
- •CONTENTS
- •EXPERTS
- •CONTRIBUTORS
- •ABBREVIATIONS
- •1 The management of chronic subdural haematoma
- •2 Glioblastoma multiforme
- •3 Spondylolisthesis
- •4 Intramedullary spinal cord tumour
- •5 Surgery for temporal lobe epilepsy
- •6 Management of lumbosacral lipoma in childhood
- •7 Idiopathic intracranial hypertension
- •8 Colloid cyst of the third ventricle
- •9 Bilateral vestibular schwannomas: the challenge of neurofibromatosis type 2
- •10 Multimodality monitoring in severe traumatic brain injury
- •11 Intracranial abscess
- •12 Deep brain stimulation for debilitating Parkinson’s disease
- •14 Trigeminal neuralgia
- •15 Cerebral metastasis
- •16 The surgical management of the rheumatoid spine
- •17 Cervical spondylotic myelopathy
- •18 Brainstem cavernous malformation
- •19 Peripheral nerve injury
- •20 Spontaneous intracerebral haemorrhage
- •21 Low-grade glioma
- •22 Intracranial arteriovenous malformation
- •INDEX
CASE
22Intracranial arteriovenous malformation
Jinendra Ekanayake
 Expert commentary Neil Kitchen
Expert commentary Neil Kitchen
Case history
A 54-year-old right-handed man was admitted with a 2-week history of left-sided headaches, visual disturbance, and difficulties with short-term memory. Examination revealed a right-sided temporal hemianopia.
Nine years previously, following a brief loss of consciousness at home, investigations had revealed a left-sided occipital arteriovenous malformation (AVM) with a 3.5-cm nidus, and associated deep venous drainage (Spetzler–Martin Grade 4). He remained entirely asymptomatic following this event, which was diagnosed as an unrelated vasovagal episode. It was initially decided that the management should be conservative, given the incidental discovery of the AVM.
 Learning point Surgical operability and the Spetzler–Martin grade
Learning point Surgical operability and the Spetzler–Martin grade
The Spetzler–Martin classification (1986) was proposed as a means of defining surgical operability [1]. It is based on three components—nidus size, venous drainage, and proximity to eloquent brain regions. Eloquent brain is given a somewhat broad description, including primary motor, sensory, visual and language cortices, as well as the thalamus, hypothalamus, brainstem, and cerebellar peduncles.
Table 22.1: The percentage of patients with a major neurological deficit (defined as hemiparesis aphasia, and/or hemianopia) increases as the Spetzler–Martin grade rises.
Table 22.1 Correlation of AVM grade with surgical results*
Grade |
No. cases |
No deficit |
|
Minor deficit |
|
Major deficit |
Death (%) |
||||
|
|
No. |
% |
|
No. |
% |
|
No. |
% |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
I |
23 |
23 |
100 |
0 |
0 |
0 |
0 |
0 |
|||
II |
21 |
20 |
95 |
1 |
5 |
0 |
0 |
0 |
|||
III |
25 |
21 |
84 |
3 |
12 |
1 |
4 |
0 |
|||
IV |
15 |
11 |
73 |
3 |
20 |
1 |
7 |
0 |
|||
V |
16 |
11 |
69 |
3 |
19 |
2 |
12 |
0 |
|||
Total |
100 |
86 |
86 |
10 |
10 |
4 |
4 |
0 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
*Exceptions to this include if the patient has repeated haemorrhages, or progressive neurological deficit, the presence of flow-related aneurysms (amenable to surgery or endovascular treatment) and steal-related deficits (amenable to endovascular treatment) [2].
The grade is the sum of the three individual components scoring AVMs from I to V (Table 22.2). A further grade has been proposed, VI, to define ‘inoperable’. Despite criticisms related to nidus
heterogeneity, eloquence, and infratentorial AVMs, the system has been prospectively validated and
remains in common usage.
(continued)
216 |
Challenging concepts in neurosurgery |
|
|
|
|
Table 22.2 Spetzler–Martin grading scale for AVMs |
|
|
|
|
|
|
|
Feature |
Score |
|
|
|
|
|
|
<3 cm |
1 |
|
|
3–6 cm |
2 |
|
|
>6 cm |
3 |
|
|
|
|
|
|
Location |
|
|
|
|
|
|
|
Non-eloquent cortex |
0 |
|
|
In/ adjacent to eloquent cortex |
1 |
|
|
|
|
|
|
Venous drainage |
|
|
|
|
|
|
|
Superficial only |
0 |
|
|
Deep |
1 |
|
|
|
|
Spetzler and Ponce have proposed a recent revision (2011) to this classification to reflect treatment strategies (Table 22.3) [2]. The grouping of the grades into a three-tier system was based on a pooled analysis of outcome in 1476 patients taken from seven surgical series. Predictive accuracies for surgical outcomes were found to similar when comparing surgical outcomes between the five-tier and threetier system [2,3].
Table 22.3 SpetzlerMartin grading scale for AVMs
Class |
Spetzler-Martin Grade |
Treatment |
|
|
|
A |
I &II |
Microsurgery |
B |
III |
Multimodality |
C |
IV & V |
No treatment* |
|
|
|
 Expert comment Unruptured AVMs—to treat or not to treat?
Expert comment Unruptured AVMs—to treat or not to treat?
Current best evidence in relation to treatment is from the ARUBA trial (A Randomised Trial in Unruptured Brain Aneurysms) reported by Mohr et al (2013) [4]. It was a prospective, parallel-design randomized controlled trial (NHS class A) which was stopped early, with a follow-up of 33 months. Eligibility was age 18 years or over, no previous haemorrhage or intervention, in patients with brain AVMs (bAVMs) considered suitable for obliteration. 226 patients were recruited between 2007–2013; 114 patients were randomised to intervention, 109 patients to best medical management (7 patients crossed to the intervention arm, without suffering a bleed). 5 were treated with surgery, 30 with embolisation, 31 with radiotherapy, 28 with a multimodality approach. The primary outcome measured were symptomatic stroke or death; secondary outcome measures were clinical impairment, defined
as 2 or higher on the modified Rankin Scale.35/114(30.7%) patients in the treatment arm vs 11/109 (10.1%) patients in the medical arm reached the primary endpoint. 17/109 (38.6%) treated patients, and 6/109 (14%) of the patients managed medically reached the secondary endpoint. The authors concluded that medical management was therefore superior to intervention for unruptured bAVM patients at 33 months.
Russin and Spetzler (2014) provided a critique of the study and its findings, citing design flaws, lack of standardisation of the treatment arm , and inadequate study detail (i.e. 726 patients were eligible, 226 patients were enrolled177 patients were managed outside of the randomisation process) [5].
Nonetheless, the findings of the ARUBA trial provide important evidence on the management of unruptured aneuryms, and is supported by the Scottish Intracranial Vascular Malformation Study (SIVMS) which was carried out between 1999–2003. This was a smaller prospective observational study, with a 3 year outcome, which found an increased risk of a poor outcome in patients receiving treatment, and those with large AVMs [6].
Case 22 Intracranial arteriovenous malformation |
217 |
On his most recent admission, CT imaging once again confirmed the presence of the AVM. There was no evidence of an intracranial bleed or brain swelling associated with the AVM.
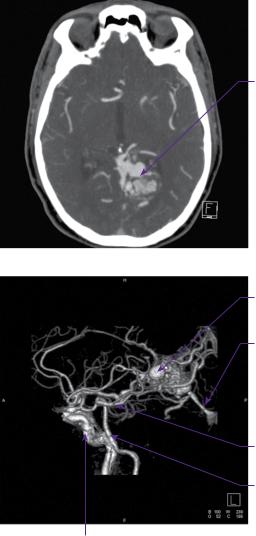
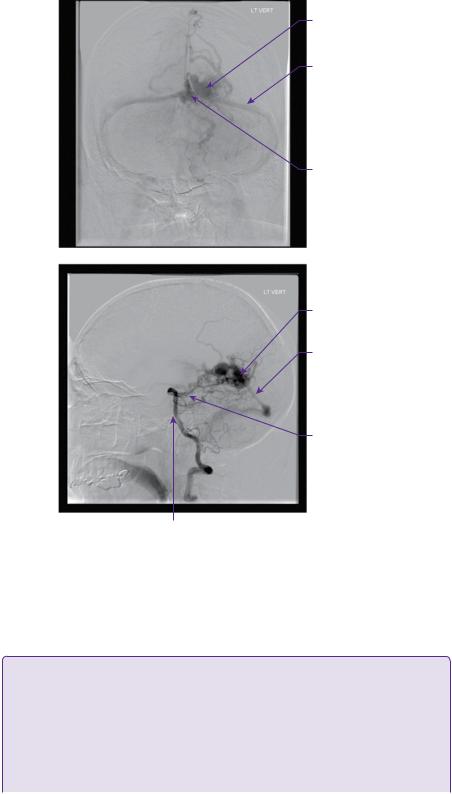
CT angiography and digital subtraction angiography (DSA) revealed that the AVM was principally supplied from the left posterior cerebral artery, with pial supply from the left anterior and middle cerebral arteries. Drainage was through a single arterialized vein into the straight sinus (Figure 22.1a–d).
(a)
Left arteriovenous malformation
(b) |
Varix within |
|
|
|
AVM |
|
Straight sinus |
Posterior cerebral artery
Basilar artery
Internal carotid artery
Figure 22.1 (a) CT angiogram axial section. There is a serpiginously-enhancing lesion in the left occipital lobe. The appearances are those of an AVM with no evidence of acute haemorrhage. (b) CT angiogram sagittal 3D reconstruction. The nidus is fed mainly by the left PCA with venous drainage into the vein of Galen. (c,d) DSA: left vertebral artery injection, anteroposterior and lateral views, respectively.
218 |
Challenging concepts in neurosurgery |
Varix within
AVM
Transverse sinus
Torcular herophili
Nidus of AVM
Straight sinus
Posterior cerebral artery
Basilar artery
Figure 22.1 Continued
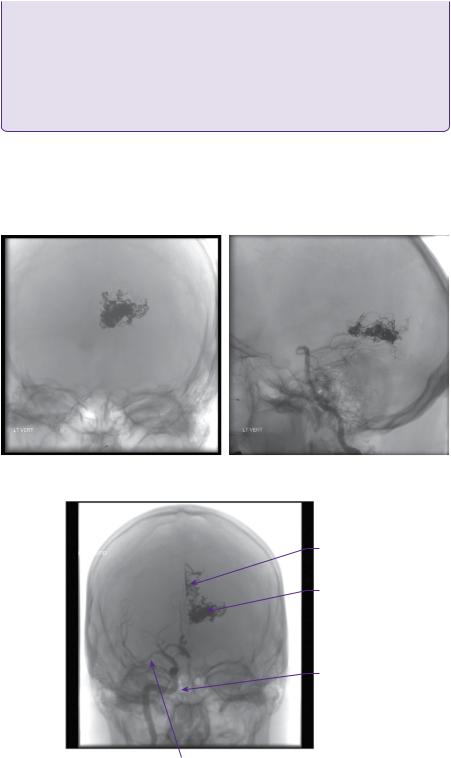
In view of the size and location of the AVM, the recommendation of the neurovascular multidisciplinary meeting was staged endovascular embolization. At the time of his first embolization, performed via internal carotid and vertebral artery catheterization, 50% of the nidus was obliterated (Figures 22.2a,b).
 Learning point Staged embolization and stereotactic radiosurgery
Learning point Staged embolization and stereotactic radiosurgery
In large lesions, which are considered to be unfavourable for surgery, staged embolization followed by
SRS has been used. Embolization has two goals:
●Targeted eradication of AVM-related aneurysms and fistulae.
●Volumetric reduction.
By reducing the size of the nidus, a larger dose of stereotactic radiation can be prescribed in order to increase the probability of nidus obliteration without increasing the risk of complications. Many
(continued)
Case 22 Intracranial arteriovenous malformation |
219 |
radiosurgery centres now avoid embolization before treatment for large AVMs, preferring instead to stage the radiosurgery either by volume (with different components of the nidus being treated on each occasion) or by dose, with suboptimal doses being used on two or more occasions.
Gobin and colleagues treated 125 inoperable AVM patients with embolization prior to definitive treatment with SRS. Embolization resulted in total occlusion in 11.2% of AVMs and, in 76%, was reduced enough to allow SRS. Subsequent SRS produced total occlusion in 65% of these cases.
No complications were associated with SRS. There was morbidity of 12.8% and a mortality of 1% following embolization. The haemorrhage rate for the partially embolized AVMs was 3% per annum [7].
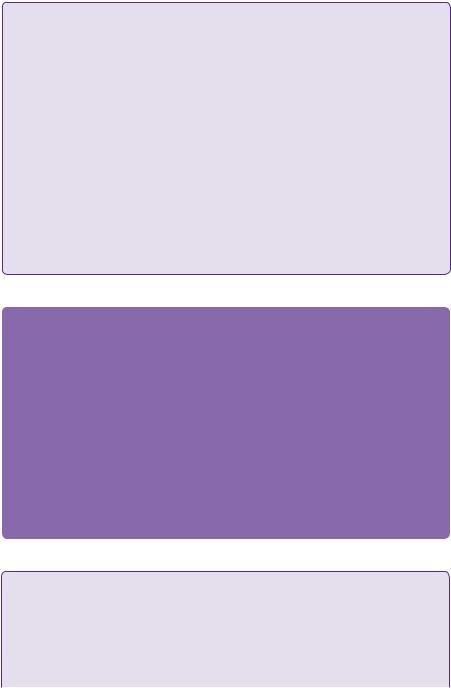
The patient subsequently underwent three further embolization procedures with a progressive reduction in the size and flow rate of the AVM (Figure 22.3).
Following a further multidisciplinary assessment, gamma knife radiosurgery was advised and subsequently delivered. Normally, follow-up after radiosurgery would
(a) |
(b) |
Figure 22.2 (a,b) Post-embolization DSA/left vertebral injection anteroposterior and lateral views.
Residual AVM
Onxy cast
Internal carotid artery
Middle cerebral artery
Figure 22.3 Post-embolization DSA/left vertebral injection, anteroposterior view.
220 Challenging concepts in neurosurgery
involve annual MRI scans as a preliminary indication of nidus obliteration. This will often require 2–3 years, but may occur up to 4 years after treatment. If there are no obvious flow voids in the area of treatment, digital subtraction angiography is indicated to confirm obliteration. Retreatment with radiosurgery is possible and would normally be recommended if obliteration has not occurred within 4 years.
 Learning point Stereotactic radiosurgery
Learning point Stereotactic radiosurgery
This is typically reserved for compact lesions, usually less than 3cm in size. It is currently the preferred treatment strategy for the majority of patients, unless there are very good clinical reasons to prefer surgery (e.g. treatment of the AVM during removal of an ICH, patient preference after careful counselling).
After treatment, which may take up to 48 months to obliterate the nidus, there is a risk of haemorrhage. Annual haemorrhage rates have been estimated at 4.8–7.9% per year for the first 2 years*, then 2.2–5% in the third, fourth, and fifth years. Notably, if the nidus is obliterated, the risk of haemorrhage is near zero. In the optimal case, i.e. small nidus, uncomplicated location, complete obliteration can be achieved in at least 90% of cases, although this decreases significantly for larger AVMs (i.e. treatment volumes of 20cm3) [4].
The technique allows a high dose of radiation to be delivered to a focal brain region, while minimizing radiation to surrounding normal brain. It is therefore ideal for centrally-located lesions, such as the brainstem, thalamus, and basal ganglia. Lim and colleagues have also demonstrated its successful
use in patients with medically intractable seizures. Furthermore, it may be used in those with multiple lesions [8], such as in familial syndromes [9,10].
*The risk of haemorrhage in the first 2 years following SRS is higher than the annual risk of haemorrhage in an untreated unruptured AVM, i.e. 2.4%.
 Expert comment Surgery for arteriovenous malformations
Expert comment Surgery for arteriovenous malformations
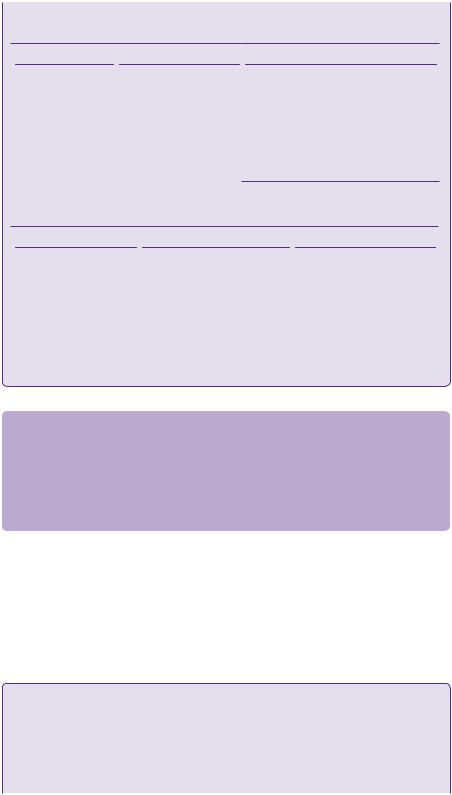
With the advent of endovascular obliteration and gamma knife radiotherapy, neurosurgical intervention is no longer the commonest modality of treatment for intracranial AVMs in most centres. The main benefit of surgery is of an immediate cure. Following dural opening, surgical technique involves delineation of the malformation, elimination of superficial feeding vessels, circumferential dissection of the nidus with control of the deep arterial pedicles and transection of the venous system. A haemosiderin or gliotic rim, or acutely haemorrhagic clot itself may provide a plane of dissection around the nidus, see figure 22.5.
It is critically important to identify arterialized veins before resection. If there is any doubt a temporary clip maybe used, as premature coagulation of a draining vein may result in catastrophic haemorrhage.
Sylvian fissure lesions are prone to en passage vessels, which must be skeletonized and preserved, with resection only of the small side branches feeding the nidus.
It is of vital importance to plan the surgical approach well in advance of surgery, using pre-operative
MRI and angiogram imaging to establish the position of probable feeding and draining vessels
 Learning point Arteriovenous malformations and driving
Learning point Arteriovenous malformations and driving
In the UK, there are very specific guidelines regarding AVMs and driving, with the diagnosis of an AVM potentially having a significant impact on the livelihood of professional drivers. Decisions are made on the background of the history and examination, e.g. incidental AVMs versus ruptured AVMs, supratentorial versus infratentorial location (Tables 22.4 and 22.5), and related seizures.
Please see the DVLA website for more information: http://www.dft.gov.uk/dvla
(continued)
Case 22 Intracranial arteriovenous malformation |
221 |
Table 22.4 Supratentorial AVMs cars/ bikes (Group 1) lorries/ large goods vehicle (LGV) (Group 2)
Pathology |
Group 1 recommendation |
|
|
Incidental |
|
No treatment |
Retain licence |
Treatment |
As for ICH presentation |
ICH presentation |
|
Craniotomy |
6/12 off driving |
Embolization/SRS |
1/12 off driving |
No treatment |
As above |
|
|
Group 2 recommendation
Permanent refusal
Refusal unless cured and 10 years seizure free
Refusal unless cured and 10 years seizure free Refusal unless cured and 10 years seizure free Permanent refusal
Table 22.5 Infratentorial AVMS
Pathology |
Group 1 recommendation |
Group 2 recommendation |
|
|
|
Incidental |
|
|
No treatment |
Retain |
Individual assessment |
Treatment |
Can drive once no disability |
Refusal unless cured/no disability |
ICH presentation |
|
|
Craniotomy |
Retain unless disability affecting |
Refusal unless cured |
|
driving |
|
Embolization/ SRS |
As above |
As above |
No treatment |
As above |
Permanent refusal |
|
|
|
 Clinical tip Risk of arteriovenous malformation rebleeding
Clinical tip Risk of arteriovenous malformation rebleeding
Several morphological and angio-architectural factors have been examined with regards to the risk of rebleeding, with a considerable degree of variability in the results [11].
●Previous rupture.
●Annual rupture rate of 6% (first 5 years)/cumulative 5-year risk 26%.
●Associated aneurysms.
●Deep venous drainage.
Discussion
AVMs were first described by Virchow in 1851, although clinical descriptions of extracranial vascular abnormalities date back to the Ebers Papyrus (c. 1500 BC). The first complete neurosurgical excision of an intracranial AVM is credited to Emile Pean in 1889 who removed a right-sided ‘central’ tumour in a 15-year-old boy with left-sided seizures.
 Learning point Arteriovenous malformations—an embryological versus developmental abnormality
Learning point Arteriovenous malformations—an embryological versus developmental abnormality
AVMs may develop during embryonic developmentthe two main hypotheses regarding their pathogenesis include:
● Embryonic agenesis of the capillary system.
● Retention of the primordial connection between arteries and veins [12].
(continued)
222 |
Challenging concepts in neurosurgery |
Despite this, they are believed to be dynamic biological entities, rather than static congenital vascular lesions.
Although certain genes are associated with the development of AVMs (e.g. ALK-1 in hereditary haemorrhagic telangectasia, (HHT)) and their rupture (e.g. ApoE e2), there is clear evidence of de novo formation and active growth post-natally. This would suggest a more complex mechanism, including environmental contributions to the expression of genes involved in angiogenesis and vascular repair. It is of note that, in certain cases, AVMs have been observed to regress and disappear completely [13].
AVMs are associated with several rare hereditary conditions including: HHT, von Hippel–Lindau disease, Sturge–Weber disease, and Wyburn–Mason syndrome. They are rarely familial, having been described in only twenty families (forty-four cases) [14]. See Figure 22.4
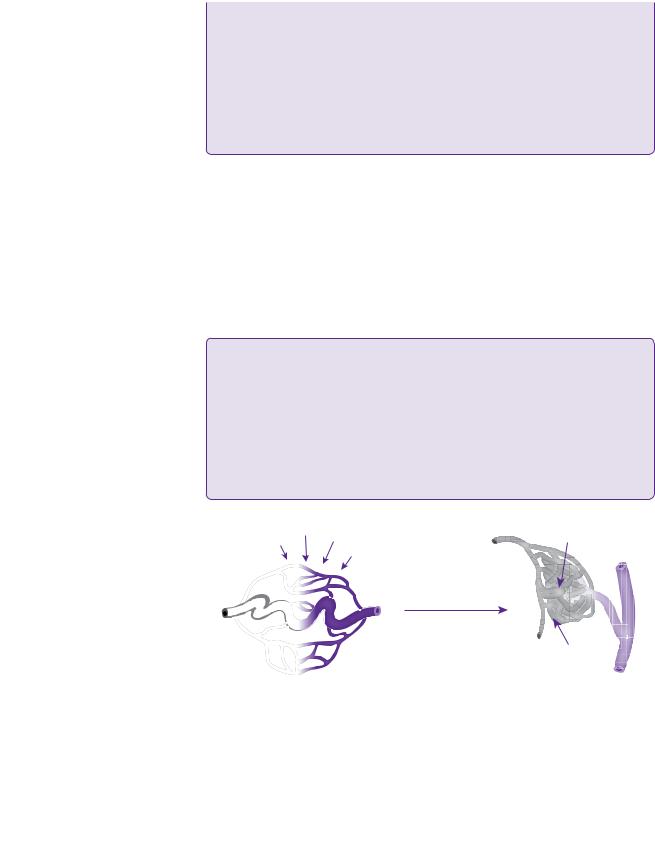
Arteriovenous malformations of the brain are complex vascular lesions with a number of associated neuroparenchymal, haemodynamic and angio-architectural changes. The fundamental abnormality is one or more direct fistulous communications between afferent feeding arteries and efferent draining veins, without the presence of an intervening capillary bed. The increased blood flow through these arteriovenous shunts causes the characteristic changes of vascular dilatation, tortuosity, and the formation of the ‘nidus’, an intervening network of vessels.
They may be found in either the brain or the dura. Note arteriovenous fistulae (AVFs) differ only in the absence of an intervening nidus.
 Learning point Arterial contributions to the nidus
Learning point Arterial contributions to the nidus
Identifying the arterial supply to the AVM is particularly important within the context of surgical disconnection prior to excision of the AVM. Valavanis (1996) described three types of supply [15].
●Direct or terminal feeders.
●Dedicated vessels terminating in the nidus, arising from main arteries.
●Indirect feeders.
●‘Satellite’ branches from an artery in close proximity to the nidus.
●‘En passant’ vessels.
●These are functional vessels and, in addition to supplying the nidus, pass through to supply normal brain tissue beyond.
Hypoxia Trauma Inflammation |
|
AVM nidus |
Angiogenesis |
|
|
|
Abnormal EC growth |
|
|
with impaired vessel |
|
|
maturation |
|
|
(+) VEGF, HIF1 , Ang2, |
|
|
MMP, IL-1, IL-6, TNF |
|
|
(–) Alk1, Eng, Smad4, Ang1 |
Feeding |
|
|
|
|
|
artery |
Teleangiectasia with dilated venule, |
|
Late AVM lesions with |
|
arterio-venous shunt without |
|
partly connected to enlarged |
|
|
|
intervening capillaries |
|
arteriole via few capillaries |
|
|
|
|
Figure 22.4 AVMs are fast-flow lesions wherein feeder arterioles shunt directly to veins without intervening capillaries. Candidate disease-associated molecules that are upregulated (+) or downregulated (−) are shown. EC, endothelial cell
(Taken from Storkebaum et al., 2011 [15])
Case 22 Intracranial arteriovenous malformation
 Expert comment The ‘nidus’
Expert comment The ‘nidus’
It is useful to consider this as a functional entity in itself. The histopathological composition is that of a vascular mass of fused arteries and veins, without an intervening capillary bed. However, the
existence of dilated perinidal capillaries forming a complex network in communication with the nidus is recognized [3].
Within the nidal mass there is brain parenchyma, which is non-functioning, gliotic, and frequently haemosiderin stained. Neuronal loss in the surrounding brain (possibly related to ‘vascular
steal’ by the AVM), together with an increase in fibrillary glia have been reported. Arterial communications may take the form of single feeding vessels, or multiple feeders in a plexiform pattern [3,16].
Clinical presentation
These are rare lesions (0.14% incidence, 0.001–0.52% prevalence), but are important as they tend to affect young patients who are frequently otherwise healthy [24].
They typically present as ICHs accounting for 2% of all strokes and 4% of intracranial haematomas overall, but up to 38% in 15–45-year-olds. Haemorrhage is associated with a 5–10% risk of death, and 30–50% chance of permanent or disabling neurological deficits [25].
Other presentations include seizures, which are the second most common presentation (20% of patients, with higher risk in large AVMs i.e. >6cm [28]), Less commonly, ipsilateral headaches, and focal neurological deficits (temporary, fixed, or progressive) in 3–10% of patients due to mass effect and haemodynamic disturbances. At least 15% of patients with AVMs at the time of detection are asymptomatic, and this figure will increase with greater access to MRI scanning [29].
CT imaging is routinely used in the acute setting of haemorrhage/ICH presentation, allowing identification of the bleed and/or infarct. Unruptured AVMs may be identified by prominent calcification (20–30%). CT angiography provides good delineation of vascular anatomy, particularly with 3D vascular reconstruction. However, digital subtraction catheter angiography remains the gold standard allowing for
223
 Learning point Epilepsy and arteriovenous malformations
Learning point Epilepsy and arteriovenous malformations
In a multicentre study of 1289 patients, seizures were the second most common presentation after haemorrhage (30% presented with generalized seizures 10% presented with focal seizures). The incidence of patients presenting with seizures in the absence of haemorrhage is between 17 and 40% [17].
Male sex, age <65 years, AVM >3cm, and temporal lobe location have shown an association with seizures [18].
 Expert comment
Expert comment
The decision to treat epileptogenic, unruptured AVMs is contentious, as is the ideal treatment modality. Surgery is not felt to represent a curative modality and, indeed, may result in new seizure onset.
 Clinical tip Risk factors for first haemorrhage
Clinical tip Risk factors for first haemorrhage
This is contentious, with a number of contradictory findings in the literature [11], primarily due to the length of follow-up. Most recently, Hernesnemi and colleagues (2008) [24,25] produced a wellvalidated study in 238 consecutive patients followed-up for a mean of 13.5 years (the most extensive study in terms of total patient years). Annual risk was 2.4%, with the highest risk in the first 5 years*.
A rough approximation of the lifetime risk for haemorrhage for an AVM can be derived as follows: Lifetime risk (%) of AVM rupture = 105 – patient age (assuming 3% annual risk of bleed).
Patient factors
● Young age: (Sex does not appear to affect the risk of rupture)
Nidus factors
● Large size.
● Deep location (i.e. deep cerebral, ventricular, periventricular). ● Deep venous drainage (exclusive).
● Infratentorial location.
(continued)

224
 Learning point ‘Steal’ phenomena
Learning point ‘Steal’ phenomena
This has been proposed as one of the mechanisms by which neurological deficits are produced by AVMs—the surrounding brain
parenchyma is rendered ischaemic due to high flow ‘shunting’ of blood into the AVM. It has since been challenged.
 Clinical tip Size and presentation
Clinical tip Size and presentation
Despite Spetzler and colleagues demonstrating higher arterial feeding pressures in smaller AVMs [30], the recent literature does not suggest that they are more likely to bleed. However, smaller AVMs are more likely to be identified following a haemorrhage, as they are less likely to produce other symptoms.
Challenging concepts in neurosurgery
In addition to the above, which were found to be independent risk factors on multivariate analysis, other authors [6,27] identified the importance of intra-nidal pressure and included the following:
●Single draining vein.
●Venous stenosis.
*Other important studies include Ondra et al. (1990) [26], and Stapf et al. (2006) [25].
evaluation of venous outflow obstruction, associated aneurysms, feeding artery/ arteries, and venous drainage. MRI/MRA enables the best assessment of the anatomical relationships to neighbouring brain structures (i.e. ‘eloquence’). Functional MRI and diffusion tensor imaging can further help to identify eloquent areas (i.e. language, motor, etc.) and associated white matter tracts, respectively.
AVMs and Epilepsy
The cause of epileptogenesis is still unclear. Focal cerebral ischaemia as a result of arteriovenous shunting has been suggested, as has ischaemia in the surrounding brain regions, as a result of steal phenomena. Gliotic brain tissue within the nidus, and in the surrounding brain regions may also contribute to secondary epileptogenesis
Nonetheless Yeh et al. operated on twenty-seven patients with epilepsy with seizure-elimination in twenty-one patients. They concluded that excision of an AVM must also include removal of the epileptogenic focus [19].
More recent studies of seizure outcomes after radiosurgery have demonstrated very promising results and deserved particular consideration (62% [20] and 80% [21]). It has been suggested that beneficial effects on seizure control occur even before complete obliteration,, although this provides the best seizure-free results However, new-onset seizures can be a complication of radiosurgery [22].
There are fewer reports of seizure control after embolization, although this likely to reflect that it primarily used as an adjunctive procedure in multimodality treatment [23].
Treatment
The treatment of AVMs uses three modalities, alone or in combination. They are microsurgery, embolization, and radiosurgery. The manner in which these are applied take into account patient factors (i.e. age, co-morbidities, mode of presentation), AVM factors (size, location, Spetzler–Martin grade), and available expertise.
Microsurgical approaches afford direct visualization of anatomy with control of feeding vessels, but are offset by the attendant risks of open surgery, together with the technical expertise required for correct vessel delineation. It potentially offers immediate and complete cure and, for some surgeons, is the preferred first line treatment for SM grade I and II AVMs, see figure 22.5. Complications following surgery have been variously classified as major and minor neurological deficits. They are related to the location of the lesion, with size, deep venous drainage, and the presence of associated aneurysms increasing the surgical risk. Complications include aphasia, hemianopia, hemiparesis, and death. A recent meta-analysis reported a morbidity of 8.6% and mortality of 3.3%, after mostly surgical treatment, in a series of 2452 patients [31]. The surgical risk for morbidity and mortality for Spetzler–Martin grade of less or equal to 3 has been reported to be 2–6.3% and 0–2%, respectively. The surgical risk for morbidity and mortality
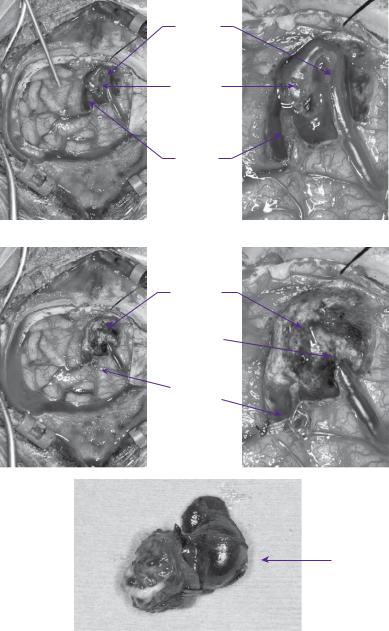
Case 22 Intracranial arteriovenous malformation |
225 |
for Spetzler–Martin grade IV and V has been reported to be 9–39% and 0–9%, respectively.
Stereotactic radiotherapy provides the least invasive treatment option. The most important factor for nidus obliteration is the dose of radiation delivered. The safe dose correlates inversely with volume, in that the larger the lesion, the smaller
Draining
vein
Nidus
of AVM
Feeding
artery
(a) |
(b) |
Resection
cavity
 Ligated vein
Ligated vein
Artery clipped
with aneurysm clip
(c) |
(d) |
Resected
AVM nidus
(e) 1cm
1cm
Figure 22.5 Intraoperative images of an occipital AVM showing cardinal anatomy (a,b) i.e. feeding artery, draining vein, and the AVM nidus. Postoperative images showing the resected AVM nidus and the residual postoperative cavity(c,d,e).

226 Challenging concepts in neurosurgery
the dose that can be given without a high risk of radionecrosis of surrounding brain. Other factors include the size of the nidus (e.g. ideally <3cm), the nidus angio-architecture (e.g. compact versus diffuse nidus), intranidal aneurysms, venous drainage, eloquent location, and previous haemorrhage. A compact nidus is a better target compared with plexiform or diffuse lesions, as they have no neural tissue inside the target volume, enabling larger does to be prescribed. It may take up to 4 years for complete obliteration of the nidus, during which time the patient continues to be at risk of haemorrhage (5% per year: see Learning point: stereotactic radiosurgery). Multiple series have been published regarding the different techniques used for radiosurgery, including proton beam, gamma knife, linear accelerator (LINAC) and CyberKnife. A recent meta-analysis of sixty-nine cohorts (twenty-two gamma knife, thirty-six LINAC) by Beijnum et al. (2011) found a 40% 2-year obliteration rate [28]. The probability of obliteration depends on the administered dose, which is influenced by the location of the AVM. Obliteration is produced by radiation-induced damage to the endothelium of the arterial wall, resulting in smooth muscle proliferation with subsequent luminal occlusion. Complications of radiosurgery include white matter oedema, seizures, radiation necrosis, and haemorrhage.
Embolization can be used as a pre-operative adjunct to surgery, leading to decreased operating times, transfusion requirements, morbidity, and mortality. As a primary modality, it offers relatively low cure rates (5–20%) with a risk of subsequent recanalization, although the use of new embolic agents, such as onyx, holds promise (15–50%). It may be offered as a primary treatment option in selected patients, i.e. for small deep-seated thalamic or basal ganglia AVMs, with one or two feeding arteries providing immediate protection against recurrent and immediate haemorrhage. Complications may be permanent or temporary, and are usually neurological deficits related to inadvertent embolization of arteries supplying healthy brain or obliteration of the venous outflow, leading to ICH. They include haemorrhage (2–5%), permanent neurological deficits (2–5%), and death (1%).
 Learning point Guidelines for treatment
Learning point Guidelines for treatment
SM I, II
●SRS.
●Microsurgery (patient preference, surgery for haematoma).
SM III
● Microsurgery/radiosurgery +/– endovascular therapy.
SM IV, V
●Observation.
●Consider tailored treatment if risk of haemorrhage/repeat.
●Bleeds.
In practice, SM grade III are the most challenging malformations to treat, representing a heterogeneous mix of pathology. This group has been subdivided into IIIA (>6cm) that are best treated with embolization and surgery, and IIIB (small, i.e. deep venous drainage and in eloquent cortex [32]) that are best treated with radiosurgery
Case 22 Intracranial arteriovenous malformation
 Learning point Aneurysms and AVMs
Learning point Aneurysms and AVMs
Aneurysms occur in approximately 20–25% of patients with AVMs. They may be incidental, in a separate vascular territory, or they may be ‘flow related’ on feeding vessels. These latter aneurysms are thought to arise secondary to the hyperdynamic circulatory state induced by the AVM. Intranidal aneurysms may also occur.
It is not clear if there is an increased risk of haemorrhage in AVMs with aneurysms. In a small study of ninety-one patients with unruptured AVMs Brown et al. demonstrated an increased risk of ICH at 1 year in patients with an AVM and an aneurysm (7% cf. 3% with only an AVM) [33].
In the presence of haemorrhage, a number of studies have suggested that the AVM is more likely to be the cause of the haemorrhage except in the posterior fossa. Intranidal aneurysms have been suggested to be associated with an increased risk of haemorrhage, as have those associated with a pedicle or feeding artery [31].
In the setting of a non-haemorrhagic presentation, aneurysms that are distal or flow-related are likely to regress with treatment of the AVM, whereas this is unlikely in those arising on the circle of Willis.
 Learning point Occlusive hyperaemia versus normal perfusion pressure breakthrough
Learning point Occlusive hyperaemia versus normal perfusion pressure breakthrough
Following AVM resection, haemorrhage and cerebral oedema is occasionally seen in the cortex surrounding the resection cavity. Two prevailing hypotheses attempt to explain this phenomenon, although it is likely that they are not mutually exclusive, potentially forming a spectrum of complex haemodynamic changes seen in and around AVMs.
The ‘normal perfusion pressure breakthrough’ hypothesis, put forward in 1978 suggests that the surrounding parenchyma of an AVM is chronically hyperperfused, with impaired autoregulation. As such, it becomes vulnerable to uncontrolled hyperaemia, oedema, and potential ‘breakthrough’ bleeding upon the restoration of normal perfusion pressures, following removal of the AVM [29].
The ‘occlusive hyperaemia’ hypothesis (1993) suggests stagnant arterial flow to the AVM, and obstruction to venous outflow of the surrounding parenchyma results in a hypoperfused and ischaemic state, which is worsened following resection [6].
A final word from the expert
The future management of these lesions will lie in better defining the natural history versus interventions in unruptured AVMs (ARUBA results), in clarifying the use of radiation in large AVMs (dose reduction/fractionation/partial volume treatments), and in the continuing evolution and assessment of endovascular technologies against the background of what is emerging as an increasingly benign disease.
The emergency or urgent treatment of ruptured AVMs requires experience and skill in determining the correct treatment sequence, and should be restricted to those centres capable of providing the full range of expertise required.
References
1.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. Journal of Neurosurgery 1986; 65 (4): 476–83.
2.Spetzler R, Ponce F. A 3-tier classification of cerebral arteriovenous malformations. Clinical article. Journal of Neurosurgery 2011; 114 (3): 842–9.
3.Houdart E, Gobin YP, Casasco A, et al. A proposed angiographic classification of intracranial arteriovenous fistulae and malformations. Neuroradiology 1993; 35: 381–5.
227
 Expert comment Multimodality treatment
Expert comment Multimodality treatment
A staged multimodality approach is often recommended for complex AVMs, or after treatment failure, on a case-by-case basis. It combines the advantages of the individual treatments, and may be the safest approach for some AVMs. However, it does result in an additive risk; Beijnum et al (2011) on meta-analysis found that embolization after SRS was associated with increased risk of haemorrhage and other complications [28].
228 |
Challenging concepts in neurosurgery |
|
|
4. |
Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional |
|
|
therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non- |
|
|
blinded, randomised trial. Lancet. 2013;383 (9917):614–621. |
|
5. |
Russin J, Spetzler R, Neurosurgery. Commentary: the ARUBA trial. Neurosurgery. 2014. |
|
|
75 (1): E96–E97 |
|
6. |
al Rodhan NR, Sundt TM, Piepgras DG, et al. Occlusive hyperaemia: a theory for the |
|
|
hemodynamic complications following resection of intracerebral arteriovenous malfor- |
|
|
mations. Journal of Neurosurgery 1993; 78 (2): 167–75. |
|
7. |
Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations |
|
|
by embolization and radiosurgery. Journal of Neurosurgery 1996; 85 (1): 19–28. |
|
8. |
Lim YJ, Lee CY, Koh JS, et al. Seizure control of gamma knife radiosurgery for non- |
|
|
hemorrhagic arteriovenous malformations. Acta Neurochirurgica 2006; 99: 97–101. |
|
9. |
Kikuchi K, Kowada M, Sasajima H. Vascular malformations of the brain in hereditary |
|
|
hemorrhagic telangiectasia (Rendu-Osler-Weber disease). Surgical Neurology 1994; 41: |
|
|
374–80. |
|
10. |
Yahara K, Inagawa T, Tokuda Y, et al. [A case of multiple cerebral arteriovenous malfor- |
|
|
mations treated by gamma knife radiosurgery]. No Shinkei Geka—Neurological Surgery |
|
|
1995; 23: 1121–5. |
|
11. |
Choi JH, Mohr JP. Brain arteriovenous malformations in adults. Lancet Neurology 2005; |
|
|
4: 299–308. |
|
12. |
Hashimoto N, Nozaki K, Takagi Y, et al. Surgery of cerebral arteriovenous malforma- |
|
|
tions. Neurosurgery 2007; 61: 375–87; discussion 387–9. |
|
13. |
Nehls DG, Pittman HW. Spontaneous regression of arteriovenous malformations. |
|
|
Neurosurgery 1982; 11: 776–80. |
|
14. |
Herzig R, Burval S, Vladyka V, et al. Familial occurrence of cerebral arteriovenous |
|
|
malformation in sisters: case report and review of the literature. European Journal of |
|
|
Neurology 2000; 7 (1): 95–100. |
|
15. |
Storkebaum E, Quaegebeur A, Vikkula M, Carmeliet P. Cerebrovascular disorders: |
|
|
molecular insights and therapeutic opportunities. Nature Neuroscience. 2011;14 (11): |
|
|
1390–1397 |
|
16. |
Valavanis A. The role of angiography in the evaluation of cerebral vascular malforma- |
|
|
tions. Neuroimaging Clinics of North America 1996; 6 (3): 679–704. |
|
17. |
Hofmeister C, Stapf C, Hartmann A, et al. Demographic, morphological, and clinical |
|
|
characteristics of 1289 patients with brain arteriovenous malformation. Stroke 2000; 31: |
|
|
1307–10. |
|
18. |
Hoh BL, Chapman PH, Loeffler JS, et al. Results of multimodality treatment for 141 |
|
|
patients with brain arteriovenous malformations and seizures: factors associated with |
|
|
seizure incidence and seizure outcomes. Neurosurgery 2002; 51: 303–9; discussion |
|
|
309–11. |
|
19. |
Yeh HS, Kashiwagi S, Tew JM, Jr, et al. Surgical management of epilepsy associated with |
|
|
cerebral arteriovenous malformations. Journal of Neurosurgery 1990; 72: 216–23. |
|
20. |
Trussart V, Berry I, Manelfe C, et al. Epileptogenic cerebral vascular malformations and |
|
|
MRI. Journal of neuroradiology.\ Journal de Neuroradiologie 1989; 16: 273–84. |
|
21. |
Yeh HS, Privitera MD. Secondary epileptogenesis in cerebral arteriovenous malforma- |
|
|
tions. Archives of Neurology 1991; 48: 1122–4. |
|
22. |
Heikkinen ER, Konnov B, Melnikov L, et al. Relief of epilepsy by radiosurgery of cerebral |
|
|
arteriovenous malformations. Stereotactic & Functional Neurosurgery 1989; 53 (3): |
|
|
157–66. |
|
23. |
Lv X, Li Y, Jiang C, et al. Brain arteriovenous malformations and endovascular treat- |
|
|
ment: effect on seizures. Interventional Neuroradiology 2010; 16: 39–45. |
|
24. |
Hernesniemi JA, Dashti R, Juvela S, et al. Natural history of brain arteriovenous malfor- |
|
|
mations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery |
|
|
2008; 63: 823–9; discussion 829–31. |
Case 22 Intracranial arteriovenous malformation |
229 |
25.Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 2006; 66: 1350–5.
26.Ondra SL, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. Journal of Neurosurgery 1990; 73 (3): 387–91.
27.Pollock BE, Flickinger JC, Lunsford LD, et al. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke 1996; 27: 1–6.
28.van Beijnum J, van der Worp HB, Buis D, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. Journal of the American Medical Association 2011; 306 (18): 2011–19.
29.Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clinical Neurosurgery 1978; 25: 651–72.
30.Spetzler RF, Hargraves RW, McCormick PW, et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. Journal of Neurosurgery 1992; 76 (6): 918–23.
31.Turjman F, Massoud TF, Vinuela F, et al. Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery 1995; 37: 856–60; discussion 860–2.
32.de Oliveira E, Tedeschi H, Raso J. Comprehensive management of arteriovenous malformations. Neurological Research 1998; 20 (8): 673–83.
33.Menghini VV, Brown RD, Jr, Sicks JD, et al. Clinical manifestations and survival rates among patients with saccular intracranial aneurysms: population-based study in Olmsted County, Minnesota, 1965 to 1995. Neurosurgery 2001; 49: 251–6; discussion 256–8.
