
- •CONTENTS
- •EXPERTS
- •CONTRIBUTORS
- •ABBREVIATIONS
- •1 The management of chronic subdural haematoma
- •2 Glioblastoma multiforme
- •3 Spondylolisthesis
- •4 Intramedullary spinal cord tumour
- •5 Surgery for temporal lobe epilepsy
- •6 Management of lumbosacral lipoma in childhood
- •7 Idiopathic intracranial hypertension
- •8 Colloid cyst of the third ventricle
- •9 Bilateral vestibular schwannomas: the challenge of neurofibromatosis type 2
- •10 Multimodality monitoring in severe traumatic brain injury
- •11 Intracranial abscess
- •12 Deep brain stimulation for debilitating Parkinson’s disease
- •14 Trigeminal neuralgia
- •15 Cerebral metastasis
- •16 The surgical management of the rheumatoid spine
- •17 Cervical spondylotic myelopathy
- •18 Brainstem cavernous malformation
- •19 Peripheral nerve injury
- •20 Spontaneous intracerebral haemorrhage
- •21 Low-grade glioma
- •22 Intracranial arteriovenous malformation
- •INDEX
CASE
6Management of lumbosacral lipoma in childhood
Martin M. Tisdall and Greg James
 Expert commentary Dominic N. P. Thompson
Expert commentary Dominic N. P. Thompson
Case history
A 13-month-old girl was referred to a tertiary paediatric neurosurgical centre from a paediatric orthopaedic service. At birth she had been noted to have a lumbosacral cutaneous lesion and bilateral foot deformity. The feet remained weak, with a tendency to collapse inward. Proximal muscle power had shown reasonable development, with sitting attained at 8 months and crawling at 1 year. There was no indication of pain relating to the lumbar lesion.
She had persistently wet nappies, with a tendency to urinary dribbling. There had been no urinary tract infections. Urodynamic studies had revealed a neurogenic bladder with weak detrusor activity, but reasonable volume and no vesico-ureteric reflux. She had been commenced on intermittent urinary catheterization. There was some evidence of neurogenic bowel dysfunction with constipation requiring aperient medication.
On examination, a midline lumbosacral lipoma was found. There was reasonable muscle bulk in all major muscle groups of the lower limbs. The feet were held in equinus. Motor examination revealed MRC Grade 5 hip extension, Grade 3 hip extension, Grade 4 knee extension and flexion, and absent plantar flexion and extension bilaterally. Sensation appeared reduced around both feet, and lower limb reflexes could not be elicited.
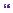 Expert comment
Expert comment
The lumbosacral lipomas are considered under the term ‘occult spinal dysraphism’ or spina bifida occulta, a category that includes, amongst others, split cord malformations, dermal sinus tracks, and fatty filum terminale. These are skin covered lesions and the term distinguishes this group of
disorders from ‘open spinal dysraphism’ or spina bifida aperta (myelomeningocele), in which there is a deficiency in the skin with exposure of the incompletely developed neural tube. The word ‘occulta’ is misleading as, in the majority of cases, there is an abnormality of the overlying skin, such as a lipomatous swelling, haemangioma, or hairy patch. These are referred to as the stigmata of spinal dysraphism. Such a finding should always alert the clinician to the possibility of an underlying spinal cord abnormality, even if there are no obvious neurological symptoms or signs.
MRI revealed a complex dysraphic malformation with an extensive transitional type lipoma extending from L2 to the sacral spinal canal (Figure 6.1).
At this stage, it appeared that the observed changes in ankle power were related to a severe motor deficit associated with the congenital dysraphic defect, rather than the effects of mechanical spinal cord tethering. As the proximal muscle groups,
60 |
Challenging concepts in neurosurgery |
 Low lying conus
Low lying conus
Transitional lipoma
Spinal bifida bony defect
Figure 6.1 Sagittal T1 and T2, and axial T1 MRIs of lumbosacral spine, demonstrating large transitional type spinal lipoma. The lipoma extends from L2 to the caudal sacral canal, and involves the conus and cauda equina extensively.
whose innervation was partly involved in the lipomatous mass, appeared to be developing. Given the complex nature of the surgical target, the inherent risks of surgical resection, and the lack of clear evidence of deterioration relating to spinal cord tethering, the initial plan was to follow-up with radiological and clinical surveillance.
The subsequent review took place at age 16 months. At this stage she had deteriorated, with evidence of lumbar pain and reduced left leg function. On examination, she had anti-gravity movements in the hips and knees, but the left leg was now weaker than the right. MRI appearances were stable. Given that she had now developed progressive symptomatology, it was decided to proceed to surgical resection of the lipomatous lesion and spinal cord detethering.
 Learning point Tethered cord syndrome
Learning point Tethered cord syndrome
Spinal lipomas may result in terminal spinal cord and cauda equina dysfunction [1–3]. The clinical manifestations of this dysfunction may be:
●Neurological: pain, sensory disturbance and neuropathic ulceration.
●Urological: impaired bladder emptying, urinary dribbling, and failure to attain continence—the neuropathic bladder.
●Orthopaedic: foot or ankle deformity, asymmetric muscle wasting, and gait disturbance.
These consequences of spinal dysraphism are sometimes termed the ‘neuro-orthopaedic syndrome’ or, more commonly, the tethered cord syndrome. The latter is probably best used to describe the clinical syndrome, rather than the radiological appearance. A low (‘tethered’) spinal cord on MRI may have no clinical sequelae.
Case 6 Management of lumbosacral lipoma in childhood |
61 |
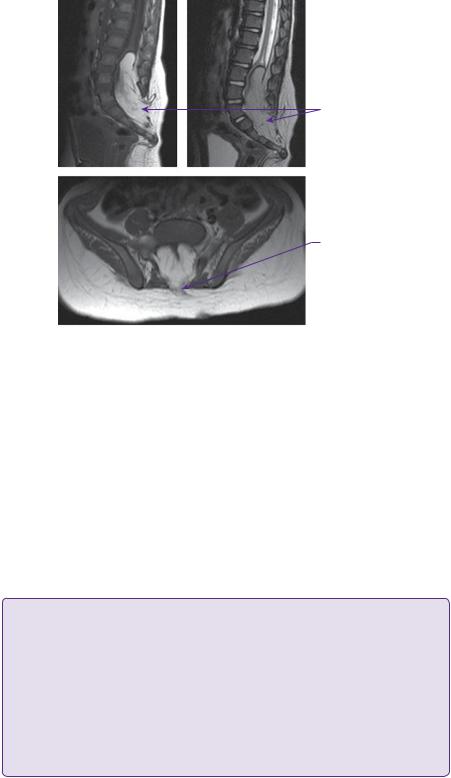
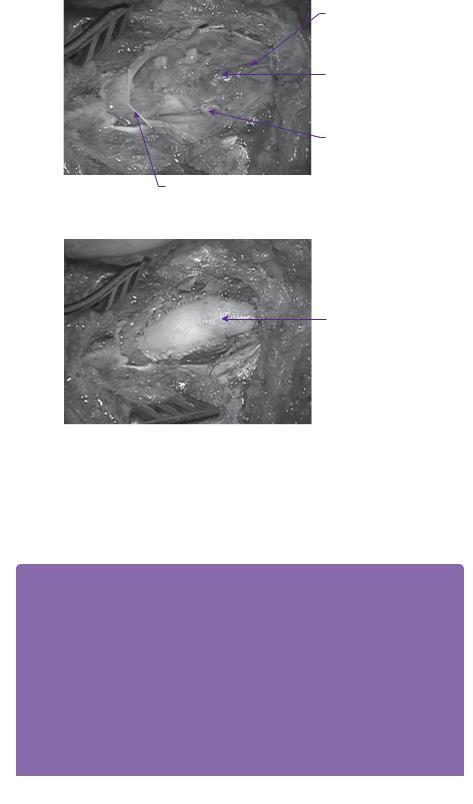
At operation, the subcutaneous component of the lipoma was initially mobilized to delineate the defect in the lumbosacral fascia. A paraspinal muscle reflection was then performed, and a wide laminectomy performed to ensure adequate exposure of the interface between the lipoma and the dura. After dural opening, the relationship of the dorsal root entry zone (DREZ) to the lipoma was established (Figure 6.2) and electrophysiological mapping was used to identify functional nerve roots. Microdissection was then used to dissect along the plane between the lipoma and the neural placode (Figure 6.3). The exposed neural placode was then neuralated (sutured in the midline) (Figure 6.4) and, finally, an expansion duraplasty was performed (using dural substitute) in an attempt to increase the volume of the thecal sac and thus reduce the risk of future retethering (Figure 6.5).
 Neural placode
Neural placode
Dorsal nerve roots
Dural edge
Figure 6.2 Operative view of transitional lipoma after dural opening. Dorsal root entry zone depicted by dotted line.
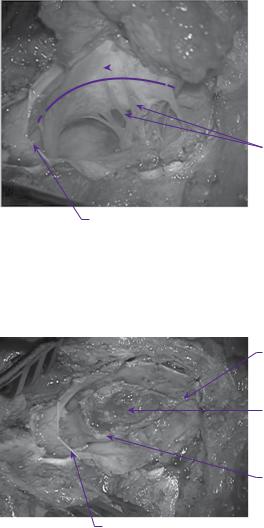
Filum terminale
Lipoma resection cavity
Edge of neural placode
Dural edge
Figure 6.3 Operative appearance after resection of lipoma.
62 |
Challenging concepts in neurosurgery |
Filum terminale
Fine non-absorbable sutures to appose edges of neural placode ‘neuralation’
Dorsal nerve roots
Dural edge
Figure 6.4 Operative appearance following neuralation of the placode.
Dural patch sutured continuously to the dural edges
Figure 6.5 Operative appearance following expansion duraplasty with dural substitute.
Post-operatively, she made a good recovery. Neurological function was unchanged, although her pain appeared to be reduced. MRI at 2 months post-operatively showed near total lipoma resection. She remains under review with regular radiological, clinical, and urological surveillance.
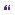 Expert comment
Expert comment
The anatomy of spinal lipomas is complex. Achieving a safe and durable untethering with minimal risk to neurological function, and avoiding post-operative wound-related complications, represents a major surgical challenge. Important principles include:
●Wide bony exposure: the attachment between the lipoma and the dura may be very lateral and a wide exposure is needed to access the dural margin
●Working from normal anatomy: the identification of normal spinal cord immediately rostral to the lesion will aid orientation and early identification of the interface between the normal spinal cord and lipoma
●Identifying the dorsal nerve roots over normal spinal cord and following the DREZ into the lesion: the spinal cord is splayed open and the lipomatous placode means that the dorsal roots are displaced laterally.
●Nerve root mapping is essential: identification of the sphincteric innervation is of particular importance.

Case 6 Management of lumbosacral lipoma in childhood |
63 |
|
|
●Lipoma resection: sharp micro-dissection is continued along the interface between the lipoma and the neural placode. The aim is maximal lipoma removal in order to reduce the risk of retethering
●Neuralation of the placode: the edges of the placode are sutured in the midline, thus reconstituting the terminal spinal cord
●A duraplasty is performed: the aim is to achieve a watertight and capacious terminal thecal sac.
Discussion
Up until the beginning of the twenty-first century, the perceived surgical wisdom has been that all lumbosacral lipomas should be treated with surgical debulking and spinal cord detethering in an attempt to prevent neurological deterioration [4]. This philosophy is based on the assumptions that inevitable deterioration will occur without surgery, and that surgery is safe and effective [5–8]. Over the last decade, evidence has emerged to challenge these assumptions. Data from paediatric neurosurgery groups at Necker-Enfants Malades, Paris [9] and Great Ormond Street, London [10] have suggested that, in the absence of neurological or urological deterioration, conservative management may have a similar or even superior outcome when compared with traditional surgical techniques.
Conversely, a single group from the United States headed by Dachling Pang have reported the results of radical total, or near total, lipoma removal, guided by meticulous neurophysiological monitoring, reconstruction of the neural placode, and duraplasty [11,12]. Their results suggest that this approach may be superior to both conservative management and traditional surgical technique.
Classification
The most widely recognized classification is that proposed by Chapman [5]. It is based on the anatomical position of the attachment of the lipoma to the spinal cord, and is divided into dorsal, caudal, and transitional types. The dorsal type is attached exclusively to the dorsal surface of the spinal cord rostral to the conus. The caudal type is attached to the termination of the spinal cord and involves the tip of the conus. The complex transitional type is intermediate between the two, and extends from the dorsal surface of the cord to the conus and cauda equina.
 Expert comment
Expert comment
The management of lumbosacral lipoma sits at a fascinating crossroads with different groups advocating diametrically opposed strategies. This debate will only be settled with the acquisition of more data. Here, we will examine the current evidence supporting these various management strategies and present our current treatment algorithm.
Epidemiology and embryology
Lumbosacral lipomas occur in approximately 1:4000 births and have a female:male ratio of 2:1 [13]. Their embryological basis remains unclear. It has been proposed that premature dysjunction, comprising early separation of neuroand cutaneous ectoderm, prior to closure of the neural tube, could expose the developing neural structures to underlying mesoderm, which adheres to it and subsequently differentiates into fat [14]. While this hypothesis has some merit and might explain the anatomy of the dorsal type lipoma, it fails to account for the formation of the more caudally-attached transitional and caudal lipomas. These subtypes have some attachment at or below the level of the conus.
 Expert comment
Expert comment
The anatomy may be further distorted by rotation of the neural placode resulting in shortening of the nerve roots on one side. Pang et al. have suggested the term chaotic lipoma as a subtype of the transitional type, in which the lipoma blends with the roots of the cauda equina and extends to lie ventral to the roots [13].
64 |
Challenging concepts in neurosurgery |
 Learning point
Learning point
Formation of the conus and cauda equina results from the incompletely understood and lateroccurring process of secondary neuralation, in which pluripotent cells situated in the tail bud, coalesce to form the secondary neural tube before attaching to the distal spinal cord. Therefore, dysjunction plays no part in the formation of the conus and, thus, an alternative pathogenesis must be considered for the caudal and transitional types of lipoma [15–17]. Co-existing malformations of the ano-rectal region and lower urinary tract are not uncommon and are in keeping with the concept of a loco-regional disorder of caudal cell mass development [18].
Natural history
The traditional view that untreated lumbosacral lipoma leads to inevitable neurological, urological, or orthopaedic deterioration has little scientific basis and, until recently, the natural history of the condition has not been accurately reported. The literature has been hampered by the inclusion of various lipomatous anomalies within the same studies, the lack of distinction between asymptomatic and symptomatic cases and a dearth of cases managed without surgical intervention. Those advocating surgical treatment for all, point to data showing that outcome for patients treated before symptom onset is superior to that for patients treated in the presence of neurological deficit and to a diminished number of asymptomatic patients in the older age groups [8,19]. Both of these arguments are inherently flawed. First, it cannot be assumed that the asymptomatic patient is simply at an earlier time point in the same disease process as the symptomatic patient and, secondly, it is not possible to know the true prevalence of asymptomatic disease in the older patient, who may not seek medical attention [20]. Surgery for an asymptomatic child is a prophylactic undertaking and so potential surgical morbidity must be carefully weighed against the perceived long-term gain.
 Expert comment Causes of neurological deterioration
Expert comment Causes of neurological deterioration
That children with spinal lipomas can develop neurological deterioration is beyond doubt [1–3], but the mechanisms underlying that deterioration are less clear. Fixation of the lower spinal cord by the lipoma may result in tractional forces as the spine grows. Experimental evidence has shown that these tractional forces can result in spinal cord ischaemia and neuronal dysfunction [21]. This
mechanical explanation, known as ‘tethering’, is the basis upon which surgical treatment is based, but other factors, which have received less attention are also likely to be involved. Histological evaluation of spinal lipomas frequently reveals much more than just adipocytes. Tissues from each of the germ cell layers have been isolated from spinal ‘lipomas’, suggesting a true malformation process [22]. Particularly in the complex transitional forms of lipoma, it is possible, if not probable, that there
is some associated neural dysgenesis. This may account for some of the congenital neurological deficits seen in patients, such as the one described in the case history above. The consequences of neural dysgenesis, for example, on bladder function may be difficult to detect in an infant and may only emerge as the child grows. Thus, what might appear to be a progressive deficit might simply be the consequences of abnormal spinal cord development unveiled by the passage of time. Finally, mass effect may have some role. The intraspinal component of the lipoma can be large and cause compression of the underlying spinal cord and nerve roots [22].
While neurological, urological, and orthopaedic deterioration in the context of spinal lipoma is well described, the natural history appears more benign than previously thought, particularly in those cases (up to 30% of lipomas) that appeared to be asymptomatic at the time of presentation. Based on the suspicion that the traditional surgical approach of lipoma debulking and spinal cord detethering was yielding unsatisfactory

Case 6 Management of lumbosacral lipoma in childhood |
65 |
results, Kulkarni et al. reported that outcome for asymptomatic patients managed conservatively with close neurological and urological surveillance [9]. Fifty-three asymptomatic cases with spinal lipoma were followed over a mean period of 4.4 years. During this period, only 25% exhibited neurological deterioration. The authors calculated that the actuarial risk of deterioration at 9 years was 33% compared with 46% for an earlier series of asymptomatic patients treated with surgery. The cohort was subsequently followed for 10 years and the rate of deterioration remained at 33%.
The group from Great Ormond Street Hospital in London has recently collected similar data from 56 asymptomatic patients [10]. They found a cumulative risk of deterioration at 2 and 10 years of 18 and 40%, respectively. Sixteen patients developed neurological symptoms and underwent subtotal resection of the lipoma with attempted detethering of the spinal cord, guided by electrophysiological monitoring. At a median post-operative follow-up of 2.5 years, nine patients experienced improvement in their pre-operative deficit and seven remained symptomatic.
 Expert comment
Expert comment
Many studies have documented the risks of late deterioration following conventional partial resection of asymptomatic spinal lipomas and, allowing for variations in the surgical series, a 40% rate of deterioration at 8 years can be anticipated [12,23,24]. These results appears no better than the natural history and suggest, therefore, that conventional surgery for asymptomatic lipomas is of questionable benefit, particularly as initial surgery not only has its own morbidity, but might increase the risk of secondary deterioration due to re-tethering.
The surgical results published by Pang et al. suggest that a more radical surgical approach might prove far more valuable. They advocate a technique comprising total or near-total resection of the lipoma, guided by meticulous neurophysiological mapping, followed by reconstruction of the neural placode and expansion duraplasty. They report progression free survival of 82.8% at 16 years for all patients and 98.4%, if only the asymptomatic cases are considered [11,12]. These results are not only far superior to previously reported surgical series, but also significantly better than the natural history.
Clearly, if these results can be reproduced by other centres, it heralds a major advance. However, the technique relies heavily on neurophysiological monitoring, which may not be available in all centres. Furthermore, it is technically demanding and carries a potentially significant risk of inflicting neurological or urological deficit, in addition to the risk of wound related complications, such as CSF leakage or pseudomeningocele formation [1,8]. As the incidence of this condition is low, it may take considerable time to develop the necessary surgical skills.
Given that a significant number of asymptomatic cases will not deteriorate, the Great Ormond Street group has tried to risk-stratify their patients. They found that, within the asymptomatic cases, female sex, transitional type lipoma, and presence of a syrinx cavity were associated with a greater risk of deterioration [10].
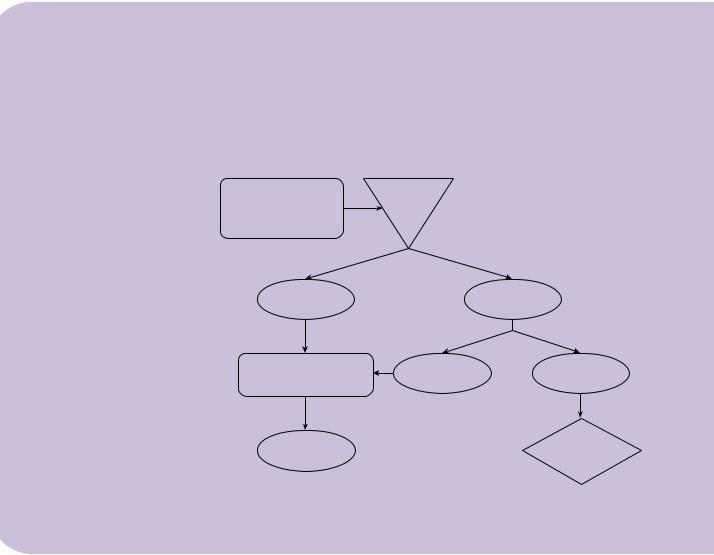
Toward a management algorithm
Initial assessment comprises clinical history, motor examination, MRI, and urological evaluation to include bladder function assessment.
Based on this evaluation, patients are divided into asymptomatic or symptomatic (i.e. with evidence of a deficit attributable to the dysraphic anomaly). Symptomatic patients are further sub-divided into those who are symptomatic with a fixed deficit
66 |
Challenging concepts in neurosurgery |
|
(e.g. an ankle or foot deformity, present since birth), termed ‘symptomatic static’, and |
|
those who are symptomatic with a new or worsening deficit, termed ‘symptomatic pro- |
|
gressive’. For the latter group with a progressive deficit (e.g. the child with new pain or |
|
new onset bladder symptoms), an attempted radical surgical intervention, as described |
|
by Pang [11,12] is undertaken. For the asymptomatic patients, a policy of close neuro- |
|
logical and urological surveillance is pursued 6-monthly until continence is established, |
|
when surveillance is reduced to annually. If, at any stage, new changes are identified |
|
and then the child is considered as symptomatic progressive and treated accordingly. |
|
For the child with a fixed deficit (symptomatic static), our present policy is to treat along |
|
the same lines as being asymptomatic, accepting that this is a particularly controversial |
|
group. Any evidence of progression would again lead us to recommend radical surgery. |
|
Further data collection will permit evaluation of this management approach and |
|
provide valuable information as to the reproducibility of the excellent results of Pang |
|
et al [11,12]. |
|
A final word from the expert |
|
One is left asking how to best manage these patients? Conventional partial debulking of |
|
asymptomatic lipomas appears to be no better than the natural history of the disease, but |
|
conservative management also carries a significant risk of deterioration. If the results of |
|
Pang [11,12] are reproduced by other groups, their radical surgical approach may become |
|
standard care for all spinal lipomas, yet we currently lack the data to endorse this fully. We |
|
suggest a management algorithm for newly-diagnosed patients with spinal lipomas, which |
|
aims to recognize the benign course of the disease in many asymptomatic patients and to |
|
provide effective long-term treatment results for those who deteriorate (Figure 6.6). |
Clinical history |
Lumbosacral |
examination |
lipoma |
MRI lumbar spine |
|
urological evaluation |
|
Asymptomatic |
Symptomatic |
Close neurological and |
Symptomatic |
Symptomatic |
urological surveillance |
static |
progressive |
Radical
Deterioration  surgical intervention
surgical intervention
Figure 6.6 Proposed management algorithm for treatment of spinal lipomas.
Taken from Wykes V, Desai D, Thompson DN. Asymptomatic lumbosacral lipomas—a natural history study. Childs Nerv
Syst, 2012; epub ahead of print.
Case 6 Management of lumbosacral lipoma in childhood |
67 |
References
1.Pierre-Kahn A, Zerah M, Renier D, et al. Congenital lumbosacral lipomas. Child’s Nervous System 1997; 13: 298–334.
2.Gourineni P, Dias L, Blanco R, Muppavarapu S. Orthopaedic deformities associated with lumbosacral spinal lipomas. Journal of Pediatric Orthopaedics 2009; 29: 932–6.
3.Kang J-K., Lee K-S., Jeun S-S., Lee I-W., Kim M-C. Role of surgery for maintaining urological function and prevention of retethering in the treatment of lipomeningomyelocele: experience recorded in 75 lipomeningomyelocele patients. Child’s Nervous System 2003; 19: 23–9.
4.Bassett RC. The neurologic deficit associated with lipomas of the cauda equina. Annals of Surgery 1950; 131: 109–16.
5.Chapman PH. Congenital intraspinal lipomas: anatomic considerations and surgical treatment. Child’s Brain 1982; 9: 37–47.
6.Hoffman HJ, Taecholarn C, Hendrick EB, Humphreys RP. Management of lipomyelomeningoceles. Experience at the Hospital for Sick Children, Toronto. Journal of Neurosurgery 1985; 62: 1–8.
7.McLone DG, Naidich TP. Laser resection of fifty spinal lipomas. Neurosurgery 1986; 18: 611–15.
8.La Marca F, Grant JA, Tomita T, et al. Spinal lipomas in children: outcome of 270 procedures. Pediatric Neurosurgery 1997; 26: 8–16.
9.Kulkarni AV, Pierre-Kahn A, Zerah M. Conservative management of asymptomatic spinal lipomas of the conus. Neurosurgery 2004; 54: 868–73.
10.Wykes V, Desai D, Thompson DN. Asymptomatic lumbosacral lipomas—a natural history study. Child’s Nervous System, 2012; 28 (10): 1731–9.
11.Pang D, Zovickian J, Oviedo A. Long-term outcome of total and near-total resection of spinal cord lipomas and radical reconstruction of the neural placode: Part I-surgical technique. Neurosurgery 2009; 65: 511–28.
12.Pang D, Zovickian J, Oviedo A. Long-term outcome of total and near-total resection of spinal cord lipomas and radical reconstruction of the neural placode, part II: outcome analysis and preoperative profiling. Neurosurgery 2010; 66: 253–72.
13.Finn MA, Walker ML. Spinal lipomas: clinical spectrum, embryology, and treatment. Neurosurgery Focus 2007; 23: E10.
14.Naidich TP, McLone DG, Mutluer S. A new understanding of dorsal dysraphism with lipoma (lipomyeloschisis): radiologic evaluation and surgical correction. AJR American Journal of Roentgenology 1983; 140: 1065–78.
15.Copp AJ, Brook FA. Does lumbosacral spina bifida arise by failure of neural folding or by defective canalisation? Journal of Medical Genetics 1989; 26: 160–6.
16.Müller F, O’Rahilly R. The primitive streak, the caudal eminence and related structures in staged human embryos. Cells Tissues Organs 2004; 177: 2–20.
17.Saitsu H, Yamada S, Uwabe C, Ishibashi M, Shiota K. Development of the posterior neural tube in human embryos. Anatomy and Embryology 2004; 209: 107–17.
18.Qi BQ, Beasley SW, Arsic D. Abnormalities of the vertebral column and ribs associated with anorectal malformations. Pediatric Surgery International 2004; 20: 529–33.
19.Oi S, Nomura S, Nagasaka M, et al. Embryopathogenetic surgicoanatomical classification of dysraphism and surgical outcome of spinal lipoma: a nationwide multicenter cooperative study in Japan. Journal of Neurosurgery: Pediatrics 2009; 3: 412–19.
20.Dorward NL, Scatliff JH, Hayward RD. Congenital lumbosacral lipomas: pitfalls in analysing the results of prophylactic surgery. Child’s Nervous System 2002; 18: 326–32.
21.Tani S, Yamada S, Knighton RS. Extensibility of the lumbar and sacral cord. Pathophysiology of the tethered spinal cord in cats. Journal of Neurosurgery 1987; 66: 116–23.
68 |
Challenging concepts in neurosurgery |
|
|
22. |
Hirsch JF, Pierre-Kahn A. Lumbosacral lipomas with spina bifida. Child’s Nervous |
|
|
System 1988; 4: 354–60. |
|
23. |
Xenos C, Sgouros S, Walsh R, Hockley A. Spinal lipomas in children. Pediatric |
|
|
Neurosurgery 2000; 32: 295–307. |
|
24. |
Colak A, Pollack IF, Albright AL. Recurrent tethering: a common long-term problem after |
|
|
lipomyelomeningocele repair. Pediatric Neurosurgery 1998; 29: 184–90. |
