
- •CONTENTS
- •EXPERTS
- •CONTRIBUTORS
- •ABBREVIATIONS
- •1 The management of chronic subdural haematoma
- •2 Glioblastoma multiforme
- •3 Spondylolisthesis
- •4 Intramedullary spinal cord tumour
- •5 Surgery for temporal lobe epilepsy
- •6 Management of lumbosacral lipoma in childhood
- •7 Idiopathic intracranial hypertension
- •8 Colloid cyst of the third ventricle
- •9 Bilateral vestibular schwannomas: the challenge of neurofibromatosis type 2
- •10 Multimodality monitoring in severe traumatic brain injury
- •11 Intracranial abscess
- •12 Deep brain stimulation for debilitating Parkinson’s disease
- •14 Trigeminal neuralgia
- •15 Cerebral metastasis
- •16 The surgical management of the rheumatoid spine
- •17 Cervical spondylotic myelopathy
- •18 Brainstem cavernous malformation
- •19 Peripheral nerve injury
- •20 Spontaneous intracerebral haemorrhage
- •21 Low-grade glioma
- •22 Intracranial arteriovenous malformation
- •INDEX
CASE
15 Cerebral metastasis
Melissa C. Werndle
 Expert commentary Henry Marsh
Expert commentary Henry Marsh
Case history
A 59-year-old woman presented to her local A&E department with a 2-week history of gradual onset worsening headache, with a few days of associated blurred vision. Her past medical history was significant for breast cancer, diagnosed in 2007, treated with bilateral mastectomies, adjuvant chemotherapy, and hormone therapy. On examination, she was alert and orientated with no focal neurological deficit. Fundoscopy revealed bilateral papilloedema.
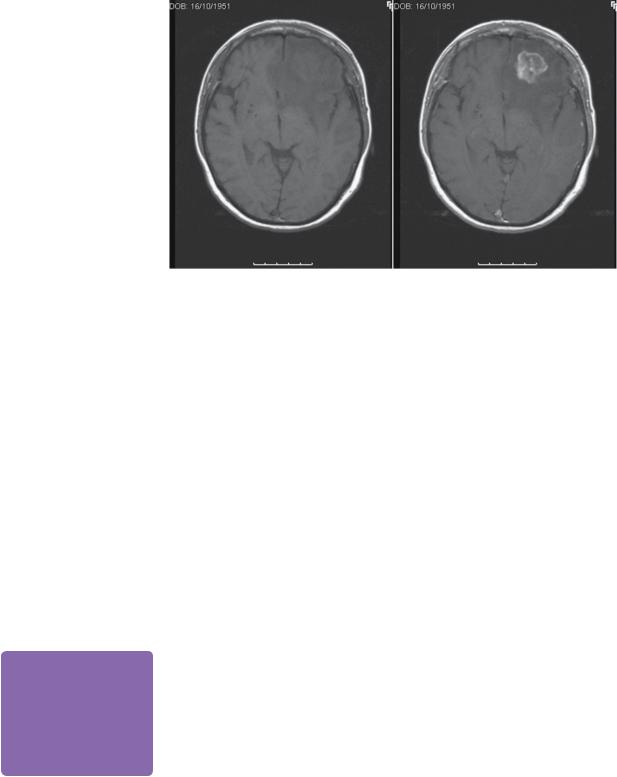
MRI preand post-contrast revealed a 2.5-cm heterogeneously enhancing, defined lesion at the grey white junction in the left frontal lobe. There was significant surrounding vasogenic oedema (Figure 15.1). CT chest/abdomen/pelvis did not reveal any other secondary spread.
Given her symptoms and the imaging appearances, the patient was commenced on dexamethasone, and treatment options were discussed.
The patient’s headaches improved with dexamethasone, but she continued to complain of blurred vision. On day 2 after admission, she underwent a left frontal craniotomy and gross total removal of the lesion.
 Expert comment Operative considerations
Expert comment Operative considerations
Most solitary metastases can be resected using an image-guided ‘mini-craniotomy’ and linear incision. From the scan, it is easy to mistakenly think that a metastasis is on the surface of the brain and yet find, on opening the dura, that the tumour is not visible, so using guidance in most cases is sensible. If the tumour is beneath the surface and must be approached via a cortical incision, it is important to realize that retractors and instruments can push the lesion away and the computer
navigation becomes unreliable. Brain swelling on opening the dura may also disorientate the surgeon with respect to image guidance. If the lesion is not easy to find it may be better to take the patient to the CT scanner and obtain a scan having left a suitable marker in place, rather than to poke blindly around in the deep white matter.
As metastases are often superficial, care must be taken with major draining veins if the lesion is adjacent to the venous sinuses, to avoid post-operative venous infarct or venous haemorrhage.
Histology revealed pleomorphic polygonal cells, forming trabeculae, acini, and cribiform patters with necrosis. The tumour was infiltrating cortex, white matter, and leptomeninges. The appearances were of a metastatic adenocarcinoma consistent with a breast origin.
The patient had an uneventful post-operative recovery and was referred to the oncologists for adjuvant therapy.
 Learning point
Learning point
Differentiating abscess from tumour on MRI
Although usually a shorter history, it can be very challenging to differentiate abscess from tumour, both clinically and on CT. MR is helpful: tumours show unrestricted diffusion, whereas
abscesses show restricted diffusion. Restricted diffusion is indicated by hyperintensity on diffusion weighted imaging (DWI), and low ADC (Apparent Diffusion Coeffecient).
 Learning point
Learning point
Dexamethasone—how it works
Dexamethasone revolutionized the outcome of surgery for brain tumours following Galicich’s seminal paper on alleviating oedema in brain tumours in 1960s [1]. The clinical improvement
in patients with symptoms and signs of oedema from tumours is often rapid and obvious. The exact mechanism of action remains an unknown; however,
the principles are a decrease in the blood–tumour barrier permeability, decreased tumoural perfusion, decreased tumoural diffusivity resulting in reduced intracranial pressure, and improved cerebral perfusion pressure.
144 |
Challenging concepts in neurosurgery |
|
|
(a) |
(b) |
 Expert comment Imaging characteristics of metastases
Expert comment Imaging characteristics of metastases
Cerebral metastases are not usually vascular. Occasionally, solid cerebellar haemangioblastomas are misdiagnosed on scanning as mets. The presence of flow voids in the tumour should alert one to this unusual possibility.
Figure 15.1 T1-weighted MR (a) preand (b) post-gadolinium images, showing left frontal isointense intrinsic lesion with surrounding oedema, which enhances heterogeneously.
Discussion
The incidence of cerebral metastases, which is the most common type of adult brain tumour, is up to 40% in patients diagnosed with a primary systemic cancer [2]. The common primary sites are lung, breast, melanoma, renal, and colorectal [3]. Most systemic treatments for primary malignancies do not cross the blood–brain barrier; therefore, we are seeing an increase in brain metastases in patients who previously would not have survived their primary disease.
Classical imaging characteristics of a cerebral metastasis are of a contrast enhancing well-defined lesion at the gray–white junction with significant surrounding oedema. Hyperdensity on CT indicates a haemorrhagic metastasis, commonly melanoma. Lesions may be single or multiple. CT underestimates the number of lesions compared with MRI (by a measure of 2–3-fold), especially lesions under 5mm in diameter, and therefore MRI is the preferred mode of imaging [4]. MRI features of the lesion are isoto mild hypointensity on T1, hyperintensity on T2, and enhancement post-gadolinium.
The current treatment options available for brain metastases are radiotherapy, stereotactic radiosurgery, and surgery (open surgical resection). The treatment combination is determined by a number of factors, which include:
●Underlying malignancy.
●Performance status.
●Location of cerebral metastasis.
●Number of cerebral metastases.
●Systemic staging and prognosis.
●Age.
Although these principles apply universally, management may vary from country to country and even from centre to centre, in terms of how aggressively the patient
Case 15 Cerebral metastasis |
145 |
Number of
metastases
Single |
|
|
|
Multiple |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Location |
|
|
|
Stereotactic radiosurgery |
||
|
|
|
or WBRT palliation |
|||
|
|
|
|
|
||
|
|
|
|
|
|
|
|
Superficial |
Deep |
|
Performance |
|
|
status |
|
|
Good |
Bad |
|
Extracranial |
|
|
disease |
|
Limited |
Extensive |
|
Surgical resection plus
WBRT
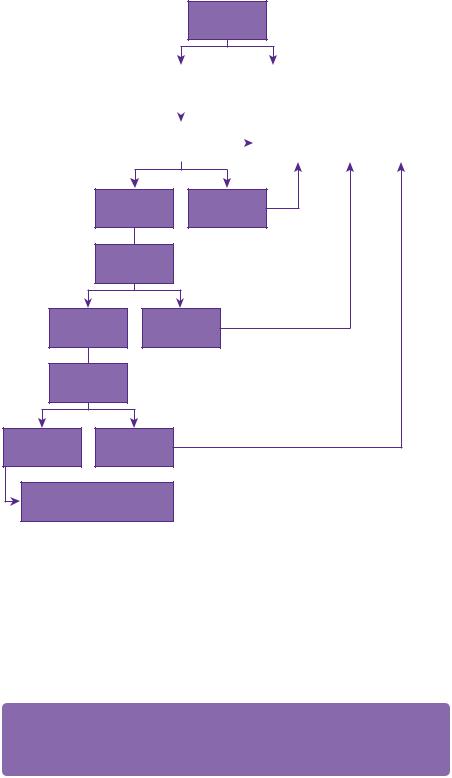
Figure 15.2 Treatment algorithm.
is treated. A challenge is to balance prolongation of life with maximizing quality of life. At times, these principles are at odds and each case must be considered individually. In general, in patients with a good performance status (Karnofsky over 70, or performance status 0, 1, 2) and controlled extracranial disease, surgical resection followed by whole brain radiotherapy confers the maximum survival [5,6] (see Figure 15.2) This is superior to whole-brain radiotherapy alone.
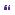 Expert comment Aim of surgery
Expert comment Aim of surgery
The aim should be to remove the metastasis completely. There is normally a good plane, and a combination of debulking and dissection of the tumour–white matter plane using a curved instrument, such as a Penfield number 2 makes this usually an easy operation.

146 |
Challenging concepts in neurosurgery |
 Evidence base
Evidence base
There are varying levels of evidence for different treatment modalities. There is a succinct series of systematic reviews published in Journal of Neurooncology [7-10]. It is important to note this review applied to good performance status patients with a single brain metastasis.
●Radiotherapy: whole brain radiotherapy is used adjunctively following surgery/stereotactic radiosurgery to decrease the rate of recurrence and increase length of time to recurrence. As a single modality treatment for palliation, whole brain radiotherapy (WBRT) improves symptoms and survival by 3 months compared with supportive care only.
●Surgery: when compared to WBRT alone, surgery (followed by WBRT) improves survival by 4–6 months.
●Stereotactic radiosurgery: stereotactic radiosurgery (SRS) plus radiotherapy is equally effective when compared to surgery plus radiotherapy (retrospective studies only) for small lesions (<3cm) with no midline shift over 1cm.
In patients with a single ‘unresectable’ metastasis, a stereotactic radiosurgery boost following WBRT improves survival compared to WBRT alone [11]. Consider SRS for unresectable or multiple metastases.
● Chemotherapy: limited benefit in brain metastases.
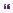 Expert comment Selection of patients for treatment
Expert comment Selection of patients for treatment
Solitary metastases on the surface of the brain in patients without a significant neurological deficit after steroid treatment and with a prognosis of more than a few months are suitable for image-guided resection. If the lesion is small and there is extensive white matter oedema, it is probably wise to give steroids for several days before surgery to reduce the risk of severe intraor post-operative brain swelling. Solitary deep lesions within the brain, if less than 3cm in size, should usually be treated with stereotactic radiotherapy. It is a question of judgement as to when stereotactic radiotherapy should be used instead of surgery.
The natural history of brain metastases, if left untreated, is a median survival in the order of weeks to months. If this heterogenous group is subdivided, those with an unfavourable combination of the aforementioned factors (underlying malignancy small cell lung cancer, poor performance status, ‘inoperable’ location, multiple lesions, extensive extracranial disease, and older age) have a prognosis of under 3 months. A ‘favourable’ combination improves median survival to 18 months [12].
A final word from the expert
With a constant improvement in treatment available for underlying primaries, where patients are now surviving longer, there is an onus on developing targeted therapies for cerebral metastases.
Anti-angiogenic therapy
Angiogenesis is an integral part of brain tumour formation, including cerebral metastases. The process is led by over-expression of the signal protein VEGF. Experimentally, obstruction of the angiogenic VEGF pathway inhibits the formation of cerebral metastases in lung, breast, and colorectal models [13], with promising results. Several anti-angiogenic agents are being studied in a number of Phase I and II human trials.

Case 15 Cerebral metastasis |
147 |
Cancer-specific targeted therapies
There are a range of agents being studied in cerebral metastases from breast, melanoma, and lung cancer. The most promising include inhibitors of (1) BRAF (v-RAF murine sarcoma viral oncogene homolog B1) for melanoma, and (2) epithelial growth factor receptor for non-small cell lung cancer [13].
References
1.Galicich JH, French LA, Melby JC. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. Lancet 1961; 81: 46–53.
2.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. Journal of Neurooncology 2005; 75(1): 5–14.
3.Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002; 94(10): 2698–705.
4.Seute T, Leffers P, ten Velde GP, et al. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI). Cancer 2008; 112(8): 1827–34.
5.Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Annals of Neurology 1993; 33(6): 583–90.
6.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New England Journal of Medicine 1990; 322(8): 494–500.
7.Gaspar LE, Mehta MP, Patchell RA, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidencebased clinical practice guideline. Journal of Neurooncology 2010; 96(1): 17–32.
8.Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. Journal of Neurooncology 2010; 96(1): 33–43.
9.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. Journal of Neurooncology 2010; 96(1): 45–68.
10.Mehta MP, Paleologos NA, Mikkelsen T, et al. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. Journal of Neurooncology 2010; 96(1): 71–83.
11.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004; 363(9422): 1665–72.
12.Jakola AS, Gulati S, Nerland US, et al. Surgical resection of brain metastases: the prognostic value of the graded prognostic assessment score. Journal of Neurooncology 2011; 105(3): 573–81.
13.Preusser M, Capper D, Ilhan-Mutlu A, et al. Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathologica 2012; 123(2): 205–22.
