
- •CONTENTS
- •EXPERTS
- •CONTRIBUTORS
- •ABBREVIATIONS
- •1 The management of chronic subdural haematoma
- •2 Glioblastoma multiforme
- •3 Spondylolisthesis
- •4 Intramedullary spinal cord tumour
- •5 Surgery for temporal lobe epilepsy
- •6 Management of lumbosacral lipoma in childhood
- •7 Idiopathic intracranial hypertension
- •8 Colloid cyst of the third ventricle
- •9 Bilateral vestibular schwannomas: the challenge of neurofibromatosis type 2
- •10 Multimodality monitoring in severe traumatic brain injury
- •11 Intracranial abscess
- •12 Deep brain stimulation for debilitating Parkinson’s disease
- •14 Trigeminal neuralgia
- •15 Cerebral metastasis
- •16 The surgical management of the rheumatoid spine
- •17 Cervical spondylotic myelopathy
- •18 Brainstem cavernous malformation
- •19 Peripheral nerve injury
- •20 Spontaneous intracerebral haemorrhage
- •21 Low-grade glioma
- •22 Intracranial arteriovenous malformation
- •INDEX

CASE
14 Trigeminal neuralgia
Isaac Phang
 Expert commentary Nigel Suttner
Expert commentary Nigel Suttner
Case history
A 59-year-old man presented to clinic with a 9-month history of recurrent excruciating left jaw and cheek pain, described as lancinating in nature. At worst, five or six episodes were clustered together per day with spontaneous resolution. Pain episodes were triggered by eating and shaving, and severe enough to limit his lifestyle and impact on his psychological well-being. The diagnosis was of trigeminal neuralgia (TN) of the V2 and V3 distributions.
Clinical neurological examination was unremarkable.
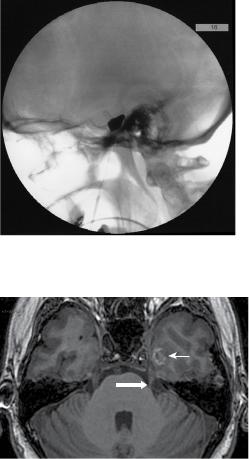
He was initially commenced on carbamezapine, but this was soon discontinued due to a rise in his liver transaminase. He was then started on gabapentin 900mg tds with phenytoin 50mg tds added for better control, although he did have some side effects of drowsiness and dizziness. He continued, however to have episodes of breakthrough pain and, after surgical assessment and discussion, opted for percutaneous balloon compression (PBC) of the left trigeminal ganglion. The procedure was performed under a short general anaesthetic. The foramen ovale was navigated using intraoperative X-ray screening with Niopam 300 radio-opaque dye and standard anatomical landmarks. A jaw twitch was noted just prior to penetration of the foramen ovale, and CSF and a small amount of venous blood were obtained once the needle was in place. Inflation of the Fogarty 4 balloon was performed for 90 seconds (Figure 14.1). He obtained complete pain relief within 24 hours of surgery and was discharged home.
He was reviewed in clinic 2 months after the procedure. Relief from the initial pain persisted and he had been weaned off all analgesia. He had transient masticatory weakness post-balloon compression, but this resolved completely.
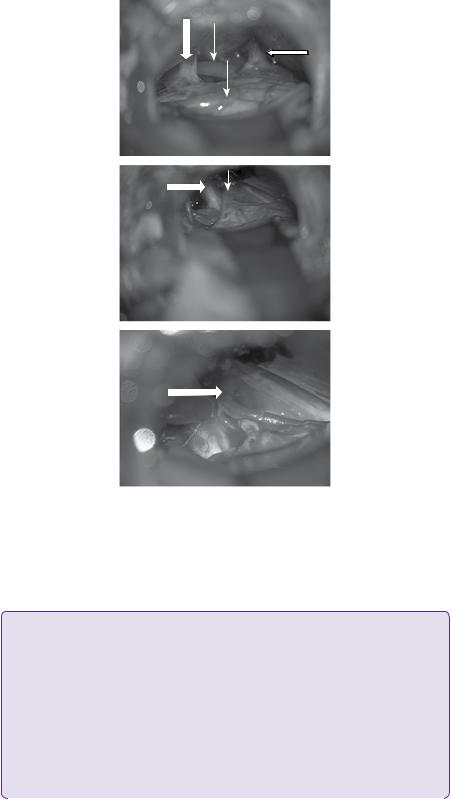
Two weeks after this assessment, however, he had a recurrence of his trigeminal neuralgia. Ultrafast gradient echo 3D MRI brain at this juncture did not show any compression of the left trigeminal nerve from a space-occupying lesion/neurovascular conflict, nor demyelinating disease. There was, however, atrophy of the left trigeminal nerve (Figure 14.2). A resolving left temporal contusion was noted adjacent to Meckel’s cave, as a result of the percutaneous balloon compression.
He now opted for microvascular decompression, as he did not want to go back onto medication. Suboccipital (retrosigmoid) craniectomy, exploration of the left trigeminal nerve from the root entry zone to Meckel’s cave, was carried out. This revealed compression from a loop of anterior inferior cerebellar artery (AICA), but also significantly from a loop of superior cerebellar artery (SCA) and a vein related to part of the root entry zone (Figure 14.3). The loop of AICA also compressed the VII/VIII complex more distally. The vein was cauterized and divided. Merocel® sponges were placed between the loop of SCA and the nerve, as well as between the
136 |
Challenging concepts in neurosurgery |
Figure 14.1 Intra-operative fluoroscopy (lateral basal skull X-ray) showing intra-operatively inflated pear-shaped Fogarty catheter for balloon compression in the management of trigeminal neuralgia.
Figure 14.2 T1-weighted axial MRI showing atrophy of left trigeminal nerve (big arrow). A resolving small contusion is noted in the adjacent temporal lobe due to inadvertent injury during prior percutaneous balloon compression (small arrow).
loop of AICA and the nerve. Recovery was uneventful and he was discharged home on post-operative day 4.
Post-operative review at 2 months revealed continued pain relief off analgesia. There was an area of subjective decrease in sensation over his left V2 region.
Discussion
The incidence of TN is approximately 4 in 100,000 [1]. The age of onset is typically in the sixth to eighth decade, and women are more affected than men with a ratio of 3:2. The right side of the face is affected more often than the left. TN occurs bilaterally in 5% of patients. Family history may be positive in 5%.
The diagnosis of TN is made on clinical history alone. There are no diagnostic tests. It can be divided into typical/classical TN (TN1) or atypical TN (TN2) [2]. Janetta [3] breaks down further into typical, atypical, and mixed TN. Mild sensory changes occur clinically in the trigeminal distribution in up to 30% of patients with TN, invariably in the area of facial pain [3]. This is despite the diagnostic criteria of the International Headache Society.
Case 14 Trigeminal neuralgia |
137 |
(a)
(b)
(c)
Figure 14.3 (a) Microvascular decompression of left trigeminal nerve. Intra-operative photograph showing a loop of AICA (small vertical arrows) coursing around the left VII/VIII complex (big vertical arrow). The superior petrosal vein is seen superiorly (horizontal arrow). (b) Microvascular decompression of left trigeminal nerve. Intra-operative photograph showing the trigeminal complex exposed
(large arrow), with a vein (small arrow) coursing over the dorsal root entry zone. (c) Microvascular decompression of left trigeminal nerve. Intra-operative photograph showing the vein divided and a loop of SCA (large arrow) abutting the trigeminal nerve.
 Learning point Definition of trigeminal neuralgia
Learning point Definition of trigeminal neuralgia
The International Classification of Headache Disorders III (ICHD-3) for trigeminal neuralgia.
I. Classical trigeminal neuralgia: diagnostic criteria
A.At least three attacks of unilateral facial pain fulfilling criteria B and C
B.Occurring in one or more divisions of the trigeminal nerve, with no radiation beyond the trigeminal distribution
C.Pain has at least three of the following four characteristics:
1.recurring in paroxysmal attacks lasting from a fraction of a second to 2 minutes
2.severe intensity
3.electric shock-like, shooting, stabbing or sharp in quality
4. precipitated by innocuous stimuli to the affected side of the face1 |
(continued) |
|
|
|
|

138 |
Challenging concepts in neurosurgery |
|
|
|
|
D.No clinically evident neurological deficit
E.Not better accounted for by another ICHD-3 diagnosis.
II. Classical trigeminal neuralgia with concomitant persistent facial pain: diagnostic criteria
A.Recurrent attacks of unilateral facial pain fulfilling criteria for classical trigeminal neuralgia
B.Persistent facial pain of moderate intensity in the affected area
C.No better accounted for by another ICHD-3 diagnosis.
Corroborating features are:
●Pain usually starts in the second or third division, affecting the cheek or the chin. In <5% of patients the first division is affected.
●Pain never crosses to the opposite side.
●Between paroxysms, the patient is usually asymptomatic, but a dull background pain may persist in some long-standing cases.
●Following a painful paroxysm, there is usually a refractory period during which pain cannot be triggered.
●The pain often evokes spasm of the muscle of the face on the affected side (tic douloureux).
●Classical trigeminal neuralgia is usually responsive, at least initially, to pharmacotherapy.
Burchiel [2] classifies trigeminal neuralgia into type 1 (TN1) where the pain is sharp, shooting, episodic pain and type 2 (TN2), where the pain is aching or throbbing more than 50% of the time, and the pain is constant. Although the natural history of trigeminal neuralgia has not been fully elucidated, it is postulated that the character of classical trigeminal neuralgia may over time change into that of type 2 pain [3].
Pathogenesis
The ignition hypothesis [4] proposes electrophysiological mechanisms, which explain the triggering, amplification, and stop mechanism of paroxysmal pain in trigeminal neuralgia. Injured neurons become hyperexcitable and generate impulses autonomously, creating ectopic pacemaker sites. Spontaneous firing may induce a burst of firing—an after-discharge in the injured neuron, with recruitment of surrounding neurons. Thus, this provides the ‘ignition’, explaining the sudden nature of the pain. The amplification is attributed to resonance of intrinsic oscillations in membrane potential of the dorsal root ganglion cells. Normally, only a few of the dorsal root ganglion cells demonstrate this characteristic, but injured cells may acquire this property. Moreover, the oscillations resonate, causing impulses to fire in a sustained manner, thus providing the sustained amplification of pain. This is encouraged by ephaptic transmission and crossed after discharge between damaged neurons. This spread of activity from large myelinated afferents to unmyelinated C nociceptors explains why innocuous stimuli elicit paroxysmal pain. The refractory period is explained by hyperpolarization due to an influx of Ca2+-activated K+ channels.
The vascular conflict theory proposed by Janetta suggests that compression of the Obersteiner–Reidleich transition zone of central to peripheral myelination of the trigeminal nerve causes demyelination of the trigeminal nerve, and hence TN, and explains the success of microvascular decompression in the treatment of TN. Histological studies of compressed trigeminal nerves in patients with TN1 show demyelination at areas of maximal compression, with adjacent areas of less severe damage [4]. Histological studies of PBC specimens in New Zealand rabbits have shown preferential destruction of large myelinated fibres serving touch, while

Case 14 Trigeminal neuralgia |
139 |
sparing the unmyelinated fibres that mediate nociceptive pain transmission [5]. The pain relief following PBC suggests that the large myelinated fibres are central to the pathogenesis of paroxysmal pain.
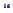 Expert comment Theories of pathogenesis of TN
Expert comment Theories of pathogenesis of TN
The precise cause and mechanisms of pain in trigeminal neuralgia are not fully understood. Most commonly pulsatile compression from neurovascular conflict peripherally at the root entry zone (REZ) of the nerve has been cited as the cause of idiopathic TN [6,7], which leads to demyelination. Tumours compressing the trigeminal nerve in the posterior fossa and demyelination of the nerve at the REZ in multiple sclerosis support a peripheral mechanism. This is confirmed histopathologically [4] and explains the ephaptic transmission between axons leading to amplification of sensory input. The Ignition hypothesis [8] attempts to explain the underlying mechanisms involved.
However, not all of the features of TN can be explained on a peripheral basis. A central origin cannot be ignored. Pain and temperature fibres extend downwards into the nucleus caudalis of the spinal nucleus of V. Lesions here produce facial analgesia supporting a central area for pain transmission [9].
 Learning point Imaging in trigeminal neuraligia
Learning point Imaging in trigeminal neuraligia
It is known that MR imaging has good sensitivity in predicting neurovascular compression [10, 11, 12]. There are two main schools of thought regarding pre-operative vascular imaging in TN. The first suggests that the lack of neurovascular compression seen on MR vascular imaging has poor
specificity and low negative predictive value [11]. Therefore, the decision to proceed to microvascular decompression should take into account both clinical and radiological findings [11] as certain vessels are beyond the resolution of contemporary imaging [13,14]. The second school of thought suggests that there is good correlation between imaging and intraoperative findings of neurovascular compression not only with high sensitivity but specificity as well [15,16] with modern MRI sequences.
 Expert comment Imaging in trigeminal neuralgia
Expert comment Imaging in trigeminal neuralgia
There are two reasons to image patients with TN. First, a secondary cause, such as multiple sclerosis or tumours should be excluded. Secondly, anatomical variation prior to microvascular decompression should be considered.
Standard MRI protocols include cisternal MRI axial and coronal T1-weighted preand postgadolinium and T2-weighted sequences. In order to best visualize the vascular conflict, heavily T2W sequences, which provide a high contrasting picture between the CSF and structures passing through the cisterns are required [9], or balanced steady state free precession (bSSFP) images. They have similar appearances, but are named according to the manufacturer of the MRI scanner, e.g.
Philips, Siemens, GE. These include balanced fast field echo (BFFE), true fast imaging with steady-state precession (FISP), and fast imaging employing steady state acquisition (FIESTA).
3D time of flight (TOF) angiography can further delineate arterial compression of the nerve, and in combination with bSSFP may increase the sensitivity and specificity [17].
Surgical treatment of trigeminal neuralgia
First-line medical therapy is carbamezapine or oxcarbazepine. Second-line treatment is based on Class III/IV evidence and includes add-on therapy with lamotrigine or a switch to lamotrigine, baclofen, or pimozide [18]. Although medical treatment works initially in about 75% of patients, it may fail over time. Surgery for TN is reserved for patients who have failed medical therapy or cannot tolerate its side effects.

140 |
|
Challenging concepts in neurosurgery |
|
||
|
Table 14.1 A comparison between the various destructive procedures for trigeminal neuralgia |
|
|||
|
|
|
|
|
|
|
|
PTR |
PGR |
PBC |
SRS |
|
Results |
99% initial pain relief |
90% initial pain relief |
30% recurrence at 10 years |
96% initial pain relief |
|
|
21% recurrence at |
23% recurrence at 11 years |
|
25% recurrence at 3 years [20] |
|
|
15 years [19] |
|
|
|
Side effects |
15% troublesome numbness |
|
3% corneal analgesia |
Comments |
Trade-off between |
|
longer-term pain relief |
|
and dysaesthesia. Requires |
|
patient co-operation |
Cisternal fibrosis due to |
Bradycardia occurs more |
Median time to pain relief is 4 |
repeated procedures may |
frequently. Ipsilateral |
weeks |
not allow glycerol into |
masticatory weakness more |
|
Meckel’s cave [22] |
frequent. |
|
|
Lower incidence of |
|
|
anaesthesia dolorosa [21] |
|
Surgery for TN can be divided into decompressive, destructive, and palliative procedures. The choice of procedure depends on the comorbidity and age of the patient, adversity to risk, pre-existing symptoms from TN and underlying cause of TN (Table 14.1). If the patient is fit enough for microvascular decompression, then this is the surgical treatment of choice, as it has the best prognosis for long-term pain relief. Destructive procedures, while usually providing immediate pain relief, is associated with residual sensory disturbances, which in the case of anaesthesia dolorosa, can be more severe than the initial TN itself.
 Learning point
Learning point
Anaesthesia dolorosa
It refers to persistent and painful anaesthesia or hyperaesthesia in the distribution of the trigeminal nerve. It is frequently described as a constant burning, crawling, or itching sensation
in the denervated area, worse than the original TN. It is a rare complication of surgical treatment for TN and is often
refractory to medical or surgical therapy.
Destructive procedures for trigeminal neuralgia
These are procedures that damage the trigeminal ganglion in Meckel’s cave. They include PBC, percutaneous thermal radiofrequency rhizotomy and percutaneous glycerol rhizotomy. Stereotactic radiosurgery (SRS) targets the root entry zone of the trigeminal nerve. With the exception of SRS, they generally provide immediate pain relief and have a higher incidence of facial numbness. However, as they ameliorate only the symptoms of TN and not the cause, recurrence of TN symptoms is fairly high. Moreover, as they rely on the destruction of trigeminal ganglion, some sensory fibres will be damaged, producing numbness and dysaesthesia in the trigeminal distribution. This may range initially from a mild dysaesthesia to anaesthesia dolorosa.
Direct comparisons between large patient cohorts have been made, showing that the initial results and recurrence rates are similar in the percutaneous rhizotomy procedures [19, 20, 21, 22]. The choice of a percutaneous procedure depends not only on the suitability of the patient, e.g. anaesthesia considerations, presence of cisternal fibrosis, or pre-existing deficits, but also on the surgeon’s experience.
Decompressive procedures for trigeminal neuralgia
Microvascular decompression (MVD) as pioneered by Janetta in 1967 [23] remains the mainstay for decompression of the trigeminal nerve. There is a vast body of surgical experience, and with advances in neuroanaesthesia, patients with comorbidities and advanced age have been treated successfully. It treats the underlying

Case 14 Trigeminal neuralgia |
141 |
pathophysiology of TN, has good immediate pain relief, and the incidence of facial numbness is less than that of destructive procedures. However, working in the cerebellopontine angle exposes the patient to the potential risks of facial weakness, diplopia, hearing disturbances, CSF leak, and cerebellar damage, in addition to the risks of a craniotomy. The incidence of pain relief off medication in the largest series thus far is 70% at 10 years. The commonest cause of compression was the SCA at 75% with the AICA at 10% [16,24].
The approach is well-described in standard neurosurgical texts and is carried out via a suboccipital/retromastoid craniotomy, exposing the junction of the transverse and sigmoid sinuses. With gentle retraction of the cerebellum, the VII/VIII complex, superior petrosal sinus and finally the trigeminal complex are identified. Decompression is achieved by insertion of a Merocel® sponge between the offending artery/vein and the trigeminal nerve. Waxing of the mastoid air cells and a watertight fascial closure minimizes the risk of CSF leak.
 Clinical tip Microvascular decompression
Clinical tip Microvascular decompression
●Once the arachnoid space is entered, patience is required for CSF drainage to aid cerebellar relaxation.
●Superomedial retraction of the cerebellum with gentle advancement of the retractor first exposes the VII/VIII complex, then the superior petrosal vein and, finally, the trigeminal complex. A superiorly placed retractor may tear bridging tentorial veins, whereas a laterally placed retractor puts undue traction on the VII/VIII complex. The goal of cerebellar retraction is to elevate it slightly towards the surgeon and not to merely compress it medially.
●Inspection of the entire length of the trigeminal nerve is necessary to look for potential pathology.
●Surface veins should be decompressed, rather than cauterized and divided due to recollateralization and, hence, recurrence of pain.
A final word from the expert
90% of recurrent TN occurs in the original distribution. Repeat percutaneous destructive procedures can be attempted if there is preservation of facial sensation. In the case of a failed microvascular decompression, Janetta recommends repeat exploration [25], with care taken to inspect for vessels that may have been displaced during surgical positioning or sponge slippage. However, Burchiel does not favour re-exploration unless there is a high degree
of suspicion of residual compression, either by high resolution MR imaging or a previous inadequate decompression [16]. Other pathology may be encountered such as granuloma secondary to sponge placement, new vessel compression and recanalization of veins.
References
1.Katusic S, Beard CM, Bergstralh E, et al. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Annals of Neurology 1990; 27(1): 89–95.
2.Burchiel KJ. A new classification for facial pain. Neurosurgery 2003; 53(5): 1164–7.
3.Janetta PJ. Typical and atypical symptoms. In: PJ Janetta (ed.), Trigeminal neuralgia (pp. 41–5). Oxford: Oxford University Press, 2011.
4.Devor M, Govrin-Lippmann R & Rappaport ZH. Mechanism of trigeminal neuralgia:
an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. Journal of Neurosurgery 2002; 96(3): 532–43.
142 |
Challenging concepts in neurosurgery |
|
|
5. |
Brown JA, Hoeflinger B, Long PB, et al. Axon and ganglion cell injury in rabbits after |
|
|
percutaneous trigeminal balloon compression. Neurosurgery 1996; 39: 993–1003. |
|
6. |
Dandy WE. Concerning the cause of trigeminal neuralgia. American Journal of Surgery |
|
|
1934; 24: 447–55. |
|
7. |
Jannetta PJ. Vascular compression is the cause of trigeminal neuralgia. APS Journal |
|
|
1993; 2(4) 217–27. |
|
8. |
Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition |
|
|
hypothesis. Clinical Journal of Pain 2002; 18(1): 40–13. |
|
9. |
Borges A, Casselman J. Imaging the trigeminal nerve. European Journal of Radiology |
|
|
2010; 74(2): 323–40. |
|
10. |
Anderson VC, Berryhill PC, Sandquist MA, et al. High-resolution three-dimensional |
|
|
magnetic resonance angiography and three-dimensional spoiled gradient-recalled |
|
|
imaging in the evaluation of neurovascular compression in patients with trigeminal |
|
|
neuralgia: a double-blind pilot study. Neurosurgery 2006; 58(4): 666–73. |
|
11. |
Vergani F, Panaretos P, Penalosa A, et al. Preoperative MRI/MRA for microvascu- |
|
|
lar decompression in trigeminal neuralgia: consecutive series of 67 patients. Acta |
|
|
Neurochirugia (Wien) 2011; 153(12): 2377–81. |
|
12. |
Yoshino N, Akimoto H, Yamada I, et al. Trigeminal neuralgia: evaluation of neuralgic |
|
|
manifestation and site of neurovascular compression with 3D CISS MR imaging and MR |
|
|
angiography. Radiology 2003; 228: 539–45. |
|
13. |
Miller J, Acar F, Hamilton B, et al. Preoperative visualization of neurovascular anatomy |
|
|
in trigeminal neuralgia. Journal of Neurosurgery 2008; 108(3): 477–82. |
|
14. |
Sekula RF, Jr, Janetta PJ. The evaluation of the pre-operative patient. In: P. Janetta (eds for |
|
|
more than one names) Trigeminal neuralgia (pp. 87–100). Oxford University Press, 2011. |
|
15. |
Leal PR, Hermier M, Souza MA, et al. Visualization of vascular compression of the |
|
|
trigeminal nerve with high-resolution 3T MRI: a prospective study comparing preopera- |
|
|
tive imaging analysis to surgical findings in 40 consecutive patients who underwent |
|
|
microvascular decompression for trigeminal neuralgia. Neurosurgery 2011; 69(1): 15–26. |
|
16. |
Elias WJ, Burchiel KJ. Microvascular decompression. Clinical Journal of Pain 2002; 18: |
|
|
35–41. |
|
17. |
Anderson VC, Berryhill PC, Sanquist DP, et al. High resolution three dimensional mag- |
|
|
netic resonance angiography and three dimensional spoiled gradient-recalled imaging |
|
|
in the evaluation of neurovascular compression in patients with trigeminal neuralgia: |
|
|
a double-blind pilot study. Neurosurgery 2006; 58(4): 666–73. |
|
18. |
Obermann M. Treatment options in trigeminal neuralgia. Therapeutic Advances in |
|
|
Neurological Disorders 2012; 3(2): 107–15. |
|
19. |
Taha JM, Tew JM, Jr, Buncher CR. A prospective 15-year follow up of 154 consecutive |
|
|
patients with trigeminal neuralgia treated by percutaneous stereotactic radiofrequency |
|
|
thermal rhizotomy. Journal of Neurosurgery 1995; 83(6): 989–93. |
|
20. |
Linskey ME, Ratanatharathorn V, Penagaricano J. A prospective cohort study of micro- |
|
|
vascular decompression and gamma knife surgery in patients with trigeminal neuralgia. |
|
|
Journal of Neurosurgery 2008; 109: 160–72. |
|
21. |
Skirving DJ, Dan NG. A 20-year review of percutaneous balloon compression of the |
|
|
trigeminal ganglion. Journal of Neurosurgery 2001; 94(6): 913–17. |
|
22. |
Kouzounias K, Lind G, Schectmann G, et al. Comparison of percutaneous balloon com- |
|
|
pression and glycerol rhizotomy for the treatment of trigeminal neuralgia. Journal of |
|
|
Neurosurgery 2010; 113(3); 486–92. |
|
23. |
Janetta PJ. Structural Mechanisms of trigeminal neuralgia. Arterial compression of the |
|
|
trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg 1967; |
|
|
26(1 pt2): 159–162. |
|
24. |
Barker FG, Jannetta PJ, Bissonette DJ, et al. The long-term outcome of microvascular decom- |
|
|
pression for trigeminal neuralgia. New England Journal of Medicine 1996; 334: 1077–84. |
|
25. |
Janetta PJ, Bissonette DJ. Management of the failed patient with trigeminal neuralgia. |
|
|
Clinical Neurosurgery 1985; 32: 334–47. |
