
- •CONTENTS
- •EXPERTS
- •CONTRIBUTORS
- •ABBREVIATIONS
- •1 The management of chronic subdural haematoma
- •2 Glioblastoma multiforme
- •3 Spondylolisthesis
- •4 Intramedullary spinal cord tumour
- •5 Surgery for temporal lobe epilepsy
- •6 Management of lumbosacral lipoma in childhood
- •7 Idiopathic intracranial hypertension
- •8 Colloid cyst of the third ventricle
- •9 Bilateral vestibular schwannomas: the challenge of neurofibromatosis type 2
- •10 Multimodality monitoring in severe traumatic brain injury
- •11 Intracranial abscess
- •12 Deep brain stimulation for debilitating Parkinson’s disease
- •14 Trigeminal neuralgia
- •15 Cerebral metastasis
- •16 The surgical management of the rheumatoid spine
- •17 Cervical spondylotic myelopathy
- •18 Brainstem cavernous malformation
- •19 Peripheral nerve injury
- •20 Spontaneous intracerebral haemorrhage
- •21 Low-grade glioma
- •22 Intracranial arteriovenous malformation
- •INDEX
CASE
10Multimodality monitoring in severe traumatic brain injury
Adel Helmy
 Expert commentary Peter J. Hutchinson
Expert commentary Peter J. Hutchinson
Case history
A 23-year-old, right-handed female presented to the A&E department following a road traffic accident. The patient was a single restrained occupant and collateral history suggested that, following loss of control of the vehicle, the driver collided with a tree at 60–70mph. There was significant intrusion into the vehicle on the passenger side resulting in a 1-hour extrication time. Medical first responders noted a GCS on scene of E1 V1 M4 (6/15) and that both pupils were small (3mm) and reactive to light. Following extrication, the patient was intubated and ventilated, and airlifted to the nearest trauma centre.
 Learning point Glasgow Coma Score
Learning point Glasgow Coma Score
The GCS was developed as a tool for the objective assessment of coma by Teasdale and Jennet in the 1970s [1]. The GCS has three distinct components, which should be reported individually, namely eyeopening (1–4), verbal (1–5), and motor (1–6) response. The best response is always used and provides a measure of global responsiveness. While the GCS has been criticized as having poor inter-observer validity in the non-expert assessor [2], it remains an important tool for both stratification of severity and prognostication following traumatic brain injury (TBI) [3].
The patient was managed in line with Advanced Trauma and Life Support (ATLS) protocols. Secondary survey did not note any further extracranial injuries. On arrival in A&E 90 minutes after injury, the patient had adequate ventilation (PO2 = 17kPa, PCO2 = 4.7kPa) and was haemodynamically stable, with a pulse rate of 85 and a blood pressure of 130/85mmHg. However, on reassessment, her pupils were found to be bilaterally fixed and dilated.
 Learning point Hyperventilation following traumatic brain injury
Learning point Hyperventilation following traumatic brain injury
Therapeutic hyperventilation can be used in the intensive care setting to reduce ICP. CO2 is an important determinant of vascular tone. Hyperventilation results in relative vasoconstriction, reduction in arterial blood volume, and a consequent reduction in ICP. However, vasoconstriction reduces cerebral blood flow and there is a trade-off between the reduction in ICP and the risk of ischaemia [4]. For this reason, outside the setting of the neurointensive care unit and appropriate monitoring
of oxygenation (such as brain tissue oxygen or jugular venous oximetry) it is not recommended [5]. Nevertheless, PCO2 should be kept in the low normal range (4.5–5.0 kPa) in those with suspected raised ICP.
92 |
Challenging concepts in neurosurgery |
Acute subdural haematoma
Diffusely swollen brain
Midline shit of (incidental) cavum septum pellucidum
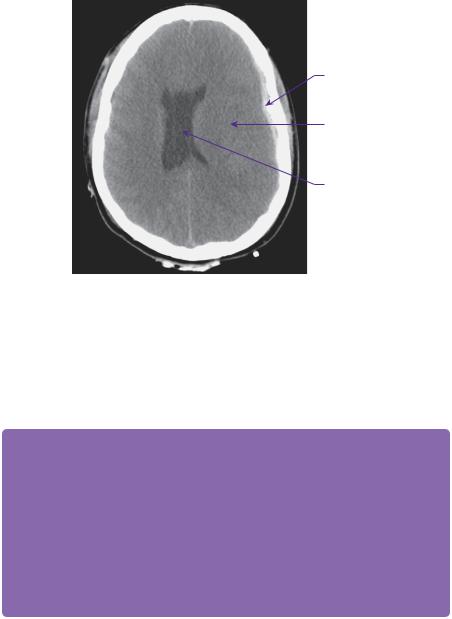
Figure 10.1 A brain window CT scan demonstrates a thin left-sided acute subdural haematoma and diffuse brain swelling.
The patient was catheterized and administered 200mL of 20% mannitol intravenously. She was transferred to the CT scanner. Figure 10.1 shows a representative axial slice on CT scan of the brain. This demonstrated a thin acute left subdural haematoma, brain swelling, but no evidence of vault or base of skull fracture. No other extracranial or spinal injuries were identified.
 Expert comment Base of skull fracture
Expert comment Base of skull fracture
Base of skull fractures can provide circumstantial evidence for the magnitude of force applied to the skull. They are sometimes missed and can be associated with several complications. CSF leak can mitigate rises in ICP by reducing intracranial CSF volume; however, they are associated with an increase in risk of meningitis. Prophylactic antibiotics do not play a role, but recent NICE guidelines
recommend the administration of pneumoccal vaccine (pneumovax) in all patient [6]. Most CSF leaks will resolve spontaneously over a few days without the need for operative intervention.
There is an increasing recognition of vascular injury to the intrapetrous carotid in base of skull fractures that traverse the carotid canal (as well as vertebral artery injury in fractures that traverse the relevant transverse foraminae) and we now recommend CT angiography in these cases. While anticoagulation may prove high risk in patients with severe TBI, endovascular interventions may still be an option if an intimal dissection is identified.
CT scan of C-spine, chest, abdomen, and pelvis did not demonstrate any other injury. The patient was immediately transferred to the theatre where a left-sided craniotomy and evacuation of subdural haematoma was carried out. The brain appeared swollen at the time of craniotomy and the bone flap was therefore not replaced. ICP, brain tissue oxygen, and microdialysis (to monitor brain chemistry) monitors were inserted in the right frontal lobe anterior to the coronal suture and the patient was returned to the intensive care unit.
The initial ICP was 18mmHg, but over the following 6 hours this gradually rose to 20–25mmHg requiring an escalation in ICP control measures, including moderate hypothermia to 35°C. Cerebral perfusion pressure (CPP) was maintained within the
Case 10 Multimodality monitoring in severe traumatic brain injury |
93 |
Radiological evidence of diffuse axonal injury
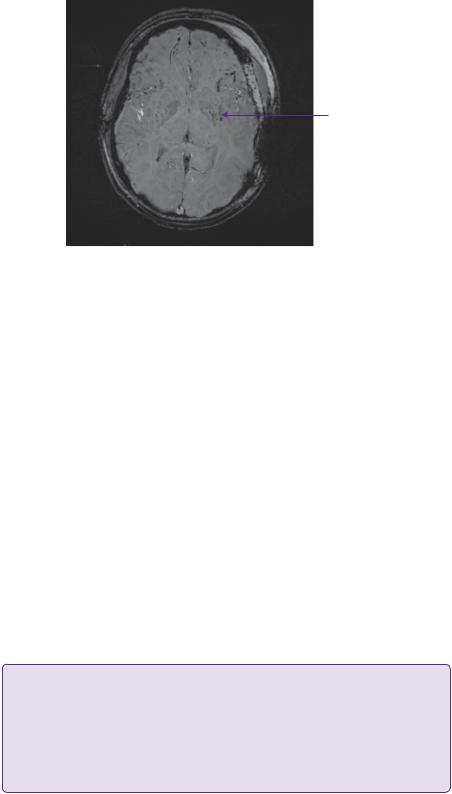
Figure 10.2 Certain MR sequences (gradient-echo, susceptibility-weighted images) are particularly sensitive to blood product and can be used to identify small petechial haemorrhages not apparent on CT or conventional MRI. The widespread dots of low signal on this image are indicative of a diffuse brain injury, which is often interpreted as diffuse axonal injury. Strictly speaking, diffuse axonal injury is a postmortem histological diagnosis with evidence of axonal retraction balls.
target range of 60–65mmHg. An MRI scan (susceptibility-weighted image) taken 24 hours post-operatively is shown in Figure 10.2.
At approximately 60 hours following craniectomy there was a sharp increase in lactate/pyruvate (L/P) ratio above 25, despite adequately controlled ICP (Figure 10.3). L/P ratio is a measure of cellular redox state and reflects the degree of anaerobic metabolism occurring within a tissue: a threshold of more than 25 is used to identify cerebral ischaemia [7]. A repeat CT scan did not reveal any further intracranial lesions, but as the brain tissue oxygen demonstrated a co-existent drop (not shown), a decision was made not to de-escalate ICP control measures.
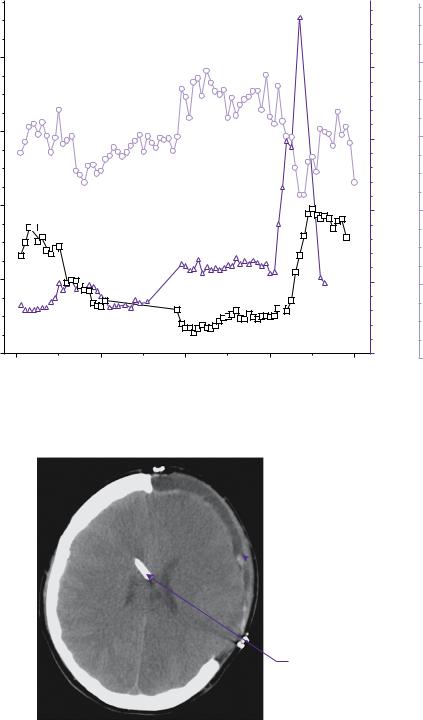
At approximately 70 hours following craniotomy there was a sharp increase in ICP to above 40mmHg requiring further escalation of ICP control measures, including cooling to 33°C, more aggressive hyperventilation to a CO2 of 4kPa and repeated boluses of 5% hypertonic saline until the serum Na+ reached 160mmol/L and the serum osmolarity reached 320mmol/L. During aggressive hyperventilation, the brain tissue oxygen tension was not permitted to drop below 15mmHg. As ICP was difficult to control an EVD was inserted at this time (Figure 10.4) as a further measure. Figure 10.5 shows the output from ICM+ demonstrating the Pulse Reactivity Index (PRx) for the patient in question. Figure 10.6 shows a plot of the PRx in relation to 5mmHg CPP bins.
 Learning point Pulse Reactivity Index
Learning point Pulse Reactivity Index
PRx is the moving correlation coefficient between mean arterial pressure (MAP) and ICP that varies from −1 to +1. The normal autoregulatory response maintains constant cerebral blood flow in the face of variations in MAP. This is achieved by vasoconstriction in the cerebral vasculature in response to an
increase in MAP. This vasoconstriction would normally reduce ICP, and in this way MAP and ICP should be inversely correlated. In this way, in the normal brain PRx should be negative. Following TBI, autoregulation can be impaired or absent such that increasing MAP causes a passive distension of the cerebral vessels, an increase in arterial blood volume and an increase in ICP. In this case, PRx will be increasingly positive [8].
94 |
Challenging concepts in neurosurgery |
ICP (mmHg) |
|
|
|
L/P ratio |
Lactate mmol/L |
|
80 |
|
|
|
200 |
8 |
|
|
|
|
|
|||
|
|
|
|
|
||
60 |
|
|
|
150 |
6 |
|
|
|
|
|
|||
40 |
|
|
|
100 |
4 |
|
|
|
|
|
|||
20 |
|
|
|
50 |
|
2 |
0 |
|
|
|
0 |
|
0 |
0 |
20 |
40 |
60 |
80 |
|
|
|
|
|||||
Hours of monitoring
Figure 10.3 ICP (left hand axis) and L/P (right hand blue axis) ratio are plotted over time since commencement of monitoring. The L/P ratio jumps from around 20–25 to 60–80 around 60 hours. This precedes an ICP spike to a plateau of 40mmHg at around 70 hours.
 Craniectomy
Craniectomy
External ventricular drain
Figure 10.4 Axial CT scan demonstrates the left-sided craniectomy and an external ventricular drain from the right frontal approach.
With thanks to Dr Karol Budohoski and Dr Marek Czosynka for providing these images.

Case 10 Multimodality monitoring in severe traumatic brain injury |
95 |
PRx PRX [ au ] CPP [ mmHg ] ICP [ mmHg ] ABP [ mmHg ]
200
150
100
50
0
60
50
40
30
20 10 0
100
90
80
70
60
50
40
1
0.5
0 -0.5 -1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
18/4 18:00 |
19/4 00:00 |
19/4 06:00 |
19/4 12:00 |
19/4 18:00 |
20/4 00:00 |
20/4 06:00 |
20/4 12:00 |
|||||||||||||||||||||||||||
Figure 10.5 Output from ICM+ software plotting arterial blood pressure (ABP), ICP, CPP, and PRx. PRx is calculated as the moving correlation coefficient between ABP and ICP.
With thanks to Dr Karol Budohoski and Dr Marek Czosynka for providing these images.
Time [%] PRx [au] PRX [ mmHg ]
CPP ICP
150
110
50
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8/4 00:00 |
|
|
|
|
|
8/4 01:00 |
|
|
|
|
8/4 02:00 |
|
8/4 03:00 |
|
|
|
|
8/4 04:00 |
|
|
|
|
8/4 05:00 |
|||||||||||||||||||||
0.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
< 40 42.50 |
47.50 52.50 |
57.50 62.50 |
67.50 |
72.50 77.50 82.50 87.50 |
92.50 97.50 |
102.5 107.5 112.5 117.5 122.5 |
127.5 132.5 |
137.5 >= 140 |
||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CPP [mmHg] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
30 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
< 50 |
|
52.50 |
57.50 |
62.50 |
67.50 |
|
72.50 |
77.50 |
|
82.50 |
87.50 |
92.50 |
97.50 |
|
|
>= 100 |
|
|||||||||||||||||||||||||||
CPP [mmHg]
Figure 10.6 Output from ICM+ software. PRx is plotted in the top panel. In the middle panel, the mean PRx value has been plotted against the CPP. A clear nadir is seen at 82.5–87.5mmHg indicating that at this value of CPP, PRx is minimized, and at this level of CPP the cerebral
vasculature is autoregulating most effectively. Some authors have suggested that this is the optimal CPP to ensure adequate delivery of metabolic substrates to the injured brain. The lower panel shows the percentage of time the patient has spent at each CPP value.
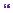 Expert comment Optimizing cerebral perfusion pressure
Expert comment Optimizing cerebral perfusion pressure
The Brain Trauma Foundation have provided guidelines on CPP targets—current guidelines suggest a range of 50–70mmHg. PRx may provide a method for individualizing CPP targets in two ways. First, if the PRx is negative and ICP is difficult to control increasing MAP can be used as a method of controlling ICP. Secondly, some authors have suggested that by targeting a CPP in which PRx can be shown to be negative may be beneficial as it provides a sufficient perfusion pressure, such that the brain can regulate its own needs physiologically.
A higher CPP target was adopted in this patient (75mmHg) resulting in improved control of ICP to below 20mmHg. After a further period of 24 hours, ICP began to fall consistently to the range of 15–20mmHg. A trial of raising CO2 to 4.5kPa from
96 Challenging concepts in neurosurgery
4kPa was attempted. This resulted in a rapid increase in ICP to 30mmHg and, therefore, PCO2 was returned to 4kPa for a further 24 hours. A repeat trial of raised CO2 was carried out at this point, which was well tolerated. This allowed further deescalation with gradual re-warming to 35°C then 37°C.
 Clinical tip De-escalation of intracranial pressure control
Clinical tip De-escalation of intracranial pressure control
Patients with raised ICP often have poor intracranial compliance, i.e. small changes in intracranial volume lead to large changes in ICP. When reducing ICP control measures a CO2 challenge is a useful method for gauging intracranial compliance and whether a patient will tolerate such de-escalation. As CO2 can be manipulated quickly and easily by alteration of minute volume it is preferable to de-escalation of other ICP control measures, such as rewarming from hypothermia. Rewarming can take several hours and if ICP were to rise, it can take several hours to re-institute. This can lead to poor ICP control for several hours, and potentially further secondary injury.
Over the following 48 hours, all ICP control measures were reversed. On stopping sedation, the patient achieved a GCS: E2, V: intubated; M5. The patient underwent tracheostomy and continued to improve over the following 2 weeks to a GCS: E4 V: tracheostomy; M6. A repeat CT scan (Figure 10.7) at this time showed a large subdural hygroma on the non-operated side. As the flap was soft and the patient was clinically improving, a decision was made to proceed to early cranioplasty and burr hole drainage of the hygroma. Figure 10.8 shows a CT scan 3 days post-cranioplasty showing resolution of the subdural hygroma.
The patient remains in a poor clinical state 6 months following injury with a Glasgow Outcome Score of 3 (severe disability). The patient has been referred for neuro-rehabilitation.
1.Presenting CT scan (brain window and bone window).
2.MRI scan (susceptibility-weighted image/gradient echo).
3.LP ratio and ICP.
CSF hygroma 
 Craniectomy
Craniectomy
Figure 10.7 Axial CT scan demonstrating CSF hygroma contralateral to the side of decompressive craniectomy.

Case 10 Multimodality monitoring in severe traumatic brain injury |
97 |
Frontal encephalomalacia
Titanium cranioplasty in situ
Figure 10.8 Axial CT scan following cranioplasty insertion. Although the subdural hygroma has resolved, the ventricles have now dilated. This is likely to be secondary to brain atrophy. Areas of low density, most marked in the right frontal lobe, indicate encephalomalacia.
4.CT scan with EVD in situ.
5.PRx trace from ICM+.
6.CPP optimal from ICM+.
7.CT scan with subdural hygroma.
8.CT scan post-cranioplasty.
Discussion
TBI is the commonest cause of death in those under 40 years of age in the UK. Although there is a long-term declining trend in severe TBI, largely related to the widespread introduction of road safety measures, such as seatbelts and cycle helmets, it still exacts a heavy economic and social toll. The management of severe TBI can provide many challenges, both to the neurosurgeon and to the neuro-intensivist. Great strides have been made by developing protocol treatment regimens for the intensive care management of ICP [9], which have led to improvements in patient outcome when compared with historical controls [10]. However, no Class 1 evidence exists for the use of ICP monitoring. In the developed world it is unlikely that there will ever be the ethical basis to run a trial in severe TBI, randomizing between ICP-driven therapy and management without ICP measurement. ICP measurement is recommended (based on the Brain Trauma Foundation Guidelines) in TBI patients presenting in coma (GCS ≤ 8), those with significant intracranial pathology on CT scan that are likely to deteriorate, and in some patients in which prolonged sedation and paralysis are required for extracranial injuries that would preclude clinical neurological monitoring.
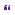 Expert comment Prognostic models
Expert comment Prognostic models
Two large clinical databases have been used to provide multivariate prediction models for outcome following TBI: CRASH-3 and IMPACT [11]. These models use admission characteristics to predict mortality and unfavourable outcome. This is potentially of key importance in risk stratification in
(continued)

98 |
Challenging concepts in neurosurgery |
|
|
|
|
clinical trials however these prognostic models must be used with caution in the clinical setting for several reasons [12]. Most importantly, prognostic models use population data to generate population statistics, but these cannot be accurately applied to a given patient. Furthermore, only admission characteristics are used within the models. This does not take into account either the clinical progress of the patient or the impact of the interventions during their clinical management. In practice, the clinical progress of a patient is used to make decisions about whether continued intervention is futile, rather than prognostic modelling of this sort.
The use of craniectomy in TBI has long been recognized as a method for reducing ICP [13]. The questions around its use centre on whether the reduction in ICP and potentially in mortality salvage patients with devastating injuries. The recently published DECRA study [14] addressed the question of whether early craniectomy could improve patient outcome by rapidly controlling ICP and preventing some of the pathophysiological consequences of raised ICP. The study randomized 155 patients to either decompressive craniectomy or medical management if they had a spike of ICP in the first 72 hours following admission. It demonstrated that there was an increased risk of unfavourable outcome in the decompressive craniectomy group (craniectomy group 70%; standard care group 42%; odds ratio 2.21) and a similar rate of mortality (19% craniectomy group, 18% medical care group). Several issues have been raised with this important study, including:
●Early randomization of patients with relatively modest ICP (15 minutes of ICP > 20mmHg).
●Imbalances between the two study groups, for example, many more patients in the decompressive craniectomy group had fixed dilated pupils (27% in decompressive craniectomy group and 12% in medical group).
●Only a tiny proportion of patients eligible for the trial (155 patients recruited from 3478 eligible) were randomized.
Nevertheless, this study highlights the fact that craniectomy should not be used in every patient, as it may not benefit all survivors of TBI.
In this particular case, the bone flap was not returned because of brain swelling and the perceived risk of further swelling, based on the mechanism of injury. The use of craniectomy after evacuation of a mass lesion is a contentious issue in its own right and one for which no clear evidence base exists.
 Expert comment Dangers and uses of decompressive craniectomy
Expert comment Dangers and uses of decompressive craniectomy
Craniectomy has several potential risks that may ameliorate some of the benefits of its effect on ICP. It can result in several problems, such as abnormalities in CSF dynamics (as seen in this case study) and a greater risk of developing kinking of the cortical veins at the margins of craniectomy [15], the syndrome of the trephined and the need for a delayed procedure (cranioplasty) to restore the skull contour. There is a risk that some patients undergoing decompressive craniectomy do not require the procedure and could be managed with medical therapy. This may have diluted out the benefits of this procedure in the DECRA study group. It is unfortunate that the randomization in this study led to so many more patients with bilateral fixed, dilated pupils in one arm of the study, which also makes the study difficult to interpret.
The Randomized Evaluation of Surgery with Craniectomy for Uncontrollable Elevation of Intra-Cranial Pressure (RESCUEicp) study (www.rescueicp.com) specifically randomized patients that have ICP refractory to medical therapy to decompressive craniectomy or further medical therapy (including barbiturate coma). This study is still recruiting and will specifically address whether decompressive craniectomy is of benefit in those patients in whom ICP is difficult to control.

Case 10 Multimodality monitoring in severe traumatic brain injury |
99 |
 Clinical tip Craniectomy
Clinical tip Craniectomy
Craniectomy can be carried out in several ways including unilateral (fronto-temporoparietal), bilateral fronto-temporoparieta leaving a bridge of bone over the sagittal sinus (‘bucket-handle’), and bifrontal. Unilateral craniectomies are recommended where there is a clear mass lesion or midline shift, while bifrontal craniectomies are recommended for diffuse brain swelling. Whatever method is used, the size of the craniectomy relates directly to the increase in intracranial volume achieved, and the risk of brain herniation causing kinking of cortical vessels and damage to the herniating brain edges. For this reason, a wide craniectomy (>12cm in diameter) is of benefit. Opening of the dura facilitates further brain expansion as does transfixing and dividing the anterior aspect of the sagittal sinus in bifrontal decompression. A layer of Surgicel® (Ethicon, Johnson and Johnson, USA) or thin dural substitute over the exposed brain creates a further layer that facilitates dissection of the scalp at the time of subsequent cranioplasty.
MRI imaging is not used routinely in the management of cerebral trauma, but it can be used to demonstrate diffuse injuries, believed to radiologically correlate with regions of diffuse axonal injury (DAI) that are not visible on CT imaging. The most sensitive MRI sequences for diffuse brain injury are those that exaggerate the signal that arises from blood products, such as gradient-echo and suscep- tibility-weighted imaging.
As well as the use of ICP monitoring, advanced monitoring techniques include brain tissue oxygenation and microdialysis monitoring. Using brain tissue oxygen probes, a value of 15mmHg regional PbO2 has been suggested as a threshold for cerebral ischaemia. Microdialysis is a technique for sampling the brain extracellular space by passing a flexible probe, lined with a semi-permeable membrane into the brain substance, which is constantly perfused with a physiological solution. Substances within the brain, such as glucose, lactate, and pyruvate, can diffuse into the catheter, and be recovered and measured at the bedside in the perfusing fluid. The ratio between L/P is used as a marker of aerobic versus anaerobic metabolism and a threshold of 25 is used to suggest anaerobic metabolism [7].
In both monitoring techniques, observational studies have demonstrated that derangements of brain tissue oxygenation [16] and microdialysis parameters [7] correlate to TBI outcome, even in a multivariate analysis. However, it is not clear which interventions are best suited to manipulate these parameters and whether targeting them will lead to improvements in outcome. Furthermore, as these are focal monitors, there is an on-going debate as to how data from these monitors should be used to guide therapy for the brain as a whole. In this case, there was a clear derangement in L/P ratio that preceded a dramatic ICP spike and provided an early warning of impending swelling.
 Expert comment Advanced monitoring techniques
Expert comment Advanced monitoring techniques
While these monitors are used in many units, specialist expertise is required to integrate the information from these monitors and individualize a given patient’s ICP and CPP targets. The data derived from these monitors must be interpreted in the context of the patient’s clinical state, as well as the position of the monitors on neuroimaging and are currently undergoing further evaluation to determine their clinical role on an individual patient intention-to-treat basis. Several other monitors exist, such as cerebral blood flow monitors and near infra-red spectroscopy. The utility of these monitors is an area of active clinical research.

100
 Expert comment Timing of cranioplasty
Expert comment Timing of cranioplasty
Early cranioplasty can minimize the complications associated with craniectomy, such as the syndrome of the trephined and abnormalities of CSF dynamics. However, there is a trade-off with the risk of infection in patients who are have been inpatients for a prolonged periods of time and may have on-going low-grade infection or harbour multidrug-resistant organisms. Most patients have a cranioplasty around the 6-month mark and a proportion will have an associated improvement during rehabilitation. There is no definitive evidence demonstrating that cranioplasty leads to a measureable improvement in outcome.
Challenging concepts in neurosurgery
Following craniectomy, CSF dynamics can change and lead to ventriculomegaly or subdural hygromas (either over the convexity or in the interhemispheric fissure). Whether these changes on CT scan represent hydrocephalus and impact on patient recovery or are a passive epiphenomenon of the change in ICP dynamics is not clear. In this case, early cranioplasty led to a partial resolution of the hygroma and the patient remains shunt free.
In this case, the patient did not have prophylactic anti-epileptic medication as there is a recognition that this does not ameliorate the long-term risk of epilepsy [17]. However, following head injury, patients are at risk of post-traumatic seizures, which can have potentially profound implications for quality of life and socioeconomic status. This applies both to patients who actually suffer from seizures, but also those deemed at risk of seizures, whose activities, e.g. driving and employment, are restricted as a result of this risk. Identifying which patients are likely to go on to develop seizures remains one of the most vexing areas of TBI management. Jennett et al. [18] in his seminal studies identified risk factors for late posttraumatic seizures, including depressed skull fractures, intracranial haematomas, early seizures (within 1 week), and patients with post-traumatic epilepsy (PTA) of more than 24 hours. Annegers et al. [19] have defined the risk of seizure according to the severity of injury with patients in the severe category given a 10% risk at 5 years post-injury. More recently, Christensen et al. [20] have shown that the risk is increased even 10 years after injury. These studies help in terms of the population risk, but it is not currently possible to accurately predict the risk for an individual patient. This is important for the ability to return to driving in the UK, particularly for bus and truck licence holders, where the risk must be deemed to be less than 2% per annum.
A final word from the expert
Maintaining the perfusion of oxygenated blood through a swollen brain is likely to decrease the morbidity and mortality associated with TBI. The aim of multi-modality cerebral monitoring is to quantify intracranial parameters believed to be upset after TBI, and guide treatment in the operating room and on the intensive care unit in order to decrease secondary brain injury. Craniectomy helps to decrease ICP, but further studies are awaited to determine how it alters long-term outcome after TBI. The overall aim would be to minimize the long-term disability and dependency of the commonly young population of severe TBI sufferers.
References
1.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2: 81–4.
2.Crossman J, Bankes M, Bhan A, Crockard HA. The Glasgow Coma Score: reliable evidence? Injury 1998; 29: 435–7.
3.MRC CRASH Trial Collaborators. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. British Medical Journal 2008; 336: 425–9.
Case 10 Multimodality monitoring in severe traumatic brain injury |
101 |
4.Coles JP, Fryer TD, Coleman MR, et al. Hyperventilation following head injury: effect on ischemic burden and cerebral oxidative metabolism. Critical Care Medicine 2007; 35: 568–78.
5.Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. XIV. Hyperventilation. Journal of Neurotrauma 2007; 24 (Suppl. 1): S87–90.
6.NationalInstitute for Health and Clinical Excellence, Head injury: Triage, assessment, investigation and early management of head injury in infants, children and adults. NICE guideline CG56, 2007. Available at: http://www.nice.org.uk/guidance/cg56
7.Timofeev I, Carpenter KL, Nortje J, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain 2011; 134: 484–94.
8.Sorrentino E., Diedler J., Kaprowicz M., et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocritical Care 2012; 16: 258–66.
9.Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. British Journal of Anaesthesia 2007; 99: 32–42.
10.Patel HC, Bouamra O, Woodford M, et al. Trends in head injury outcome from 1989 to 2003 and the effect of neurosurgical care: an observational study. Lancet 2005; 366: 1538–44.
11.Steyerberg EW, Mushkudiani N, Perel P, Butcher I, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Medicine 2008; 5: e165; discussion e165.
12.Helmy A, Timofeev I, Palmer CR, et al. Hierarchical log linear analysis of admission blood parameters and clinical outcome following traumatic brain injury. Acta Neurochirurgia (Wien) 2010; 152: 953–7.
13.Kocher T. Hirnerschütterung, hirndruck und chirurgische eingriffe bei hirnkrankheiten. In: H Nothnagel (ed.) Specielle pathologie und therapie, Vol. 9 (pp. 1–457). Vienna: Hölder, 1901.
14.Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. New England Journal of Medicine 2011; 364: 1493–502.
15.Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurgery Focu S 2009; 26: E7.
16.van den Brink WA, van Santbrink H, Steyerberg EW, et al. Brain oxygen tension in severe head injury. Neurosurgery 2000; 46: 868–76; discussion 876–8.
17.Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. Journal of Neurosurgery 1999; 91: 593–600.
18.Jennett B, Teather D, Bennie S. Epilepsy after head injury. Residual risk after varying fit-free intervals since injury. Lancet 1973; 2: 652–3.
19.Annegers JF, Hauser WA, Coan SP, et al. A population-based study of seizures after traumatic brain injuries. New England Journal of Medicine 1998; 338: 20–24.
20.Christensen J, Pedersen MG, Pedersen CB, et al. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet 2009; 373: 1105–10.
