
- •CONTENTS
- •EXPERTS
- •CONTRIBUTORS
- •ABBREVIATIONS
- •1 The management of chronic subdural haematoma
- •2 Glioblastoma multiforme
- •3 Spondylolisthesis
- •4 Intramedullary spinal cord tumour
- •5 Surgery for temporal lobe epilepsy
- •6 Management of lumbosacral lipoma in childhood
- •7 Idiopathic intracranial hypertension
- •8 Colloid cyst of the third ventricle
- •9 Bilateral vestibular schwannomas: the challenge of neurofibromatosis type 2
- •10 Multimodality monitoring in severe traumatic brain injury
- •11 Intracranial abscess
- •12 Deep brain stimulation for debilitating Parkinson’s disease
- •14 Trigeminal neuralgia
- •15 Cerebral metastasis
- •16 The surgical management of the rheumatoid spine
- •17 Cervical spondylotic myelopathy
- •18 Brainstem cavernous malformation
- •19 Peripheral nerve injury
- •20 Spontaneous intracerebral haemorrhage
- •21 Low-grade glioma
- •22 Intracranial arteriovenous malformation
- •INDEX
CASE
21 Low-grade glioma
Deepti Bhargava
 Expert commentary Michael D. Jenkinson
Expert commentary Michael D. Jenkinson
Case history
A 34-year-old right-handed man presented to A&E with a single generalized tonic clonic seizure lasting 3 minutes. In the post-octal period, he recovered to a normal conscious level with no focal neurological deficits.
He was generally fit and well, a non-smoker with no significant family history. However, direct questioning revealed that he had suffered occasional headaches for the past 6 months, with exacerbation in the mornings, and he felt generally lethargic.
Routine bloods were normal. A contrasted CT head undertaken at this stage revealed a uniformly hypodense, ill-defined, non-enhancing mass lesion in the left temporoparietal region, expanding the overlying gyrus, causing a degree of midline shift (Figure 21.1).The appearances were consistent with an intrinsic primary lesion, probably a low-grade glioma. Biopsy was offered and declined by the patient. Management comprised anti-epileptic medication (phenytoin 300mg), and a ‘wait and watch’ policy was instituted with an initial 6-month scan followed by annual review of disease that remained stable both clinically and radiologically.
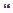 Expert comment
Expert comment
Starting anti-epileptic drugs (AEDs) after the first seizure, with consideration of surgical treatment of the epileptogenic focus early in patients with poor seizure control on medication, are two recommendations we make for low-grade glioma.
Surveillance imaging
There are no guidelines for surveillance imaging in patients being managed conservatively. MRI is the modality of choice for follow-up and should include T1 +/– gad, T2, and FLAIR. Six-monthly MRI is probably sufficient. Perfusion MRI can be useful for predicting malignant transformation. A joint or concurrent neurosurgery/neuro-oncology low-grade glioma clinic is desirable—this minimizes clinic appointments for the patient, and avoids duplication of follow-up imaging. Once all active treatment options have been exhausted, management should be transferred to the palliative care team.
After 6 years, the patient developed gradual onset expressive dysphasia over a period of 2 months. Radiologically, the tumour remained unchanged. He was seizure free on medication and did not have any adverse drug effects. After discussion, the patient consented to tissue diagnosis. The lesion being superficial, but in eloquent cortex, stereotactic biopsy from the most posterior part of the lesion was undertaken.

206 |
Challenging concepts in neurosurgery |
Midline shift
Left temperoparietal space-occupying lesion
Figure 21.1 T1-weighted axial MRI with gadolinium shows a non-enhancing diffuse large temporoparietal lesion with minimal surrounding oedema and midline shift.
 Clinical tip Brain biopsy: techniques, limitations, and yields
Clinical tip Brain biopsy: techniques, limitations, and yields
Open biopsy
Under general anaesthesia via a small craniotomy. The biopsy may be:
●Excisional, resecting the whole lesion.
●Incisional, removing a tissue block without disturbing the cyto-architecture.
●Needle aspiration, where the tissue architecture is disturbed, yielding material for cytology.
Open biopsy is most suitable for superficial lesions, particularly where meningeal biopsy is also required. It may be difficult to localize the region of interest in ill-defined lesions. The diagnostic yield is variable and depends on the diagnosis.
Stereotactic biopsy
Under general or local anaesthesia, via a burr hole using 3-D co-ordinates from a contrasted CT/ MRI scan. Stereotaxy may either be frame based or frameless, using neuronavigation techniques. This technique is most suitable for deep/eloquent/small/heterogeneous lesions. It should not be
undertaken if the patient has a bleeding diathesis or low platelet count. The diagnostic accuracy using stereotactic biopsy is between 82 and 99 % [1] and is better for cases with enhancing lesions.
The diagnostic yield of a biopsy can be improved with the use of an intra-operative smear/ frozen section. Smearing tissue on a microscope slide reveals lesion cytology. Freezing a block of fresh tissue and cutting thin sections with a cryotome allows the tissue architecture to be preserved, and demonstrates the histological appearances. Generally, after sending fresh tissue, the surgeon waits for the results before either closing the wound or obtaining more tissue if necessary. The accuracy of frozen section or of a smear is comparable, but intra-operative smears are quicker and provide additional cytology characteristics to aid the formal pathology diagnosis [2,3].
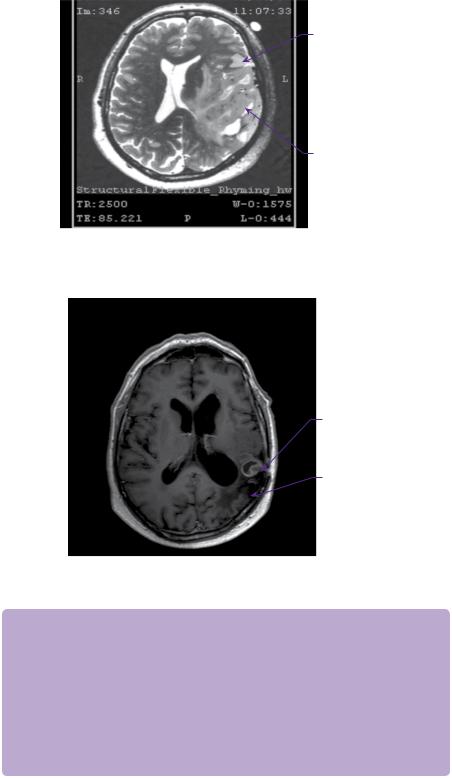
Biopsy revealed mildly atypical neoplastic glial cells with a fibrillary and myxoid background consistent with grade 2 oligodendroglioma. The case was discussed and a decision made to offer the patient procarbazine-lomustine (CCNU)-vincristine chemotherapy. This decision took into account the location within eloquent brain and his Karnofsky score of over 90. The disease remained clinically and radiologically stable for another 8 years. However, there was then obvious growth of the tumour on contrasted MRI scan, although it remained non-enhancing (Figure 21.2) and there was recurrence of his seizures.
Functional MRI for motor and language mapping (Figure 21.3) was undertaken. This revealed close proximity of the tumour to speech pathways.
The patient subsequently underwent awake craniotomy and subtotal resection of low-grade glioma.
Case 21 Low-grade glioma |
207 |
Activated speech pathway
Left temperoparietal space-occupying lesion
Figure 21.2 T1-weighted axial MRI with gadolinium contrast showing small irregular enhancing lesion in the resection cavity at 10 months.
Region of enhancement
Resection cavity
Figure 21.3 T2-weighted axial functional MRI shows close proximity of the tumour to speech pathways.
 Clinical tip Awake craniotomy with speech mapping
Clinical tip Awake craniotomy with speech mapping
The operation commences with the patient intubated and under general anaesthesia. The cortical surface is exposed give a 2-cm clearance around the tumour margin. The anaesthetist then lightens the sedation and the patient is extubated. Electrical stimulation of the cortex is performed, while the patient interacts generally with tasks of naming and numbering. Each site is tested three times. Tumour resection is undertaken with the patient awake and any areas where electrical stimulation hampers speech are spared with a 1-cm margin. White matter mapping is also possible to attempt preservation of relevant tracts. Once the desired resection is achieved, the patient is re-intubated for craniotomy closure. Functional deficits, if any, after such resections are typically transient and, although they may be encountered immediately in the post-operative period in up to a third of patients, only 1.6% are left with permanent deficits after 6 months [4].
208 |
Challenging concepts in neurosurgery |
 Expert comment
Expert comment
Although fMRI informs us of the areas involved in the speech pathways, awake craniotomy and intraoperative mapping is still required for speech preservation in the clinical setting.
Histology revealed grade 2 oligodendroglioma without any features of malignancy. Post-operatively, he developed transient expressive dysphasia, right homonymous hemianopia, and mild paraesthesia in right upper limb.
As resection was limited due to the presence of functional tissue, a post-operative MRI scan revealed a sizable residue. Adjuvant radiotherapy was administered, after which he suffered mild cognitive and memory deficits. He was referred for neurorehabilitation, where he made good progress.
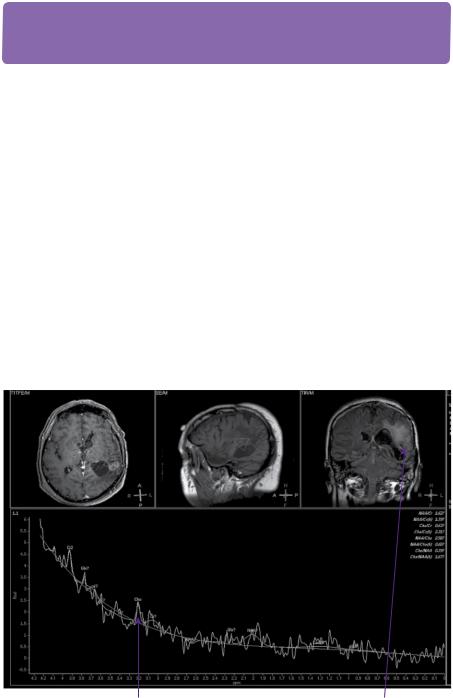
However, after initial improvement, a further increase in seizure frequency occurred and right upper limb sensory symptoms worsened over a period of the next 10 months. Repeat MRI scan at 10 months revealed an area of enhancement in the resection cavity (Figure 21.2). A change of grade was further suggested on MR spectroscopy (Figure 21.4).
The consensus view of the MDT was that he would not benefit from further surgery or radiotherapy. The patient currently has a KPS of 80 and is receiving temozolamide chemotherapy.
Choline peak |
Region of interest for |
on spectogram |
voxelated spectroscopy |
Figure 21.4 MR spectroscopy imaging with metabolic signature of enhancing voxels showing increased cellularity (increased choline: Cho), indicating likely change of grade.

Case 21 Low-grade glioma |
209 |
 Learning point Metabolic markers in MR spectroscopy (Table 21.1)
Learning point Metabolic markers in MR spectroscopy (Table 21.1)
Table 21.1 Metabolic markers in MR spectroscopy
Metabolites |
Clinical significance in brain tumours |
|
|
NAA |
Neuronal marker, lower in primary brain (glial) tumours. NAA/Cr ratio |
(N-acetyl-aspartate) |
has inverse relation to grade of glioma |
Cho (choline) |
Marker of cellular proliferation, increases with tumour grade in glioma. |
|
Cho/Cr ratio direct relation to grade of glioma |
Cr (creatinine) |
Stable brain metabolite in grey and white matter, used as internal |
|
reference for determining metabolite ratios |
Lipid |
Marker of necrosis or proliferation in absence of necrosis |
Lactate |
Marker of anaerobic glycolysis. Lipid and lactate increase with grade |
|
of glioma, combined lipid–lactate peak: reliable marker to distinguish |
|
Grade II, III, and IV glioma |
|
|
Discussion
Low-grade gliomas are diffusely infiltrating brain tumours, comprising WHO grade II astrocytomas, grade II oligodendrogliomas, and oligoastrocytomas. Despite the name, low-grade gliomas are not benign lesions. These tumours have growth potential and can undergo malignant transformation to high grade tumours. Death is usually due to malignant change. With recent advances in neurobiology, diagnostic imaging, surgical technology, and adjuvant therapies, the management of these tumours has undergone a paradigm shift [5].
Low-grade gliomas comprise 15% of all primary brain tumours. They are more common in Caucasian men, the most common age at onset being the fourth decade. Primarily supratentorial in adults, these lesions have a predilection for the insula and the supplementary motor area [6]. Exposure to ionizing radiation is the only definitive causative factor [7]. They are more commonly seen in patients with neu- rofibromatosis-1 and Li–Fraumeni syndrome, but no familial tendencies have been noted. IgE levels have been found to have an inverse relation to glioma risk and survival [6].
Histological characteristics determine the classification of these lesions and have a direct bearing on prognosis. Astrocytomas are thought to be the more aggressive with a median survival of 6–9 years; oligodendrogliomas have a relatively better prognosis with a median survival of 9–12 years. The mixed oligoastrocytomas fare somewhere in between [1]. Recent studies show that oligoastrocytomas are monoclonal indicating a common lineage for these tumour subtypes. Amongst astrocytomas, there are three further histological subtypes—fibrillary, protoplasmic, and gemistocytic. The fibrillary variety is most common. A high number of gemistocytes indicates less time to progression and a higher risk of upgrading to higher WHO grades.

210 |
Challenging concepts in neurosurgery |
 Learning point Histological sub-typing of diffuse low-grade glioma (Table 21.2)
Learning point Histological sub-typing of diffuse low-grade glioma (Table 21.2)
Table 21.2 Histological sub-typing of diffuse low-grade glioma
Astrocytoma |
Oligodendroglioma |
Oligoastrocytoma |
Image
 Expert comment
Expert comment
The three most relevant favourable genetic markers include
1p/19q loss, MGMT promoter hypermethylation, and IDH1 mutation. Testing for these is increasingly being made available in the NHS. While 1p19q simply suggests better outcome, IDH and MGMT mutations are important in predicting the response to temozolamide chemotherapy.
 Learning point
Learning point
Lack of enhancement on MR is not necessarily predictive of low-grade as there is a 50% error rate in diagnosis on imaging grounds alone. The risk of anaplasia in non-enhancing lesions increases with age. Pre-operative metabolic imaging to guide stereotactic biopsy improves diagnostic accuracy up to 100% [6].
Morphology |
Low to moderate |
Uniform cells with |
|
cellularity, and minimum |
round nuclei and |
|
cellular and nuclear |
scant cytoplasm—fried |
|
atypia |
egg appearance. |
|
|
Loose fibrillary matrix |
|
|
traversed by delicate |
|
|
branching vessels, |
|
|
dividing the tumour into |
|
|
pseudolobules—chicken |
|
|
wire pattern |
Biomarkers |
GFAP, vimentin, S-100 |
No specific markers, |
|
protein |
identified by absence of |
|
|
typical astrocytic markers |
Genetics |
Heterozygosity on |
1p19q co-deletion is |
|
17p and heterozygosity |
most common, seen in |
|
of TP53 |
40–70% oligos |
Mixed areas of astrocytic and oligodendroglial differentiation
No specific markers
30–70% have 1p19q c0-deletion, 30% have TP53 and 17p mutation. All cells in a given tumour are monoclonal
Genetic profiling has further advanced our knowledge of these subtypes and their clinical behaviour. Of clinical relevance, P53 and PDGF mutations are associated with astrocytomas and with upgrading. 1p19q loss of heterozygosity is associated with oligodendroglial lineage. Tumours with this mutation have better response rates to temozolamide chemotherapy. IDH mutations are associated with better prognosis; IDH1 is more common in astrocytomas and IDH2 in oligodendrogliomas. Methylation of MGMT can be found in all histological subtypes and is again associated with a better response to temozolamide [6].
Seizures, often simple partial, complex partial, or generalized tonic clonic, are the most common presentation in 80–90% of patients with low grade glioma (LGG), followed by headaches and focal deficits. Close proximity to the cortex is likely to be responsible for this picture.
Typical radiological findings are of a diffuse, hypodense lesion on CT. Structural MRI, which has been traditionally used for diagnosing LGG’s, shows a hypo-intense lesion on T1, and hyperintense on T2 and FLAIR sequences, but patchy enhancement is seen in 15–39% [7]. Cystic degeneration and calcification are common in oligodendroglial tumours.

Case 21 Low-grade glioma
Advanced imaging techniques have helped to further define tumour characteristics. Low-grade gliomas are hypometabolic on PET. MR spectroscopy shows high choline (increased membrane synthesis), low N-acetyl aspartate (neuronal marker), and no lactate or lipid (no necrosis).
Voxels with choline peaks offer targets for biopsy. MR spectroscopy can also be used in follow-up to distinguish recurrence or change of grade from radiation necrosis, both of which present with enhancement on MRI. Phosphocoline increases with cell density, while glycerophosphocholine increases with Ki-67 proliferation index [8].
The prognosis of LGGs is variable. Based on a meta-analysis, the following factors have been shown to decrease survival:
●Age over 40.
●Tumour size ≥6cm.
●Tumour crossing the midline.
●Neurologic deficits.
●KPS ≤70.
●Enhancement on imaging.
●Residual tumour volume greater than 1cm.
Rate of tumour growth on serial imaging is the most reliable indicator of time to progression and likelihood of malignant change [9]. Class I evidence supports age over 40 years and presence of pre-operative neurological deficits as adverse prognostic factors. Evidence for neuro-imaging findings is Class II [10].
While wait and watch was an accepted policy in management of these tumours, recent evidence has challenged this approach. Modern protocols start with anticonvulsants for symptom control and steroids if there are features of raised ICP [9].
Further management is guided by both patient and tumour characteristics. If the lesion is diffuse and in highly eloquent cortex MRI-guided stereotactic biopsy is offered. Molecular profiling of the tumour accompanies this. Biopsy may be followed by procarbazine carvincrisitine vincrisitine (PCV) or temozolamide chemotherapy, especially if the tumour is 1p19q or MGMT mutant, if the tumour demonstrates ‘high risk’ features or if radiotherapy is not appropriate.
If the tumour has relatively discrete margins in brain tissue, where a macroscopic resection will be possible without compromising function, maximum safe surgical resection of all hyperintense tissue on FLAIR sequences should be attempted. This will improve overall and progression-free survival and gives improved seizure control, as well as providing better tumour sampling for histology. Total/near total resection decreases the incidence of recurrence, the risk of malignant transformation, and improves progression-free survival (PFS) and overall survival (OS) (Class III) [11]. Complex partial and generalized tonic clonic seizures, as well as seizures of short duration responsive to AEDs are best controlled with surgery. Extent of resection of the tumour also has important impact on seizure control with gross total resection achieving much better control than partial/subtotal resection [12]. Use of fMRI, awake craniotomy, intra-operative imaging, and cortical and sub-cortical mapping are all useful adjuncts in achieving the surgical goal.
211
 Clinical tip
Clinical tip
Anticonvulsants may increase or decrease the metabolism of chemotherapeutic agents
(cytochrome p 450). In patients with a single seizure, immediate treatment with AEDs reduces seizure frequency. There is no role for prophylactic AEDs.

212 |
Challenging concepts in neurosurgery |
 Expert comment
Expert comment
Surgical adjuncts in the macroscopic resection of low grade gliomas (LGGs)
●Intra-operative cortical stimulation mapping is typically used for speech and motor mapping. The technique is detailed in the description of awake craniotomy. Risks of permanent damage are substantially reduced.
●Intra-operative tractography/subcortical electrical mapping is performed in a similar manner to cortical mapping. The technique is most useful for tumours involving the optic radiation, speech tracts, and descending motor fibres in that order.
●Intra-operative ultrasound correlates poorly with MRI and generally results in larger post-operative residual tumour volumes when compared with MRI directed resections. It may have a role in centres, where intra-operative MRI is not available or the patient has a contraindication for MRI scanning.
●Intra-operative MRI is the most useful for LGG, as it may be difficult to differentiate glioma intra-operatively from normal brain tissue. Sequential intra-operative MR is ideal. Operative time is substantially increased, but the need for early second surgery is curtailed and better tumour resection is possible. In the ideal setting, one would use intra-operative MR with direct cortical stimulation mapping to achieve maximal functional resection.
 Clinical tip
Clinical tip
Identification and resection of the epileptogenic focus through electrocorticography is likely to enhance seizure control after resection. Long duration (multifocality) and hippocampal atrophy suggest poor seizure control post-operatively. Seizure control is an index for quality of life in these patients, but surgery is not targeted at epileptogenic foci for those with minimal seizure frequency or well-controlled disease on medication. In patients with good seizure control post-operatively, seizure recurrence usually indicates tumour progression.
The timing and role for adjuvant/neo-adjuvant therapy in these patients is being extensively researched. According to current evidence early (primary adjuvant) versus delayed (upon recurrence) radiotherapy does not bestow any overall survival advantage. Although improved PFS was demonstrated for patients treated with immediate RT, this did not translate into improved OS (Class I)- EORTC 22845 (reference here).
The benefit of progression-free survival gained from early radiotherapy is offset by the resultant neurotoxicity. As such radiotherapy is mainly reserved for high risk cases or upon tumour progression. Optimal dosage is considered to be 50–54Gray since studies show no difference when compared with a higher dose [EORTC 22844 and NCCTG investigated higher v/s lower dose of RT showed no advantage for higher doses (Class I)] and the benefit is reduced neurotoxicity [9].
Traditionally, chemotherapy was not thought to be very effective in this setting. As detailed above, genetic profiling of these tumours has changed this perspective. Temozolamide and PCV regimens are both being extensively investigated in multicentre trials. RTOG 9802 investigated RT plus early chemotherapy versus chemotherapy at progression. PFS, but not OS were improved (Class I). However, beyond 2 years, the addition of PCV to RT conferred a significant OS and PFS advantage, reducing the risk of death by 48% and progression by 55%, suggesting a delayed benefit for chemotherapy. Grade 3–4 toxicity was higher amongst patients receiving RT + PCV (67 versus 9%; Class I).
Patient wishes and aspirations are always important, and need to be considered in all decision making, as is the discussion of all cases in the neuro-oncology multidisciplinary meeting.

Case 21 Low-grade glioma |
213 |
A final word from the expert
The optimal management of low-grade glioma remains to be defined. The majority of tumours are located within eloquent brain and affect young people. Management aims to stabilize tumour, whilst preserving quality of life. Four points summarize the future direction of LGG management:
●There is a paradigm shift towards early aggressive resection to functional limits defined by intra-operative brain mapping to encompass the FLAIR signal abnormality.
●Functional remodelling of eloquent brain regions allows further tumour resection.
●Neo-adjuvant chemotherapy can reduce tumour size to enable complete resection of the FLAIR signal.
●Supra-complete tumour resection with a 2-cm margin beyond the FLAIR signal abnormality may potentially ‘cure’ low-grade glioma, but long-term outcome data are awaited.
References
1.Greenberg M, Duckworth EA, Arredondo N (6the edn). Handbook of Neurosurgery. Thieme Medical Publishers, Incorporated, November 2005.
2.Woodworth GF, McGirt MJ, Samdani A, et al. Frameless image-guided stereotactic brain biopsy procedure: diagnostic yield, surgical morbidity, and comparison with the frame-based technique. Journal of Neurosurgery 2006; 104 (2): 233–7.
3.Hayden R, Cajulis RS, Frias-Hidvegi D, et al. Intraoperative diagnostic techniques for stereotactic brain biopsy: cytology versus frozen-section histopathology. Stereotactic Functional Neurosurgery 1995; 65 (1–4): 187–93.
4.Sanai N, Berger MS. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics 2009; 6 (3): 478–86.
5.Potts MB, Smith JS, Molinaro AM, et al. Natural history and surgical management of incidentally discovered low-grade gliomas. Journal of Neurosurgery 2012; 116 (2): 365–72.
6.Sanai N, Chang S, Berger MS. Low-grade gliomas in adults (Review). Journal of Neurosurgery 2011; 115 (5): 948–65.
7.Cavaliere R, Lopes MB, Schiff D. Low-grade gliomas: an update on pathology and therapy. Lancet Neurology 2005; 4: 760–70.
8.McKnight TR, Smith KJ, Chu PW, et al. Choline metabolism, proliferation, and angiogenesis in nonenhancing grades 2 and 3 astrocytoma. Journal of Magnetic Resonance Imaging 2011; 33 (4): 808–16.
9.Gilbert MR, Lang FF. Management of patients with low-grade gliomas. Neurology Clinic 2007; 25: 1073–88.
10.Pignatti F, van den Bent MJ, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low grade glioma. Journal of Clinical Oncology 2002; 20: 2076–84.
11.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. Journal of Clinical Oncology 2008; 26: 1338–45.
12.Englot DJ, Berger MS, Barbaro NM, et al. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. Journal of Neurosurgery 2011; 115 (2): 240–4.
