
- •CONTENTS
- •EXPERTS
- •CONTRIBUTORS
- •ABBREVIATIONS
- •1 The management of chronic subdural haematoma
- •2 Glioblastoma multiforme
- •3 Spondylolisthesis
- •4 Intramedullary spinal cord tumour
- •5 Surgery for temporal lobe epilepsy
- •6 Management of lumbosacral lipoma in childhood
- •7 Idiopathic intracranial hypertension
- •8 Colloid cyst of the third ventricle
- •9 Bilateral vestibular schwannomas: the challenge of neurofibromatosis type 2
- •10 Multimodality monitoring in severe traumatic brain injury
- •11 Intracranial abscess
- •12 Deep brain stimulation for debilitating Parkinson’s disease
- •14 Trigeminal neuralgia
- •15 Cerebral metastasis
- •16 The surgical management of the rheumatoid spine
- •17 Cervical spondylotic myelopathy
- •18 Brainstem cavernous malformation
- •19 Peripheral nerve injury
- •20 Spontaneous intracerebral haemorrhage
- •21 Low-grade glioma
- •22 Intracranial arteriovenous malformation
- •INDEX
CASE
11 Intracranial abscess
Ciaran Scott Hill
 Expert commentary George Samandouras
Expert commentary George Samandouras
Case history
A 20-year-old right-handed man presented to the Emergency department with a 4-week history of right-sided earache associated with a foul smelling purulent discharge. He had suffered from intermittent ear discharge since childhood, but he had been well for the previous year. The current episode had been treated with a 1-week course of antibiotics by the general practitioner without any effect. The patient then developed general malaise, positional headaches, and was now describing intermittent horizontal vertigo, the sensation of movement as if the environment were spinning. There were no meningitis symptoms. He had no headache, neck stiffness, or photophobia. His past medical history was otherwise unremarkable.
On examination, there was an erythematous, boggy swelling over the right mastoid process. The right external auditory meatus was completely occluded by pus and the pinna was pushed anteriorly.
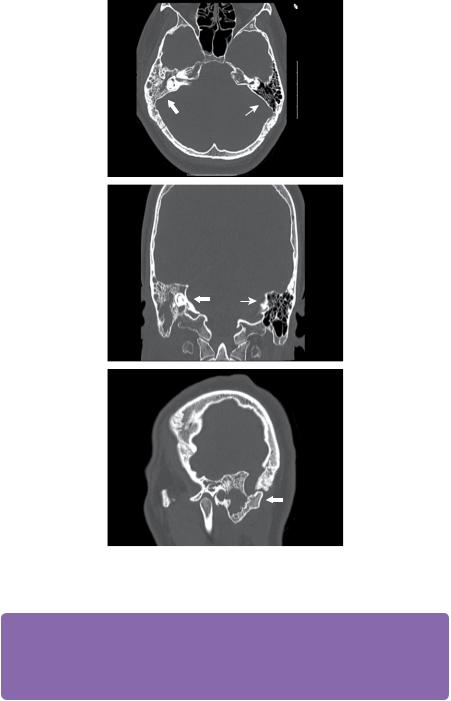
The patient was admitted under the ear, nose, and throat surgeons who requested routine laboratory investigations and a microbiology swab that was sent for microscopy, culture, and sensitivity. A CT scan was performed and the CT images are shown in Figure 11.1.
A diagnosis of mastoiditis was made and the patient was placed on the emergency theatre list for an exploratory mastoidectomy. However, the next day the patient was noted to have developed a mild right-sided hemiparesis and was referred to neurosurgery. Review of the CT scans (Figure 11.2) with brain windows demonstrated a hypodensity of the right cerebellum in association with subtle triventricular hydrocephalus and displacement of the IVth ventricle.
It was felt these images were consistent with cerebritis and a T1, T2 and T2 FLAIR MR scan was requested (Figure 11.3). Additionally, a T1 scan with contrast (Figure 11.4), diffusion-weighted imaging (Figure 11.5) and magnetic resonance venography (MRV) was performed (Figure 11.6).
 Expert comment
Expert comment
The role of steroids remains controversial in the literature, with some studies supporting their use, while others advocate against them.
Cerebral oedema is a major cause of morbidity and mortality in patients with brain abscess. When the patient is on a targeted antibiotic treatment, administration of dexamethasone, in the presence of oedema on imaging, is often an essential part of the patient’s management. Long-term use should be discouraged.
Rapidly deteriorating patients referred from district general hospitals requiring urgent treatment can have, prior to transfer, administration of broad spectrum antibiotics and dexamethasone after obtaining blood cultures.
 Expert comment
Expert comment
The classic clinical triad of headache, high temperature, and focal neurological deficit occurs in <50% of cases. When no obvious source of infection is identified an extensive septic work up is mandatory.
104 |
Challenging concepts in neurosurgery |
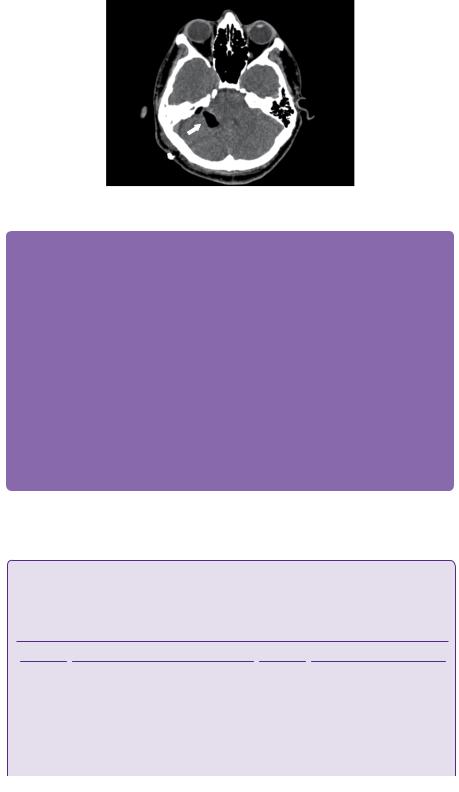
Figure 11.1 CT scan with bone windows demonstrates a right-sided opacification of the mastoid air cells with bony expansion in the inferior aspect and bony sclerosis superiorly (white block arrows). The left mastoid process is well aerated and normal in appearance (white line arrows).
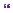 Expert comment
Expert comment
Cortical thrombophlebitis is a major cause of cortical neurological deficits in patients with white matter brain abscesses. This may be the result of occlusion of specific veins, such as the vein of Labbé or a diffuse process involving cortical territory. Involvement of deep venous systems, although not as common can occur.
The imaging studies demonstrated cerebellar and right mastoid abscesses in keeping with an otogenic origin. The MRV showed patent sinuses and large veins, with no signs of lateral sinus thrombosis (Figure 11.6). Cultures obtained from the ear canal swab grew Group A beta haemolytic streptococci and Pseudomonas and the patient was started on intravenous ceftriaxone, 2g bd, and clindamycin, 600mg qds.
Case 11 Intracranial abscess |
105 |
Figure 11.2 Non-enhanced CT scan with brain windows show an ill-defined right cerebellar hypodensity (white block arrows).
 Learning point Stages of brain abscess formation
Learning point Stages of brain abscess formation
Stages of brain abscess formation as defined by Britt et al. (pathological) and Osborn et al. (radiological) (see Table 11.1) [1,2].
Table 11.1 Stages of brain abscess
Stage |
Day |
Microscopic features |
MRI T1 |
MRI T1 + contrast |
|
|
|
|
|
Early |
0–3 |
Acute inflammatory reaction |
Poorly-defined hypo/ |
Patchy enhancement |
cerebritis |
|
with polymorphonuclear |
isointense lesion |
|
|
|
leukocytes. Fibroblasts appear |
|
|
|
|
and angiogenesis begins. |
|
|
Late |
4–9 |
Necrosis. Macrophage |
Hypointense centre |
Intense irregular rim |
cerebritis |
|
recruitment. Neovascularization and iso/hyperintense |
enhancement |
|
|
|
and associated vasogenic |
rim |
|
|
|
oedema. Fibroblastic collagen |
|
|
|
|
deposition. |
|
|
Early |
10–13 |
Progressive central necrosis |
Centre becomes |
Well-defined, thin-walled |
capsule |
|
and collagen deposition in |
more hyperintense |
capsule |
|
|
capsule. Peripheral gliosis. |
than CSF and rim |
|
|
|
|
more hyperintense |
|
|
|
|
than white matter |
|
Late |
14+ |
Reduction in inflammatory |
capsule |
|
cells. Multiple layers of |
|
|
collegen capsule form |
|
|
surrounded by increasing |
|
|
numbers of reactive astrocytes. |
|
|
|
Capsule thickens and |
Thick capsule with |
cavity may collapse |
possible cavity collapse. |
|
Capsule is thicker on |
|
cortical side and thinner |
|
on ventricle side. |
|
|
Samandouras G. The Neurosurgeon's Handbook. 2011. Oxford University Press.
106 |
Challenging concepts in neurosurgery |
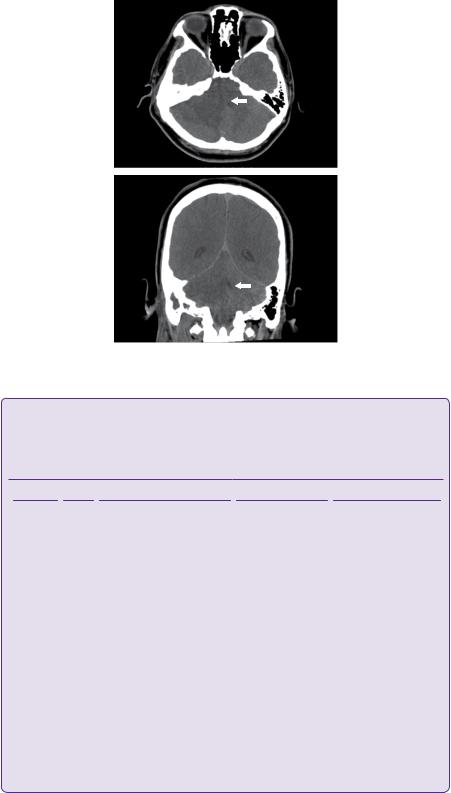
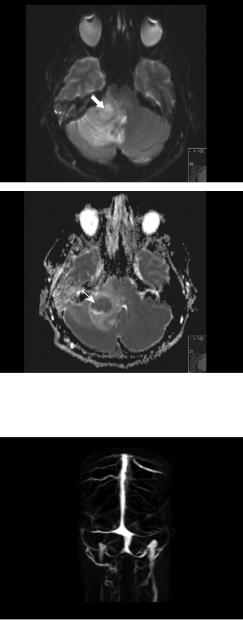
Figure 11.3 MR T1, T2, and T2 FLAIR images, top to bottom, demonstrate a lesion near the right cerebellopontine angle (CPA) involving the right cerebellar peduncle and abutting the brainstem (white block arrow). The perifocal oedema affects the cerebellum, particularly the vermis, the pons, and midbrain (white line arrows). The classical T2 hypointense rim caused by the susceptibility artefacts of a maturing abscess is demonstrated (dotted arrow).
In this case the radiological features and clinical timeline are consistent with the late capsule phase.
 Clinical tip
Clinical tip
Cases referred to neurosurgeons are often at the late abscess stage. When a cerebritis stage abscess is suspected, microbiologists often request CSF analysis. This should be discouraged, as it is not only dangerous in the presence of mass effect, but provides low diagnostic yield. CSF findings when obtained, typically show normal glucose, raised protein, and raised WCC (1–1000/mm3) with lymphocytes predominating.
Case 11 Intracranial abscess |
107 |
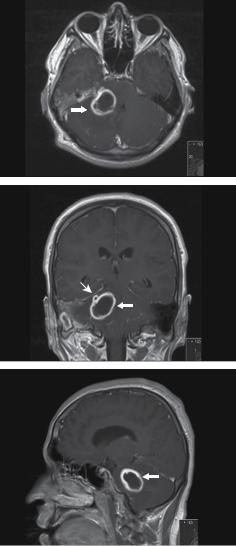
Figure 11.4 MR T1 after gadolinium administration show a smooth, well-demarcated right cerebellar ring-enhancing 3 × 3 × 2.5cm lesion with a thin wall (white block arrow). The hypointense surrounding area is consistent with oedema. On the coronal views (middle) a second ring enhancing ‘daughter’ lesion is seen in contact with the lesion superiorly (white line arrow). There is also ring-enhancement (1.8 × 1.5cm) in the right mastoid. There is moderate enhancement of the right cerebellar tentorium.
The patient was taken to theatre urgently for aspiration of the abscess. In the lateral position and without image-guided neuronavigation, a Dandy cannula was inserted aiming just lateral to the right CP angle. Pus was aspirated at the first attempt, but at the second attempt frank blood was aspirated. The resulting haemorrhage was difficult to control and, therefore, it was decided to convert the burr hole to a small posterior fossa craniectomy. The haemorrhage was finally controlled and it was felt by the operating surgeon that he could uneventfully remove the capsule that was prominent in the operative field. After dissection, the abscess capsule was excised. The post-operative CT is shown in Figure 11.7.
108 |
Challenging concepts in neurosurgery |
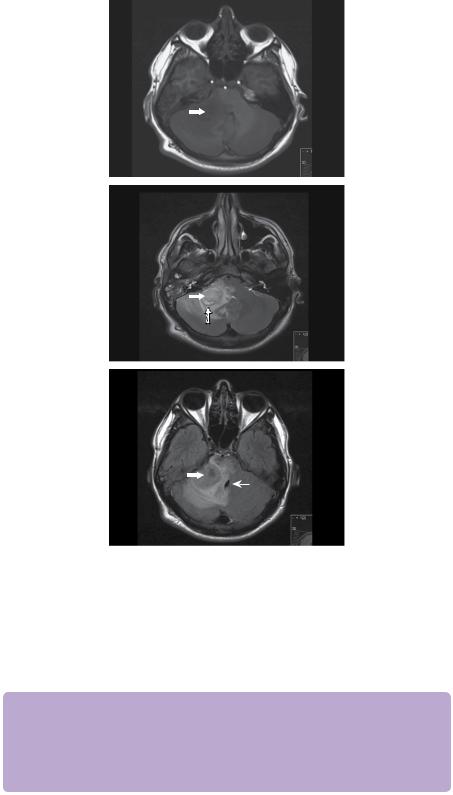
Figure 11.5 Diffusion-weighted MRI shows a high signal lesion in the right CPA with restricted diffusion (white block arrow). This is confirmed with the ADC map below that shows a central region of low signal (white line arrow).
Figure 11.6 MRV. There is no evidence of thrombosis. The transverse sinuses bilaterally are gracile and hypoplastic, but the sigmoid sinuses are of normal calibre. There is incidental anatomical variation as the superior sagittal sinus divides caudally into two branches that drain to the internal jugular veins.
Post-operatively he was unable to abduct his right eye, but other movements were unaffected. There was also a complete loss of right-sided facial cutaneous sensation in V1–V3 distribution and an associated palsy of the muscles of mastication. He was noted to have developed a right-sided House–Brackmann Grade 5 lower motor neurone facial paresis. These findings were consistent with lesions of the abducens, trigeminal, and facial nerves.
Case 11 Intracranial abscess |
109 |
Figure 11.7 Enhanced axial CT shows complete capsule excision and a small amount of intra-operative air.
 Expert comment
Expert comment
This case demonstrates that free-hand aspiration has a limited role in the management of cerebral abscess, even in large or very superficial lesions.
Image guidance systems, either frameless or frame based are very useful in achieving target acquisition and optimum aspiration of the centre of the volumetric space allowing maximum removal of the infective material and planning of a minimally invasive trajectory.
An additional benefit of image guidance systems is the stabilization of a fixed trajectory. Hand-held probes, even with minute hand movements, inadvertently and unnecessarily widen the tract disturbing or damaging neural tissue at the walls of the tract.
The decision to excise the abscess capsule should be planned, especially when the capsule is adjacent to eloquent areas, and is usually indicated when, despite repeated aspirations and targeted antibiotic treatment, there is no radiological resolution and no clinical improvement of the patient.
The capsule of the abscess is adherent and tough and is not similar to the soft capsule of a metastatic lesion or of a circumscribed meningioma.
Even removal of the capsule does not guarantee eradication of the abscess as recurrences have been observed after complete abscess capsule removal.
Although not a common practice, the stereotactic insertion of an Ommoya reservoir to allow repeated aspiration and antibiotic infiltration has been described [3].
 Learning point House–Brackmann classification
Learning point House–Brackmann classification
The original House–Brackmann classification of facial nerve weakness is shown in Table 11.2 [4].
Table 11.2 House–Brackmann classification
Grade |
Description of facial weakness |
Score |
Percentage motor function |
|
|
|
|
1 |
None |
8/8 |
100 |
2 |
Slight |
7/8 |
76–99 |
3 |
Moderate with full eye closure |
5–6/8 |
51–75 |
4 |
Moderate with incomplete eye closure |
3–4/8 |
26–50 |
5 |
Severe |
1–2/8 |
1–25 |
6 |
Complete |
0/8 |
0 |
|
|
|
|
(continued)

110 |
Challenging concepts in neurosurgery |
|
|
|
|
 Clinical tip
Clinical tip
Temporal lobe abscesses secondary to middle ear infection are
best managed operatively in conjunction with the ear, nose, and throat (ENT) surgeon. Petrosectomy or mastoidectomy are often necessary and, ideally, when indicated, should be performed at the same operative session as the abscess drainage.
The overall score is calculated by measuring the movement of the mid-portion of the superior aspect of the eyebrow in a superior direction and the movement of the angle of the mouth laterally. The eye is scored from 0 to 4 with one point given for each 0.25cm of cephalic movement. The mouth is also scored out of 4 with 1 point given for each 0.25cm of lateral movement. The maximum score is 8. This does not consider the sensory or parasympathetic innervation of the facial nerve. A graphical
version was produced by Lazarini et al., this is simple to use and offers the advantage of speed over the tabulated scale [5].
Two days later he underwent a mastoidectomy and tympanoplasty. A pneumatized mastoid cavity full of granulation tissue was drilled to healthy bone. The facial nerve was decompressed by opening its bony canal. Within 2 weeks, his cranial neuropathies had improved with only mild weakness of mastication, normal facial sensation, minimal diplopia, and a House–Brackman Grade 3 facial weakness remaining. The intra-operative pus samples were sterile and a peripherally inserted central catheter (PICC) line was inserted so the patient could receive 6 weeks of intravenous antibiotics.
Discussion
The earliest successful series of posterior fossa intracranial operations were those of the Sir William Macewen over 100 years ago. The original technique involved blind drainage of a cerebellar abscess through a trephined opening in the temporal mastoid bone [6,7]. Macewen was also perhaps the first surgeon to champion the use of the electric burr, a tool that in combination with the operating microscope and suction irrigation would allow surgeons to unlock the complexities of the skull base. Mastoidectomies were popularized by the German otologist Hermann Schwartze and modified to achieve their current form under William F. House [8].
Chronic suppurative otitis media is a longstanding infective disease of the middle ear. It is usually easily treated in the early stages with antibiotics, with or without myringotomy. If treatment is delayed or ineffective the complications can be severe. Most intracranial complications develop in patients with a chronically discharging ear. The complications that are the primary concern to neurosurgeons are extraaxial (such as subdural empyema) or intra-axial (such as brain abscess). A brain abscess is a focal suppurative process that involves the brain parenchyma. At least 50% of all adults brain abscesses are thought to be otogenic in origin [9]. The possible causes of brain abscesses are outlined in Table 11.3.
Table 11.3 Aetiology of brain abscess from The Neurosurgeon’s Handbook by G. Samandouras (reproduced with permission).
Primary infection |
Micro–organisms |
Frontal sinus |
Aerobic and anaerobic streptococci |
|
Strep milleri, Bacteroides species, Haemophilus species, Enterobacteriaceae, |
|
Staph. aureus |
Middle ear/mastoid bone |
Aerobic and anaerobic streptococci |
|
Bacteroides fragilis, Enterobacteriaceae, Pseudomonas aeruginosa |
Haematogenous spread |
Polymicrobial Bacteroides species, Streptococcus species |
Penetrating trauma |
Staph. aureus, Clostridium species, Bacillus species, Enterobacteriaceae |
|
|
Case 11 Intracranial abscess
Spread of contiguous infection from an otogenic source to the brain is thought to be a key cause of cerebellar abscesses and has been found in up to 93% of cases [10]. Intracranial entry can occur by a number of pathways.
The management of brain abscesses from an otogenic origin is controversial. Otological infections that spread to the brain lie at the interface of neurosurgery, ENT, and microbiology. Treatments include pure pharmacological management, single or repeat aspirations, capsule excision, and extensive ENT procedures.
 Evidence base
Evidence base
Staged operative approach
The classical approach to intracranial complications of chronic supperative otitis media is first to treat the intracranial disease (with either aspiration or capsule excision) and then remove the
offending source via mastoidectomy at a later date. This was described by Joe Pennybacker in 1948 who reported eighteen cases of otogenic cerebellar abscesses. Interestingly, he notes that only two of the nine survived in the pre-antibiotic era, whereas after penicillin was introduced eight out of nine survived, underlining the importance of antimicrobial therapy [13] (Class IV evidence). In 1981, Shu-Yuan Yang reported 400 cases of brain abscess (115 cerebellar) treated over 20 years in China without the aid of CT imaging. They found no difference in mortality between simple aspiration or capsule excision (Class IV evidence). In 2011, a review of 973 brain abscesses (38.6% otorhinogenic) over a 20-year period in Durban, South Africa recommended abscess drainage and separate eradication of infection source. This study was limited by its lack of direct comparison with other
strategies [14]. A review of the literature pertaining to aspiration versus capsule excision over a 78-year period by Ratnaike et al. favoured aspiration because of a 6.6% mortality rate versus 12.7% in the capsule excision group [15]. However, the validity of this final conclusion is questionable because abscesses location, aetiology or adjuvant therapy was not addressed. A modern consensus document on treatment of bacterial brain abscesses states that the type of surgical approach does not appear to be critical in determining outcome but that the speed of the therapeutic operation, including surgery, appear to be the more decisive factors for the final outcome [16] (Class V evidence).
Combined approach (neurosurgery and ENT surgeons)
The location of cerebral abscesses that originate in the ear is remarkably constant. In twenty-six cases of otogenic abscesses that were treated over 15 years all were found immediately adjacent to the petrous temporal bone. In the pre-CT era, this consistency of location allowed blind drainage. More recently, it has facilitated a concurrent approach to the abscess and mastoid infection through a single incision [17]. Morwani & Jayashankar propose a single stage, transmastoid approach as a safe treatment modality for otogenic intracranial abscesses [18] (Class IV evidence). They retrospectively reviewed sixty-one patients who had undergone transmastoid abscess drainage and concurrent tympanomastoidectomy (canal wall up or down depending on pathology). Follow-up was for a minimum of 24 months. Their mortality was 3%, there was a 6% complication rate (CSF leak or
meningitis), and a 3% abscess recurrence rate. They conclude that this is a safe and effective treatment strategy. This view is also supported by the work of Singh and Maharaj who found lower mortality (13% versus 36%) when procedures were combined or performed within 12 hours of each other [19] (Class IV evidence). Kurien et al. also adopted concurrent craniotomy and mastoidectomy, and in their report of thirty-six patients found this to be a safe procedure [20] (Class IV evidence). It has been suggested that early surgical intervention is important to achieve a good outcome and transtemporal drainage of the abscess allows eradication of the primary mastoid disease at the same time as treating the intracranial complications [11,21].
Non-surgical management
Wanna et al. have suggested that an initial non-surgical approach to otogenic intracranial abscess with 6 weeks of broad-spectrum antibiotics (vancomycin, ceftriaxone, and metronidazole) and a shorter intravenous steroid course is safe and effective (Class IV evidence) [12]. They reserve surgical
(continued)
111
 Learning point Routes of intracranial infection from the middle ear
Learning point Routes of intracranial infection from the middle ear
●Direct spread through a bone defect in the tegmen tympani (the very thin layer of temporal bone that separates the tympanic cavity from the middle cranial fossa) or via Trautmann’s triangle (demarcated by the angle between the sigmoid sinus, the superior petrosal sinus and the osseous labyrinth).
●A retrograde thrombophelbitis of the emissary veins may allow communication through the skull into the venous sinuses and then to the brain parenchyma [11,12].

112
 Expert comment
Expert comment
In the absence of definitive diagnosis and clinical deterioration, atypical causes of abscess should be considered, including TB, Nocardia, and fungal infections, such as Aspergillus or Mucorales order fungi.
Nocardia responds to sulphonamides with or without trimethoprim. Aspergillus responds to voriconazole and mucormycosis to amphotericin B.
Challenging concepts in neurosurgery
intervention for patients with an abscess that is expanding with mass effect, despite therapy, or shows a poor response to treatment. They also state that neurosurgical intervention is indicated if the abscess looks likely to rupture into the ventricles as this is a recognized poor prognostic marker, with up to 80% mortality. Interval mastoidectomy is performed once the intracranial disease is stable unless neurosurgical intervention is needed, in which case it is performed as a combined procedure wherever possible. It has been suggested that perhaps mastoidectomy can also be avoided in these patients. In separate studies, Kenna et al. and Dagan et al. treated mastoiditis with daily aural toilet and intravenous antibiotics [22,23]. This approach has not been widely adopted and Wanna et al. urge caution, given the severe potential complications of mastoiditis, including sinus thrombosis and further abscess development. The results of Wanna et al. in ten consecutive patients with intracranial
complications from chronic supperative otitis media (four temporal abscesses, one cerebellar abscess, four sagittal sinus thrombosis, and one subdural empyema) showed 0% mortality and no recurrence. Mean hospital stay was 6.4 days. Although firm evidence is lacking, it has been suggested that medical treatment alone may be more successful if it is begun during the cerebritis stage, if the lesion is less than 2.5cm, if GCS is >12, and a specific organism is known (Class V evidence) [16,24]. Other authors have found higher mortality rates with Hsiao et al. reporting an overall case fatality rate of 48% in thirty-one cases of brain abscess that were managed non-operatively. They identified a low GCS as a key poor prognostic marker in these patients (Class IV evidence) [25].
The optimum antibiotic regimen has not been firmly established, but an ‘Infection in Neurosurgery’ working party review in 2000 suggested ampicillin, metronidazole, and ceftazidime (or gentamicin), as the first line empirical therapy (Class V evidence) [26].
A final word from the expert
To date, there has not been a blinded, randomized trial comparing the different treatment approaches to intracranial abscesses. Neither has there been any meta-analysis of the existing evidence. There remains clinical equipoise as to the most effective strategy for treating otogenic posterior fossa brain abscesses. However, in the presence of a large posterior fossa abscess at earlyor late-stage capsule, stereotactic aspiration to obtain diagnosis and reduce the microbial load appears to be a reasonable initial approach within the context of multidisciplinary management.
References
1.Britt RH, Enzmann DR, Yeager AS. Neuropathological and computerized tomographic findings in experimental brain abscess. Journal of Neurosurgery 1981; 55(4): 590–603.
2.Osborn AG, Salzman KL, Barkovich AJ. Diagnostic imaging: brain. Salt Lake City: Amirsys, 2004.
3.Shen H, Huo Z, Liu L, et al. Stereotatic implantation of Ommaya reservoir in the management of brain abscesses. British Journal of Neurosurgery. 2011; 25(5): 1–5.
4.House JW, Brackmann DE. Facial nerve grading system. Otolaryngology—head and neck surgery 1985; 93(2): 146–7.
5.Lazarini P, Mitre E, Takatu E, et al. Graphic visual adaptation of House-Brackmann facial nerve grading for peripheral facial palsy. Clinical Otolaryngology 2006; 31(3): 192–7.
6.Canale DJ. William Macewen and the treatment of brain abscesses: revisited after one hundred years. Journal of Neurosurgery 1996; 84(1): 133–42.
7.Macewen SW. Pyogenic infective diseases of the brain and spinal cord. Basingstoke: Macmillan, 1893.
Case 11 Intracranial abscess |
113 |
8.Sunder S, Jackler RK, Blevins NH. Virtuosity with the Mallet and Gouge: the brilliant triumph of the ‘modern’ mastoid operation. Otolaryngologic Clinics of North America. 2006; 39(6): 1191.
9.Syal R, Singh H, Duggal K. Otogenic brain abscess: management by otologist. Journal of Laryngology & Otology 2006; 120(10): 837–41.
10.Hsu CW, Lu CH, Chuang MJ, et al. Cerebellar bacterial brain abscess: report of eight cases. Acta Neurologica Taiwanica 2011; 20(1): 47–52.
11.Alaani A, Coulson C, McDermott AL, et al. Transtemporal approach to otogenic brain abscesses. Acta Otolaryngologica 2010; 130(11): 1214–19.
12.Wanna GB, Dharamsi LM, Moss JR, et al. Contemporary management of intracranial complications of otitis media. Otology & Neurotology 2010; 31(1): 111.
13.Pennybacker J. Cerebellar abscess: treatment by excision with the aid of antibiotics. Journal of Neurology, Neurosurgery, and Psychiatry 1948; 11(1): 1.
14.Nathoo N, Nadvi S.S., Narotam PK, & van Dellen JR. Brain abscess: management and outcome analysis of a computed tomography era experience with 973 patients. World neurosurgery. 2011; 75(5): 716–726.
15.Ratnaike TE, Das S, Gregson BA, et al. A review of brain abscess surgical treatment,78 years: aspiration versus excision. World Neurosurgery 2011; 76(5): 431–6.
16.Arlotti M, Grossi P, Pea F, et al. Consensus document on controversial issues for the treatment of infections of the central nervous system: bacterial brain abscesses. International Journal of Infectious Diseases 2010; 14: S79–92.
17.Penido NDO, Borin A, Iha LCN, et al. Intracranial complications of otitis media: 15 years of experience in 33 patients. Otolaryngology-Head and Neck Surgery. 2005; 132(1): 37–42.
18.Morwani K, Jayashankar N. Single stage, transmastoid approach for otogenic intracranial abscess. Journal of Laryngology and Otology 2009; 123(11): 1216.
19.Singh B, Maharaj TJ. Radical mastoidectomy: its place in otitic intracranial complications. The Journal of Laryngology & Otology 1993; 107(12): 1113–18.
20.Kurien M, Job A, Mathew J, et al. Otogenic intracranial abscess: concurrent craniotomy and mastoidectomy—changing trends in a developing country. Archives of Otolaryngology—Head and Neck Surgery 1998; 124(12): 1353.
21.Hippargekar P, Shinde A. Trans-mastoid needle aspiration for otogenic brain abscesses. Journal of Laryngology & Otology 2003; 117(5): 422–3.
22.Kenna MA, Bluestone CD, Reilly JS, et al. Medical management of chronic suppurative otitis media without cholesteatoma in children. Laryngoscope 1986; 96(2): 146–51.
23.Dagan R, Fliss DM, Einhorn M, et al. Outpatient management of chronic suppurative otitis media without cholesteatoma in children. Pediatric Infectious Disease Journal 1992; 11(7): 542–546.
24.Erdofüan E, Cansever T. Pyogenic brain abscess. Neurosurgery Focus 2008; 24(6): E2.
25.Hsiao SY, Chang WN, Lin WC, et al. The experiences of nonoperative treatment in patients with bacterial brain abscess. Clinical Microbiology and Infection 2011; 17(4): 615–20.
26.De Louvois EB, Bayston R, Lees PD, et al. The rational use of antibiotics in the treatment of brain abscess. British Journal of Neurosurgery 2000; 14(6): 525–30.
27.Kocherry XG, Hegde T, Sastry KVR, et al. Efficacy of stereotactic aspiration in deep-seated and eloquent-region intracranial pyogenic abscesses. Neurosurgical Focus 2008; 24(6): 13.
28.Senft C, Seifert V, Hermann E, et al. Surgical treatment of cerebral abscess with the use of a mobile ultralow-field MRI. Neurosurgical Review 2009; 32(1): 77–85.
