stenosis with several sharp plaques and an ulceration (arrow) on CE-MRA (b) to an extent matching DSA (a)
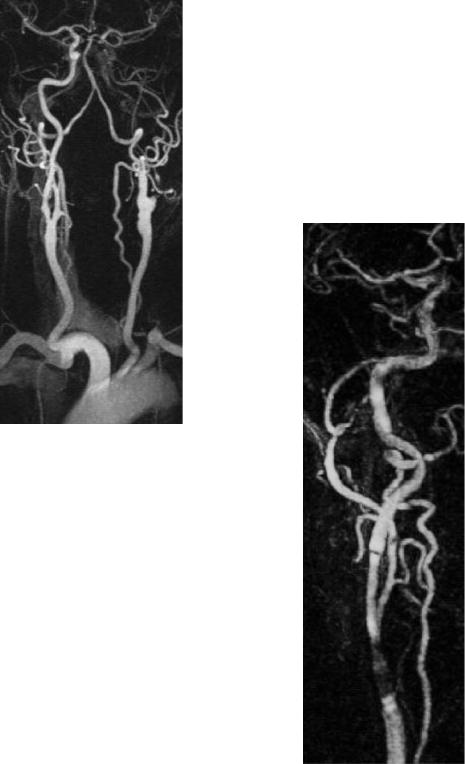
Using MRA techniques with high spatial resolution, the correlation with DSA in the estimation of stenosis grades is excellent, with a tendency towards mild overestimation (Cosottini et al. 2003). By means of physical principles, however, a quantification of atherosclerotic stenoses with CE-MRA alone is not exactly possible. Even if there are no signal losses within a voxel, the spatial resolution is not sufficient to transfer the NASCET and ESCT quantification criteria to MRA. If, however, MRA is combined with ultrasound, the grading of carotid stenosis is reliable (Friese et al. 2001a). A remaining limitation of CE-MRA is still the diagnosis of very short, web-like stenoses (Fig. 5.15).
Subtotal stenoses greatly reduce post-stenotic flow and are called pseudoocclusions, since they are difficult to differentiate from true occlusions even with Doppler ultrasound (without the use of ultrasound contrast media). There are only a few studies on this problem with limited numbers of patients. In the majority of cases, CE-MRA could differentiate stenosis from occlusion (Friese et al. 2001a; Remonda et al. 2002) (Fig. 5.16). In these
Fig. 5.14. Tandem stenoses of the left ICA demonstrated with CE-MRA. Approximately 80% proximal stenosis and high grade distal stenosis near the carotid canal (arrows)
a |
b |
Fig. 5.15. High grade, very short web-like stenosis of ICA on high resolution CEMRA (b) compared to pre-interventional DSA (a). MRA depicts abnormality , but fails to sufficiently delineate the web. DSA indication should be discussed if ultrasound and MRA do not match
Vascular Anatomy and Pathology |
89 |
guished from a plaque under certain conditions. This is especially true for the carotid bifurcation. In suggestive examination situations, conventional spin echo sequences could provide the evidence of thrombus. These sequences, however – including fat signal suppression and presaturation pulses – are time consuming and are not principally scheduled in standard examination protocols. Furthermore, the results are not always conclusive. Without the use of specially designed HF coils and sequence protocols the differentiation of thrombus against plaque remains difficult (Figs. 5.8, 5.9). Vessel wall
Fig. 5.16. Typical pseudoocclusion on CE-MRA. Note subtotal left proximal ACI stenosis (arrow). The distal vessel is collapsed and very thin, but relatively visible up to the circle of Willis
cases, it is less difficult to detect slow flowing blood in the distal ICA than not to confuse tiny extraintracranial collaterals with residual post-stenotic flow in the ICA. This problem also occurs preferentially in MRA techniques with low spatial resolution. Thus, more false positive than false negative results occur.
After stent PTA, metallic artifacts hinder evaluation of restenosis due to metallic artifacts with all MRA sequences. Even with detailed knowledge of artifacts, a reliable assessment is possible only in few cases. The severity of artifacts is mainly dependent on stent material (Fig. 5.17) where steel stents disturb imaging more than those from nitinol (Cavagna et al. 2001; Teng et al. 2004).
Although MRI also depicts vessel walls, the detection of an intravascular thrombus remains a diagnostic dilemma. On CE-MRA the intraluminal signal gap due to a thrombus can not be distin-
Fig. 5.17. Post-stent CE-MRA. Substantial signal loss due to metallic artifacts (a steel containing wall-stent was used). Note typical ring-like extinctions at proximal and distal tips of the stent. While CE-MRA did not really contribute to the problem of in-stent restenosis, ultrasound was normal in this case
90 |
D. Petersen and S. Gottschalk |
lesions that have a configuration and localization untypical for a plaque should be suspected as thrombus. Intramural hematomas in cases of arterial dissections are much easier to recognize, as described later.
5.3.1.3
Intracranial Stenoses
The depiction of intracranial arterial stenosis by MRA requires a high sensitivity resolution technique, while the importance of an exact grading by MRA is not defined yet. A preventive anti-calcula- tion for stenosis of proximal intracranial segments exceeding 50% is still a matter of debate (Sherman 2002).
In the diagnosis of intracranial stenosis, TOFMRA is definitely superior to PC-MRA protocols. Oelerich et al. (1998) found a sensitivity of 87% for 3D TOF-MRA in intracranial stenoses. The correlation with DSA was 78%, and other authors found a correlation with MRA up to 88% (Dagirmanjian et al. 1995). While sensitivity is relatively high, false negative findings are rare, so that with sufficient examination quality, a good negative prediction is reached.
Despite high anatomical resolution TOF-MRA does not allow an authentic quantification of intracranial vessel stenoses. Stenoses are commonly overestimated, especially in small vessels (Figs. 5.18, 5.19). Turbulences and velocity displacement effects depending on flow velocity and the tortuosity of a vessel cause false positive stenoses. Typical localizations of pseudostenoses are the carotid siphon and the bent entrance into the carotid canal. In CE-MRA the contrast medium limits spin saturation effects and very short echo times reduce the negative signal effect of turbulent flow patterns, whereby false positive intracranial stenosis can be identified (Gottschalk et al. 2002). Currently, the spatial resolution of CE-MRA as obtained with sufficient signal-to-noise ratio and without relevant venous contrast overlap in the limited acquisition time, is still significantly lower than that of TOF-MRA. Therefore, CE-MRA should be used intracranially as an add-on and not as a substitute for TOF-MRA.
In many instances TOF-MRA provides indirect indicators on collateral circulation at the level of the circle of Willis. While the anatomic conditions for collateral circulation can be studied, the recognition
a |
b |
c
Fig. 5.18a-c. TOF-MRA versus DSA correlation in intracranial stenoses. Occlusion of the right MCA and only mild stenosis of the left one on DSA (b, c), while signal loss on CE-MRA (a) should not lead to an overestimation of stenosis grade
of collateral flow itself is limited (Hoksbergen et al. 2003).
Preliminary studies of collateral circulation in high grade stenoses or occlusions using ultra fast dynamic MRA with temporal resolution in the range of a second did show delayed contrast enhancement in the affected vascular territory, but did not provide relevant additional information compared with conventional MRI and perfusion techniques probably due to the reduced spatial resolution (Wetzel et al. 2001). A dedicated analysis of collateral circulations, especially extra-intracranially, is still the domain of DSA as far as the exact depiction of anatomical connections is of importance. If the exact anastomotic vascular anatomy is not of primary interest, the collateral supply is better determined by MR perfusion techniques.
Vascular Anatomy and Pathology |
91 |
Patients with atherosclerosis present with stenoses and irregularities of vessel walls and elongated and ectatic arteries. Extreme dilative atherosclerotic pseudoaneurysms can typically occur with wall adherent thrombus. The differentiation of flow and thrombus signal can be difficult, although its age
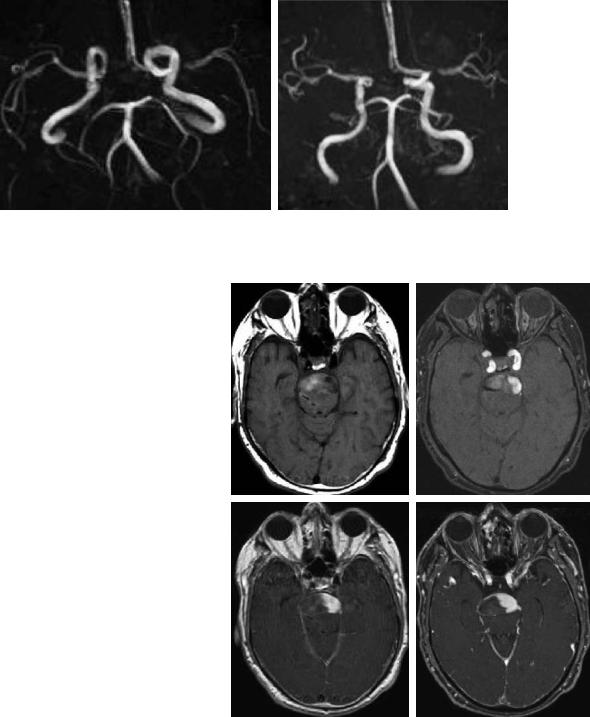
can fairly be estimated from the signal intensity in T1and T2*-weighted images. If a high T1 signal of thrombus interferes with flow signal of TOF-MRA, then post-contrast TOF or post-contrast spin echo sequences or a CE-MRA should be performed in addition (Fig. 5.20).
a |
b |
Fig. 5.19a,b. Multiple intracranial stenoses: TOF-MRA (a,b) with Doppler ultrasound correlation. High grade stenosis of left MCA (M1) was confirmed (>400 cm/s), suspected right M2 stenosis was estimated as mild by ultrasound and a left PCA stenosis was suspected only on MRA
a |
b |
Fig. 5.20a–d. Subacute |
thrombus formation |
|
in a large fusiforme aneurysm of the basilar |
|
|
artery. Parts of the thrombus are still isoin- |
|
|
tense to brain, while others are moderately |
|
|
hyperintense on spin echo T1-weighted image |
|
|
(a). Residual flow within the vessel is outlined |
|
|
against thrombus much better on post-con- |
|
|
trast T1-weighted spin echo (c) and post-con- |
|
|
trast TOF-MRA (d) than on unenhanced TOF- |
|
|
MRA (b) |
c |
d |
92 |
D. Petersen and S. Gottschalk |
5.3.2
Arterial Dissection
In young patients, non-atherosclerotic lesions of intraand extracranial vessels are relevant causes for strokes (Bogousslavsky and Pierre 1992). An arterial dissection is an intimal lesion with hemorrhage into the vessel wall. A constriction of the vessel lumen can range from a minimal stenosis to total occlusion. Dissections occur post-traumatically or spontaneously, in conjunction with hypertension or with migraine, often with fibromuscular dysplasia or with other connective tissue diseases (Guillon et al. 1998). In contrast to atherosclerosis the carotid bulb is spared in most cases and the manifestations occur near the skull base or intracranially. Multiple simultaneous dissections of the ICA and vertebral arteries are possible. Besides the ICA , intracranial dissections can affect the MCA, the vertebral arteries and the basilar artery in rare instances. The typical angiographic image of a dissection is characterized by an increasing vessel stenosis. In typical localizations, the image is often suggestive, but not proof of an underlying dissection. Occasionally a false lumen, a pseudoaneurysm or a minimal lesion with intimal flap is detectable. Dissections can cause acute or subacute ischemic strokes and persisting pseudoaneurysms can remain as a source of embolism.
The examination strategy in MRA has to concentrate not only on the depiction of the vessel lumen by CE-MRA in analogy to DSA. The evidence of a dis-
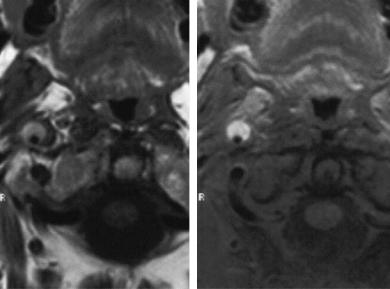
section on MRA is given rather by the assessment of an intramural hematoma. Using noninvasive angiographies, dissections are recognized with best sensitivity in TOF-MRA (Levy et al. 1994; Oelerich et al. 1999). The depiction is easy, if a mural hematoma is already signal intense on T1-weighted images and provides a typical falciform configuration on axial slices below the skull base. An additional fat saturated T1 spin echo sequence facilitates the correct diagnosis (Fig. 5.21). Hematomas, however, provide short T1 signal not in the early phase, but only after 4–6 days. Acute hematomas are isointense on T1weighted spin echo images and in the source images of TOF-MRA compared with brain and soft tissue and need to be analyzed accurately (Fig. 5.22). In cases of vessel occlusion or high grade stenosis, CEMRA may demonstrate a pointed tapering of the vessel (Carr et al. 2002).
In the clinical setting, one should start with a TOFMRA and an unenhanced T1-weighted sequence with fat saturation if possible, subsequently followed by a CE-MRA. It is important to mention that projection images and even single source images of CE-MRA constantly fail to show the intramural hematomas with high signal, even if very bright on TOF images (CE-MRA sequences are principally T1weighted, but they are optimized for very short T1 values as they occur during the first pass of arterial contrast boluses). The typical bright signal of intramural hematoma is only seen on spin echo images or the source images of TOF-MRA (Fig. 5.23). Stenoses in patients without risk factors or in unusual distal
a |
b |
Fig. 5.21a,b. Subacute dissection of the extracranial right ICA. Spin-echo T1weighted images pre- (a) and post-fat saturation (b)
Vascular Anatomy and Pathology |
93 |
Fig. 5.22a–d. Time course of post-traumatic carotid dissection. Spin echo T1-weighted (a,c) and TOF single slices (b,d). The typically bright hematoma and stenosis are well seen 12 days after the trauma (c,d). On day 4, however, (a,b) there is already stenosis on both images, but signal intensity of hematoma is not yet markedly increased
a |
b |
c |
d |
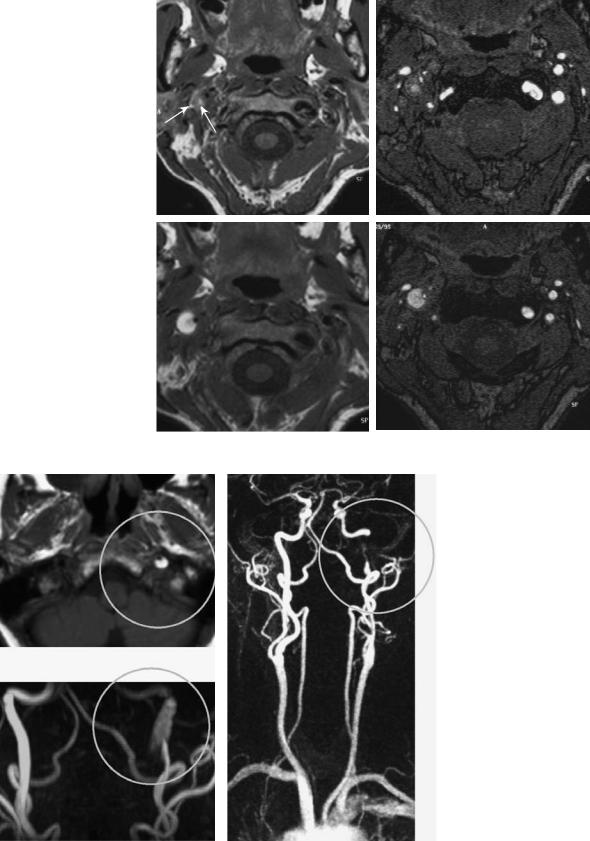
a
b
c
Fig. 5.23a–c. Carotid dissection. While the characteristic intramural hematoma is easily recognized as bright on TOF-MRA source images (a) and MIP (b), CE-MRA (c) outlines the long high grade stenosis of the distal extracranial vessel, but not the hematoma itself
94 |
D. Petersen and S. Gottschalk |
localizations should always raise the suspicion of dissections and therefore be examined with the above mentioned adequate methods.
Smooth dissections (Lucas et al. 2000) or less impressive vessel lesions, especially in elongated vessels of the posterior circulation, are diagnostically problematic. In these cases, a DSA is sometimes necessary. False positive findings arise if vascular steps in secondary reconstructions of MR angiographic data sets are mistaken as pathologic wall lesions, which predominantly occur with insufficient spatial resolution.
5.3.3
Fibromuscular Dysplasia
Fibromuscular dysplasia (FMD) is a disease of medium-sized vessels, beginning in young to middle-aged patients with a predominance among females (Begelman and Olin 2000). The exact etiology is not clear, an association with a lack of alpha 1-antitrypsin is discussed as an indicator to a genetically mediated risk (Schievink et al. 1996). The radiological appearance of FMD depends on the histological type of disease and affects mainly the muscularis media, more rarely isolated the adventitia or the intima. FMD manifests through dissections or hyperplasia of the vessel walls with resulting stenosis. Stenosis and dilations with thinning of vascular walls alternate over longer distances, occasionally with aneurysms or luminal duplications. On DSA, this type of lesions was described as “string of beads”. The carotid artery is often affected bilaterally, mostly sparing the bifurcation. There is an elevated incidence of intracranial aneurysms and additionally arteriovenous fistulae can occur (Mettinger and Ericson 1982; Osborn and Anderson 1977; Zimmerman et al. 1977).
Compared to high-resolution examinations of the vessel wall for plaque imaging there are no dedicated studies for the analysis of vessel wall alterations in FMD. MRA can provide evidence of dissections with high sensitivity and of alternating arterial dilatation and stenosed vessels. MRA may also to help identify the string of beads pattern. In source images of TOF-MRA, segmental changes in arterial wall thickness, however, are detected to only a limited extent. If the analysis is performed on the basis of CE-MRA source images, one should consider the restricted spatial resolution. Reconstruction artifacts have to be excluded, which can simulate vessel wall pathology.
MRA is recommended for FMD, particularly with regard to the cervical region (Heiserman et al. 1992; Link et al. 1996), but due to the frequently subtle lesions, DSA should still be considered for FMD (see Fig. 5.10) (Hurst 1996).
5.3.4
Moya Moya Phenomenon
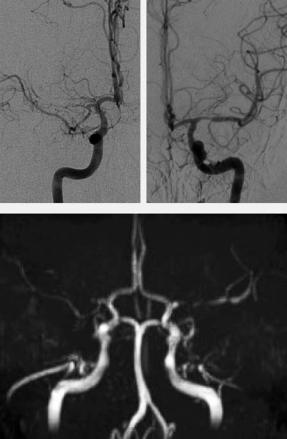
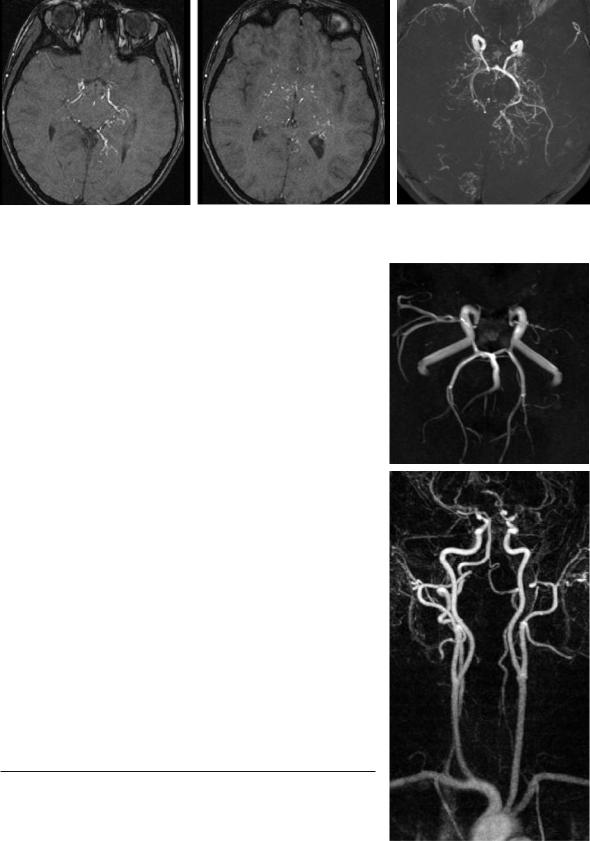
Moya Moya disease is a chronic cerebral arteriopathy with progressive stenoses of the distal ICA mainly found in children and young adults (Chiu et al. 1998; Giroud et al. 1997). This idiopathic disease is rarely found outside of Asia. Typically, the progressive occlusion of the distal intracranial ICA causes the dilatation of small cloud-like collateral vessels around the circle of Willis. The angiographic image, however, is not specific for the idiopathic disease, but rather displays an etiologically unspecific pattern of collateral circulation, which can always develop when a stenosis of the distal ICA slowly progresses. This is specifically true for atherosclerosis and FMD. Since the grading of the stenosis is crucial for radiological diagnosis and very tiny collaterals have to be studied, a definite and reliable evaluation is most suitable with DSA. Nevertheless, MRA can also show stenoses and depict the collaterals to a certain degree (Fig. 5.24), if these vessels are dilated. In many of these cases with significantly slowed down flow with preand post-stenotic signal decrease, TOF-MRA is not able to sufficiently define the precise degree of stenosis nor to differentiate high grade stenosis from an occlusion (Fig. 5.25).
5.3.5 Vasculitis
With regard to the classification of primary and secondary forms of CNS vasculitis and the difficulties of confirming the diagnosis, we refer to specific literature (Younger 2004). The amount of vascular alterations might be relevant for the diagnosis, but the role of the pattern of vascular lesions for differential diagnosis is of different importance. The disease becomes manifest in different localizations and in differently sized group of arteries.
Takayasu arteritis affects medium-sized and large arteries, specifically the aorta and the supraaortic branches. The disease is usually not seen in intracranial vessels nor in the temporal artery. Although for a long time DSA was accepted as a diagnostic
Vascular Anatomy and Pathology |
95 |
a |
b |
c |
Fig. 5.24a–c. Moya Moya syndrome. TOF-MRA demonstrates on source images (a,b) and MIP image (c ) multiple small collateral vessels and distal occlusions of the carotids
gold standard, MRI and MRA obviously provide the advantage not only to depict stenoses, but also to provide signs of inflammatory vessel disease. This provides new criteria for therapy monitoring. An appropriate examination consists of a CE-MRA, as well as acquisition of spin echo images (Andrews et al. 2004).
Primary angiitis of the CNS causes inflammation particularly in small leptomeningeal vessels, whereas larger vessels can also be affected. Systemic variants of vasculitis and secondary arteritis of the
CNS affect mostly small or medium-sized arteries
a
to different degrees, and occasionally the venous system is also afflicted as in Behcet disease. Segmental stenoses are frequently found, often not including bifurcations and alternating with arterial dilatations. This pattern is not definitively specific and can also be seen in atherosclerosis. Even with an optimal MRA technique, DSA still remains necessary for the depiction of tiny vessel lesions (Fig. 5.26).
5.3.6
Stenoses of Other Etiologies
Vasospasms following subarachnoid hemorrhages are a frequent and highly relevant complication with significant morbidity. Spasms occur in the proximal
Fig. 5.25a,b. Moya Moya syndrome. Due to limited resolution as well as turbulence, |
|
TOF-MRA (a) and CE-MRA (b) do not enable definitive quantification of obstruc- |
|
tions at the level of the distal intracranial carotids: TOF might be diagnosed as |
|
left occlusion and right high grade stenosis, while CE-MRA demonstrates bilateral |
|
enhancement of MCA branches |
b |
