
- •Table of Contents
- •Copyright
- •Dedication
- •Introduction to the eighth edition
- •Online contents
- •List of Illustrations
- •List of Tables
- •1. Pulmonary anatomy and physiology: The basics
- •Anatomy
- •Physiology
- •Abnormalities in gas exchange
- •Suggested readings
- •2. Presentation of the patient with pulmonary disease
- •Dyspnea
- •Cough
- •Hemoptysis
- •Chest pain
- •Suggested readings
- •3. Evaluation of the patient with pulmonary disease
- •Evaluation on a macroscopic level
- •Evaluation on a microscopic level
- •Assessment on a functional level
- •Suggested readings
- •4. Anatomic and physiologic aspects of airways
- •Structure
- •Function
- •Suggested readings
- •5. Asthma
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Treatment
- •Suggested readings
- •6. Chronic obstructive pulmonary disease
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach and assessment
- •Treatment
- •Suggested readings
- •7. Miscellaneous airway diseases
- •Bronchiectasis
- •Cystic fibrosis
- •Upper airway disease
- •Suggested readings
- •8. Anatomic and physiologic aspects of the pulmonary parenchyma
- •Anatomy
- •Physiology
- •Suggested readings
- •9. Overview of diffuse parenchymal lung diseases
- •Pathology
- •Pathogenesis
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Suggested readings
- •10. Diffuse parenchymal lung diseases associated with known etiologic agents
- •Diseases caused by inhaled inorganic dusts
- •Hypersensitivity pneumonitis
- •Drug-induced parenchymal lung disease
- •Radiation-induced lung disease
- •Suggested readings
- •11. Diffuse parenchymal lung diseases of unknown etiology
- •Idiopathic pulmonary fibrosis
- •Other idiopathic interstitial pneumonias
- •Pulmonary parenchymal involvement complicating systemic rheumatic disease
- •Sarcoidosis
- •Miscellaneous disorders involving the pulmonary parenchyma
- •Suggested readings
- •12. Anatomic and physiologic aspects of the pulmonary vasculature
- •Anatomy
- •Physiology
- •Suggested readings
- •13. Pulmonary embolism
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic evaluation
- •Treatment
- •Suggested readings
- •14. Pulmonary hypertension
- •Pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic features
- •Specific disorders associated with pulmonary hypertension
- •Suggested readings
- •15. Pleural disease
- •Anatomy
- •Physiology
- •Pleural effusion
- •Pneumothorax
- •Malignant mesothelioma
- •Suggested readings
- •16. Mediastinal disease
- •Anatomic features
- •Mediastinal masses
- •Pneumomediastinum
- •Suggested readings
- •17. Anatomic and physiologic aspects of neural, muscular, and chest wall interactions with the lungs
- •Respiratory control
- •Respiratory muscles
- •Suggested readings
- •18. Disorders of ventilatory control
- •Primary neurologic disease
- •Cheyne-stokes breathing
- •Control abnormalities secondary to lung disease
- •Sleep apnea syndrome
- •Suggested readings
- •19. Disorders of the respiratory pump
- •Neuromuscular disease affecting the muscles of respiration
- •Diaphragmatic disease
- •Disorders affecting the chest wall
- •Suggested readings
- •20. Lung cancer: Etiologic and pathologic aspects
- •Etiology and pathogenesis
- •Pathology
- •Suggested readings
- •21. Lung cancer: Clinical aspects
- •Clinical features
- •Diagnostic approach
- •Principles of therapy
- •Bronchial carcinoid tumors
- •Solitary pulmonary nodule
- •Suggested readings
- •22. Lung defense mechanisms
- •Physical or anatomic factors
- •Antimicrobial peptides
- •Phagocytic and inflammatory cells
- •Adaptive immune responses
- •Failure of respiratory defense mechanisms
- •Augmentation of respiratory defense mechanisms
- •Suggested readings
- •23. Pneumonia
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features and initial diagnosis
- •Therapeutic approach: General principles and antibiotic susceptibility
- •Initial management strategies based on clinical setting of pneumonia
- •Suggested readings
- •24. Bacterial and viral organisms causing pneumonia
- •Bacteria
- •Viruses
- •Intrathoracic complications of pneumonia
- •Respiratory infections associated with bioterrorism
- •Suggested readings
- •25. Tuberculosis and nontuberculous mycobacteria
- •Etiology and pathogenesis
- •Definitions
- •Pathology
- •Pathophysiology
- •Clinical manifestations
- •Diagnostic approach
- •Principles of therapy
- •Nontuberculous mycobacteria
- •Suggested readings
- •26. Miscellaneous infections caused by fungi, including Pneumocystis
- •Fungal infections
- •Pneumocystis infection
- •Suggested readings
- •27. Pulmonary complications in the immunocompromised host
- •Acquired immunodeficiency syndrome
- •Pulmonary complications in non–HIV immunocompromised patients
- •Suggested readings
- •28. Classification and pathophysiologic aspects of respiratory failure
- •Definition of respiratory failure
- •Classification of acute respiratory failure
- •Presentation of gas exchange failure
- •Pathogenesis of gas exchange abnormalities
- •Clinical and therapeutic aspects of hypercapnic/hypoxemic respiratory failure
- •Suggested readings
- •29. Acute respiratory distress syndrome
- •Physiology of fluid movement in alveolar interstitium
- •Etiology
- •Pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Treatment
- •Suggested readings
- •30. Management of respiratory failure
- •Goals and principles underlying supportive therapy
- •Mechanical ventilation
- •Selected aspects of therapy for chronic respiratory failure
- •Suggested readings
- •Index

and alveolar distending pressure to no more than 30 cm H2O. As noted above, the use of a low tidal volume ventilatory strategy in patients with ARDS has been demonstrated to decrease mortality, whereas the use of higher levels of PEEP has not resulted in decreased mortality versus using lower levels of PEEP.
Use of a low tidal volume ventilatory strategy in patients with ARDS has been demonstrated to decrease mortality.
Another major adverse effect of positive-pressure ventilation is potential impairment of cardiovascular function. At least two mechanisms are thought to play a role. The first involves a decrease in venous return to the heart. Whereas the normally negative intrathoracic pressure during inspiration promotes venous return from the periphery, positive inspiratory pressure from a ventilator impedes venous return. The hemodynamic consequences of low cardiac output and blood pressure are even more likely when the patient is also receiving PEEP and is somewhat volume depleted. In many cases, judicious administration of fluids can restore the effective intravascular volume and reverse the adverse hemodynamic consequences of positive-pressure ventilation.
The second mechanism involves an increase in pulmonary vascular resistance. When alveolar volume is increased with positive-pressure mechanical ventilation, alveolar vessels are compressed, compromising the overall cross-sectional area of the pulmonary vascular bed. As a result, pulmonary vascular resistance and the workload placed on the right ventricle increase. Right ventricular output is potentially compromised, and the right ventricle may dilate. This shifts the interventricular septum toward the left ventricular cavity, also impairing left ventricular filling and stroke volume.
Management of patients receiving positive-pressure ventilation, particularly those with hypoxemic respiratory failure who require PEEP, is complicated. Many factors interact in a complex way, specifically oxygenation, cardiac output, and fluid status. Optimal care requires both sophisticated patient monitoring and substantial expertise from the team responsible for patient care. Such care is necessary for proper support of vital functions and to minimize the complications of therapy.
Selected aspects of therapy for chronic respiratory failure
Chronic ventilatory support
Working with patients who have chronic irreversible respiratory or neuromuscular disease and require continuous long-term ventilatory support involves difficult clinical decisions. The first question is whether the patient wishes “to be on a machine” for the rest of his or her life. Some patients clearly wish to prolong life even if it means permanent ventilatory support; others choose not to be dependent on a ventilator for the remainder of their lives. When the patient chooses to be maintained on a ventilator, support usually is given by positive-pressure ventilation administered through a tracheostomy tube. The care of some patients can be handled at home with the proper support of family and visiting healthcare personnel. Management of other cases continues in chronic care hospitals or other facilities equipped to care for such patients.
A subgroup of patients with chronic respiratory insufficiency does not require continuous ventilatory support but benefits from nocturnal assistance with ventilation. These patients often have chronic neuromuscular or chest wall disease accompanied by chronic hypercapnia. More recent data suggest that some patients with COPD and hypercapnia may also benefit from chronic nocturnal ventilation. Although the degree to which respiratory muscle fatigue contributes to the hypercapnia experienced by these
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

patients is not clear, at least part of the rationale for using nocturnal ventilatory support is to afford these patients a number of hours each day when their inspiratory muscles are allowed to rest. After a period of nocturnal rest, the respiratory muscles presumably are better able to handle the work of breathing during the day, and daytime hypercapnia may be improved.
When ventilatory support is needed only during the night, it is generally preferable to avoid a chronic tracheostomy. Several options are available, and the most appropriate depends on the particular patient. Positive pressure can be administered at night through nasal pillows, a mouthpiece, or a mask (i.e., NIPPV). Much less commonly, lung inflation can be achieved by negative-pressure ventilation, intermittent negative pressure applied outside the chest wall, causing it to expand and the lungs to inflate. The original type of negative-pressure ventilator was the “iron lung,” used for ventilatory support from the late 1920s through the polio epidemics of the 1950s. The two most common types of negative pressure ventilators currently used are the raincoat (or “poncho”) ventilator and the cuirass (or “chest shell”) ventilator. Unlike the iron lung, which enclosed the entire body below the neck, raincoat and cuirass ventilators do not enclose or limit movement of the lower half of the body.
Continuous chronic ventilatory support is typically provided through a tracheostomy tube, whereas chronic nocturnal ventilatory support is provided using noninvasive positive-pressure ventilation.
Lung transplantation
First performed successfully in 1983, lung transplantation is an option for some patients with severe and disabling chronic pulmonary disease. However, availability of a lung transplant is limited, primarily because suitable donor organs are scarce, and difficulties with posttransplant infections and chronic rejection limit the long-term utility of the procedure. The most common clinical problems leading to lung transplantation are COPD (including α1-antitrypsin deficiency), idiopathic pulmonary fibrosis, cystic fibrosis, and pulmonary arterial hypertension.
Several types of transplantation can be performed: single lung, bilateral lung, lobar transplantation from living donors, and heart-lung transplantation. Although single lung transplantation allows more potential recipients to receive a donor lung than bilateral lung transplantation, survival is better following bilateral lung transplantation, and there has been a trend away from single lung and toward bilateral lung transplantation. For patients with cystic fibrosis, in whom chronic bilateral pulmonary infection complicates their lung disease, bilateral lung transplantation is essential to avoid infection of the new lung by spillover of infected secretions from a remaining diseased native lung. When severe cardiac disease accompanies end-stage lung disease, combined heart-lung transplantation may be required. The most recent lung transplantation technique is lobar transplantation from living donors. In this technique, which is used primarily in younger patients with cystic fibrosis, the recipient is given bilateral implants of a lower lobe from each of two living donors.
In many ways, the lung transplant patient trades the primary lung disease for another disease state: that of the transplant recipient. The major potential complications of lung transplantation fall under the general categories of rejection and infection. Because of the risk of rejection, patients are routinely given immunosuppressive drugs, such as prednisone, mycophenolate mofetil (or azathioprine), and tacrolimus (or cyclosporine), as a regimen to prevent rejection. Nevertheless, acute or chronic rejection can occur despite maintenance immunosuppression. Acute rejection is often characterized by fever, impairment of pulmonary function and gas exchange, and pulmonary infiltrates on chest radiograph. Episodes typically occur during the first several months after transplantation and are difficult to differentiate from infection on clinical grounds alone. Acute rejection is treated by short-term intensification of the immunosuppressive regimen, especially with increased doses of corticosteroids. Chronic rejection is

usually manifested as bronchiolitis obliterans, which is characterized by progressive inflammation, fibrosis, and obstruction of small airways. The physiologic consequence of this process is progressive airflow obstruction, which typically is unresponsive to augmentation of immunosuppressive therapy. As a result, bronchiolitis obliterans is the major cause of graft failure and death occurring later in the course after lung transplantation. Pharmacologic treatment of severe bronchiolitis obliterans has been disappointing, and the main treatment option for posttransplant patients with this syndrome is repeat transplantation.
The major complications occurring after lung transplantation are rejection and infection.
Progressive airflow obstruction from bronchiolitis obliterans is thought to represent chronic transplant rejection.
Another major complication of lung transplantation is infection, the risk of which is greatly increased by the need for immunosuppressive therapy. In some cases, organisms (e.g., bacteria, cytomegalovirus) accompanied the donor organ, and development of a complicating infection was precipitated by immunosuppression and impairment of the recipient’s defense mechanisms. Patients are also subject to the variety of opportunistic infections common to patients with impaired cell-mediated immunity, including other viruses, fungi, and Pneumocystis. Finally, there is also an increased risk of malignancy in lung transplant recipients, notably with what has been called posttransplant lymphoproliferative disease, a type of malignant expansion of lymphocytes that is usually related to Epstein-Barr virus infection and frequently can be controlled by reducing the intensity of immunosuppression.
Accompanying the growing experience with lung transplantation over the past decade has been a modest improvement in survival. Survival is approximately 75% to 80% at 1 year after transplantation; however, median survival is only approximately 5 to 7 years. Lung transplantation is an accepted but expensive therapeutic option for a highly selected group of patients, and future improvements in donor organ preservation and immunosuppression may lead to improved outcomes and broader application of the procedure.
Suggested readings
Mechanical ventilation
Abrams D, Schmidt M, Pham T, Beitler J.R, Fan E, Goligher E.C., et al. Mechanical ventilation for acute respiratory distress syndrome during extracorporeal life support. Research and practice American Journal of Respiratory and Critical Care Medicine 2020;201: 514-525.
Azoulay E, Lemiale V, Mokart D, Nseir S, Argaud L, Pène F., et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: The HIGH randomized clinical trial JAMA 2018;320: 20992107.
Baldomero A.K, Melzer A.C, Greer N, Majeski B.N, MacDonald R, Linskens E.J., et al.
Effectiveness and harms of high-flow nasal oxygen for acute respiratory failure: An evidence report for a clinical guideline from the American College of Physicians Annals of Internal Medicine 2021;174: 952-966.
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
Bourke S.C, Piraino T, Pisani L, Brochard L. & Elliott M.W. Beyond the guidelines for noninvasive ventilation in acute respiratory failure: Implications for practice Lancet Respiratory Medicine 2018;6: 935-947.
Briel M, Meade M, Mercat A, Brower R.G, Talmor D, Walter S.D., et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis JAMA 2010;303: 865-873.
Brodie D, Slutsky A.S. & Combes A. Extra-corporeal life support for adults with respiratory failure and related indications: A review JAMA 2019;322: 557-568.
Chandra D, Stamm J.A, Taylor B, Ramos R.M, Satterwhite L, Krishnan J.A., et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008 American Journal of Respiratory and Critical Care Medicine 2012;185: 152-159.
Curley G.F, Laffey J.G, Zhang H. & Slutsky A.S. Biotrauma and ventilator-induced lung injury: Clinical implications Chest 2016;150: 1109-1117.
Davidson A.C, Banham S, Elliott M, Kennedy D, Gelder C, Glossop A., et al. BTS/ICS guideline for the ventilator management of acute hypercapnic respiratory failure in adults Thorax 2016;71: ii1ii35.
Drake M.G. High-flow nasal cannula oxygen in adults: An evidence-based assessment
Annals of the American Thoracic Society 2018;15: 145-155.
Fan E, Del Sorbo L, Goligher E.C, Hodgson C.L, Munshi L, Walkey A.J., et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome American Journal of Respiratory and Critical Care Medicine 2017;195: 1253-1263.
Fan E, Zakhary B, Amaral A, McCannon J, Girard T.D, Morris P.E., et al. Liberation from mechanical ventilation in critically ill adults. An official ATS/ACCP clinical practice guideline Annals of the American Thoracic Society 2017;14: 441-443.
Goligher E.C, Dres M, Patel B.K, Sahetya S.K, Beitler J.R, Telias I., et al. Lungand diaphragm-protective ventilation American Journal of Respiratory and Critical Care Medicine 2020;202: 950-961.
Junhasavasdikul D, Telias I, Grieco D.L, Chen L, Gutierrez C.M, Piraino T., et al. Expiratory flow limitation during mechanical ventilation Chest 2018;154: 948-962.
Klompas M. Potential strategies to prevent ventilator-associated events American Journal of Respiratory and Critical Care Medicine 2015;192: 1420-1430.
McConville J.F. & Kress J.P. Weaning patients from the ventilator New England Journal of Medicine 2012;367: 2233-2239.
Munshi L, Mancebo J. & Brochard L.J. Noninvasive respiratory support for adults with acute respiratory failure New England Journal of Medicine 2022;387: 1688-1698.
Oczkowski S, Ergan B, Bos L, Chatwin M, Ferre M, Gregoretti C., et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure European Respiratory Journal 2022;59: 2101574.
O’Gara B, Fan E. & Talmor D.S. Controversies in the management of severe ARDS: Optimal ventilator management and use of rescue therapies Seminars in Respiratory and Critical Care Medicine 2015;36: 823-834.
Ou X, Hua Y, Liu J, Gong C. & Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: A meta-analysis of randomized controlled trials CMAJ 2017;189: E260E267.
Patel B.K. & Kress J.P. The changing landscape of noninvasive ventilation in the intensive care unit JAMA 2015;314: 1697-1699.
Putensen C, Theuerkauf N, Zinserling J, Wrigge H. & Pelosi P. Meta-analysis: Ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury
Annals of Internal Medicine 2009;151: 566-576.
Qaseem A, Etxeandia-Ikobaltzeta I, Fitterman N, Williams J.W,Jr, Kansagara D., et al: Clinical Guidelines Committee of the American College of Physicians. Appropriate use of high-flow nasal oxygen in hospitalized patients for initial or postextubation management of acute respiratory failure: A clinical guideline from the American College of Physicians Annals of Internal Medicine 2021;174: 977-984.
Rello J, Lisboa T. & Koulenti D. Respiratory infections in patients undergoing mechanical ventilation Lancet Respiratory Medicine 2014;2: 764-774.
Rochwerg B, Brochard L, Elliott M.W, Hess D, Hill N.S, Nava S., et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure
European Respiratory Journal 2017;50: 1602426.
Sahetya S.K, Goligher E.C. & Brower R.G. Setting positive end-expiratory pressure in acute respiratory distress syndrome American Journal of Respiratory and Critical Care Medicine 2017;195: 1429-1438.
Scala R. & Pisani L. Noninvasive ventilation in acute respiratory failure: Which recipe for success? European Respiratory Review 2018;27: 180029.
Schjørring O.L, Klitgaard T.L, Perner A, Wetterslev J, Lange T, Siegemund M., et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure New England Journal of Medicine 2021;384: 1301-1311.
Slutsky A.S. History of mechanical ventilation. From Vesalius to ventilator-induced lung injury American Journal of Respiratory and Critical Care Medicine 2015;191: 1106-1115.
Subirà C, Hernández G, Vázquez A, Rodríguez-García R, González-Castro A, García C., et al.
Effect of pressure support vs T-piece ventilation strategies during spontaneous breathing trials on successful extubation among patients receiving mechanical ventilation: A randomized clinical trial JAMA 2019;321: 2175-2182.
Thille A.W, Muller G, Gacouin A, Coudroy R, Decavèle M, Sonneville R., et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: A randomized clinical trial JAMA 2019;322: 1465-1475.
Tobin M.J, Laghi F. & Jubran A. Narrative review: Ventilator-induced respiratory muscle weakness Annals of Internal Medicine 2010;153: 240-245.
Support of chronic respiratory failure
Ergan B, Oczkowski S, Rochwerg B, Carlucci A, Chatwin M, Clini E., et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD European Respiratory Journal 2019;54: 1901003.
Hannan L.M, Dominelli G.S, Chen Y.W, Darlene Reid W. & Road J. Systematic review of non-invasive positive pressure ventilation for chronic respiratory failure Respiratory Medicine 2014;108: 229-243.
Hind M, Polkey M.I. & Simonds A.K. Homeward bound: A centenary of home mechanical ventilation American Journal of Respiratory and Critical Care Medicine 2017;195: 1140-
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
1149.
Jacobs S.S, Krishnan J.A, Lederer D.J, Ghazipura M, Hossain T, Tan A.M., et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society clinical practice guideline American Journal of Respiratory and Critical Care Medicine 2020;202: e121e141 [Erratum in: Am J Respir Crit Care Med. 2021;203:10451046].
MacIntyre E.J, Asadi L, Mckim D.A. & Bagshaw S.M. Clinical outcomes associated with home mechanical ventilation: A systematic review Canadian Respiratory Journal 2016;2016: 6547180.
Macrea M, Oczkowski S, Rochwerg B, Branson R.D, Celli B, Coleman J.M., et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline American Journal of Respiratory and Critical Care Medicine 2020;202: e74e87.
Sahetya S, Allgood S, Gay P.C. & Lechtzin N. Long-term mechanical ventilation Clinics in Chest Medicine 2016;37: 753-763.
Wilson M.E, Dobler C.C, Morrow A.S, Beuschel B, Alsawas M, Benkhadra R., et al.
Association of home noninvasive positive pressure ventilation with clinical outcomes in chronic obstructive pulmonary disease: A systematic review and meta-analysis JAMA 2020;323: 455-465.
Lung transplantation
Ahmad S, Shlobin O.A. & Nathan S.D. Pulmonary complications of lung transplantation
Chest 2011;139: 402-411.
Kotloff R.M. & Thabut G. Lung transplantation American Journal of Respiratory and Critical Care Medicine 2011;184: 159-171.
Leard L.E, Holm A.M, Valapour M, Glanville A.R, Attawar S, Aversa M., et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation Journal of Heart and Lung Transplantation 2021;40: 1349-1379.
Levine D.J. & Hachem R.R. Lung allograft rejection Thoracic Surgery Clinics 2022;32: 221229.
Mahajan A.K, Folch E, Khandhar S.J, Channick C.L, Santacruz J.F, Mehta A.C., et al. The diagnosis and management of airway complications following lung transplantation Chest 2017;152: 627-638.
Mitilian D, Sage E, Puyo P, Bonnette P, Parquin F, Stern M., et al. Techniques and results of lobar lung transplantations European Journal of Cardio-Thoracic Surgery 2014;45: 365369.
Schaffer J.M, Singh S.K, Reitz B.A, Zamanian R.T. & Mallidi H.R. Singlevs double-lung transplantation in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis since the implementation of lung allocation based on medical need
JAMA 2015;313: 936-948.
Seiler A, Klaghofer R, Ture M, Komossa K, Martin-Soelch C. & Jenewein J. A systematic review of health-related quality of life and psychological outcomes after lung transplantation Journal of Heart and Lung Transplantation 2016;35: 195-202.
Solomon M, Grasemann H. & Keshavjee S. Pediatric lung transplantation Pediatric Clinics
of North America 2010;57: 375-391.
van der Mark S.C, Hoek R.A.S. & Hellemons M.E. Developments in lung transplantation over the past decade European Respiratory Review 2020;29: 190132.
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

Appendix A: Sample problems using respiratory equations
A comatose patient with no spontaneous respiration is placed on mechanical ventilation with the following settings:
Tidal volume (VT) = 1000 mL Respiratory frequency (f) = 10 breaths/min
Inspired O2 concentration = 40% (FiO2 = 0.4) The following measurements are made:
Arterial PCO2 (PaCO2) = 40 mm Hg Mixed expired PCO2 (PECO2) = 30 mm Hg Arterial PO2 (PaO2) = 95 mm Hg
1.Calculate minute ventilation ( 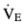 ), dead space–to–tidal volume ratio (VD/VT), alveolar volume (VA), and alveolar ventilation (
), dead space–to–tidal volume ratio (VD/VT), alveolar volume (VA), and alveolar ventilation (  ).
).
2.If extra tubing with a volume of 250 mL were added to the system in a position such that it provided additional dead space, what would be the new VD/VT?
3.With the new system as described in Question 2, what would be the new PaCO2?
4.Going back to the original conditions (without added extra tubing), the ventilator settings are changed to new settings:
VT = 500 mL
f = 20 breaths/min
a.Calculate the new  , VA,
, VA,  , and VD/VT.
, and VD/VT.
b.What would happen to PaCO2 on the new settings?
c.What would you expect PECO2 to be if you now measured it?
5.Using the original ventilator settings and arterial blood gases as given, calculate the alveolararterial difference in partial pressure of oxygen (AaDO2).
6.After the patient is improved, arterial blood gases measured with the patient breathing room air are as follows:
PO2 = 75 mm Hg PCO2 = 40 mm Hg
pH = 7.40 Calculate AaDO2.
7. The next day, the patient’s arterial blood gas values on room air are as follows: PO2 = 80 mm Hg
PCO2 = 20 mm Hg

pH = 7.55 What is AaDO2?
Answers
1. 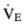 = 1000 mL/breath × 10 breaths/min = 10, 000 mL/min = 10 L/min VD/VT = (40 mm Hg − 30 mm Hg)/40 mm Hg = 0.25
= 1000 mL/breath × 10 breaths/min = 10, 000 mL/min = 10 L/min VD/VT = (40 mm Hg − 30 mm Hg)/40 mm Hg = 0.25
VA = VT − VD = 1000 mL − (0.25 × VT) = 1000 mL − 250 mL = 750 mL  = VA × f = 750 mL/breath × 10 breaths/min = 7.5 L/min
= VA × f = 750 mL/breath × 10 breaths/min = 7.5 L/min
2.New VD = 250 mL + 250 mL = 500 mL New VD/VT = 500 mL/1000 mL = 0.5
3.Because PaCO2 is inversely proportional to  , the new PaCO2 can be calculated from the old
, the new PaCO2 can be calculated from the old
and the new  (assuming
(assuming  remains constant).
remains constant).
As per Problem 1, old 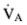 = 7.5 L/min
= 7.5 L/min
New VA = 1000 mL − new VD = 1000 mL − 500 mL = 500 mL
New |
= 500 mL/breath × 10 breaths/min = 5 L/min |
New |
= 2/3 × old |
New PaCO2 = 3/2 × old PaCO2 = 3/2 × 40 mm Hg = 60 mm Hg 4. On the basis of the new settings:
a.  = 500 mL/breath × 20 breaths/min = 10 L/min VA = 500 mL − 250 mL = 250 mL
= 500 mL/breath × 20 breaths/min = 10 L/min VA = 500 mL − 250 mL = 250 mL
 = 250 mL/breath × 20 breaths/min = 5 L/min
= 250 mL/breath × 20 breaths/min = 5 L/min
VD/VT = 250 mL/500 mL = 0.5 |
|
|
||
b. New PaCO2 is inversely proportional to the ratio of the new |
to the old |
. |
||
New |
/old |
= (5 L/min)/(7.5 L/min) = 2/3 |
|
|
New PaCO2 = 3/2 × old PaCO2 = 3/2 × 40 mm Hg = 60 mm Hg
c.Because VD/VT = (PaCO2 − PECO2)/PaCO2, substitute the known values and solve the equation for PECO2.
0.5= (60 mm Hg − PECO2)/60 mm Hg
PECO2 = 30 mm Hg
5.PAO2 = (0.4 × 713 mm Hg) − (40 mm Hg/0.8) = 285 mm Hg − 50 mm Hg = 235 mm Hg AaDO2 = PAO2 − PaO2 = 235 mm Hg − 95 mm Hg = 140 mm Hg
6.PAO2 = 150 mm Hg − (40 mm Hg/0.8) = 100 mm Hg
AaDO2 = 100 mm Hg − 75 mm Hg = 25 mm Hg = 150 mm Hg − (20 mm Hg/0.8) = 125 mm Hg
AaDO2 = 125 mm Hg − 80 mm Hg = 45 mm Hg
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

Appendix B: Pulmonary function tests: Guidelines for interpretation and sample problems
This appendix provides an outline of a simplified approach to interpreting pulmonary function tests and gives several examples of test results presented as unknown problems. Because details of the interpretation of these tests may vary among laboratories, the approach here focuses on the general concepts rather than the specific details, providing a step-by-step approach to analyzing pulmonary function tests. The concepts underlying this step-by-step approach are covered in the relevant section on pulmonary function tests in Chapter 3.
Analysis of pulmonary function tests
1.Examination of lung volumes:
a.A decrease in total lung capacity (TLC) generally indicates the presence of a restrictive pattern. However, TLC measured by helium dilution (as opposed to body plethysmography) may also be artificially depressed when there are poorly communicating or noncommunicating regions within the lung (e.g., in bullous lung disease).
b.Are lung volumes symmetrically reduced (i.e., are TLC, residual volume [RV], functional residual capacity [FRC], and vital capacity [VC] all decreased to approximately the same extent)? If so, this suggests diffuse parenchymal lung disease as the cause of the restrictive pattern. A low diffusing capacity also supports the diagnosis of diffuse parenchymal lung disease as the cause of the restrictive pattern.
c.A relatively preserved RV and a normal diffusing capacity suggest another cause of restrictive disease, such as neuromuscular or chest wall disease. Poor effort from the patient may also create this type of pattern.
2.Examination of the mechanics—that is, flow rates measured from the forced expiratory spirogram:
a.A decrease in the ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) indicates obstruction. In some cases of airflow obstruction, both FEV1 and FVC are reduced by approximately the same extent, and FEV1/FVC may be preserved. Clues to the presence of obstructive disease in this setting are a low forced expiratory flow from 25% to 75% of vital capacity (FEF25%–75%), a normal to high TLC with a high ratio of RV to TLC, and the configuration of the flow-volume curve.
b.Interpretation of FEF25%–75% (also called maximal midexpiratory flow [MMF]):
(1)FEF25%–75% is subject to more variability than most other measurements obtained during a forced expiration, so guidelines for normal values are less well established.
(2)When lung volumes are low, FEF25%–75% can also be decreased without necessarily
indicating coexisting airflow obstruction. Therefore, in the presence of decreased lung volumes, a low FEF25%–75% indicates obstruction, primarily if the decrease in FEF25%– 75% is out of proportion to the decrease in lung volumes.
(3) Taking into account the aforementioned qualifications, FEF25%–75% may be a relatively sensitive measurement for airway obstruction. An isolated abnormality in FEF25%–75%
has sometimes been considered a marker for early or very mild airflow obstruction, theoretically reflecting “small airway disease.”
c.The criteria for a significant response to a bronchodilator, indicating at least partial reversibility of airflow obstruction, are an improvement over baseline of either the FEV1 or FVC by 10% of the predicted value. Patients with asthma characteristically fulfill at least one of these criteria, as do some patients with chronic obstructive pulmonary disease (COPD) who have a reversible component to their disease.
3.Interpretation of the flow-volume curve:
a.An obstructive pattern is reflected by decreased flow relative to lung volume, generally accompanied by a “scooped out” or “coved” appearance to the descending part of the expiratory curve (see Fig. 3.21).
b.A restrictive pattern is characterized by decreased volumes (i.e., narrowing of the curve along the volume or X-axis) and relatively preserved flow rates. The flow rates often appear increased relative to the small lung volumes, producing a tall, narrow curve.
4.Interpretation of diffusing capacity of the lung for carbon monoxide (DLCO):
a.Ensure that the value has been corrected for the patient’s hemoglobin level. If not, the value will be falsely low if the patient is anemic.
b.A decrease in the diffusing capacity reflects disease affecting the alveolar-capillary membrane (decreased surface area for gas exchange and/or abnormal thickness of the membrane) or a decrease in pulmonary capillary blood volume.
c.An increase in the diffusing capacity can reflect increased pulmonary capillary blood volume or erythrocytes within alveolar spaces (pulmonary hemorrhage).
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

Answers
1. All measurements of lung volume (TLC, VC, FRC, RV) are significantly decreased, indicative of
restrictive disease. FEV1 and FVC are decreased because of low lung volumes, but FEV1/FVC is
preserved. This finding, along with the fact that FEF25%–75% is not decreased out of proportion to the decrease in lung volumes, indicates there is no obstruction. Diffusing capacity is decreased, suggesting that the restrictive disease is secondary to an abnormality of the pulmonary parenchyma rather than a result of chest wall or neuromuscular disease. The flow-volume curve is tall and narrow, consistent with a restrictive pattern. Diagnosis: Diffuse parenchymal lung disease secondary to pulmonary sarcoidosis.
2. FEV1 and FVC are both decreased. Because FEV1 is decreased more than FVC, FEV1/FVC is
decreased. FEF25%–75% is also decreased. These values are indicative of obstructive lung disease. TLC is normal, and RV and FRC are increased. RV/TLC ratio is also increased. Therefore, there is no restriction, but the high RV/TLC ratio indicates there is “air trapping, ” as is often expected with airflow obstruction. The diffusing capacity is decreased, reflecting loss of alveolar-capillary bed. The flow-volume curve shows an obstructive pattern characterized by a striking decrease in flow rates, well seen throughout most of the expiratory curve after the initial peak flow rate. This combination of significant airflow obstruction with normal or increased volumes and a low diffusing capacity suggests emphysema.
3.TLC and FRC are reduced, indicating restrictive disease. RV is relatively preserved. FEV1 and FVC both are decreased, but FEV1/FVC ratio is preserved. There is no evidence for coexisting
obstructive disease. Diffusing capacity is normal, suggesting the alveolar-capillary bed is preserved. The flow-volume curve is relatively tall and narrow, without any evidence of obstructive disease. Diagnosis: Restrictive pattern secondary to chest wall disease (kyphoscoliosis).
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

Appendix C: Arterial blood gases: Guidelines for interpretation and sample problems
The following guidelines are meant to expand on the material presented in Chapter 3 and to simplify the interpretation of arterial blood gas values. Because memorizing a “cookbook” approach can sometimes be counterproductive if the reason why the approach is being used is not clear, these guidelines are meant to supplement a basic understanding of the underlying physiologic principles.
Numerous formulas are used to assess the appropriateness of compensation for a primary acid-base disorder. These formulas are particularly useful for suggesting whether a mixed acid-base disorder is present. Table C.1 lists commonly used formulas that predict the expected degree of respiratory compensation for a primary metabolic problem and metabolic compensation for a primary respiratory problem. These formulas relate arterial PCO2 and measured HCO3−. However, measured values from arterial blood gases include arterial PCO2 and pH, not serum HCO3−. Therefore, to use the formulas in the table, one must either measure serum HCO3− (as part of serum electrolyte values) or use a value calculated from PCO2 and pH according to the Henderson-Hasselbalch equation.

Table C.1
Expected Compensation for Primary Acid-Base Disorders
Primary Disorder |
Compensatory |
Expected Magnitude of Response |
|
Response |
|||
|
|
||
Metabolic |
↓ PCO2 |
PCO2 = 1.5 × (HCO3−) + 8 ± 2 |
|
acidosis |
|
|
|
Metabolic |
↑ PCO2 |
PCO2 increases 6 mm Hg for each 10 mEq/L increase in |
|
alkalosis |
|
HCO3− |
|
|
|
|
|
Respiratory |
↑ HCO3− |
Acute: HCO3− increases 1 mEq/L for each 10 mm Hg |
|
acidosis |
|
increase in PCO2 |
|
|
|
Chronic: HCO - increases 3.5 mEq/L for each 10 mm |
|
|
|
3 |
|
|
|
Hg increase in PCO2 |
|
Respiratory |
↓ HCO3− |
Acute: HCO3− falls 2 mEq/L for each 10 mm Hg |
|
alkalosis |
|
decrease in PCO2 |
|
|
|
Chronic: HCO − falls 5 mEq/L for each 10 mm Hg |
|
|
|
3 |
|
|
|
decrease in PCO2 |
Modified from Narins, R. G., & Emmett, M. (1980). Simple and mixed acid-base disorders: A practical approach. Medicine (Baltimore), 59, 161–187. © by Williams & Wilkins, 1980.
Alternatively, one can use other guidelines relating PCO2 and pH values. Because these latter guidelines are based on direct measurements obtained with arterial blood gases—and because they are relatively easy to remember—they are used in the method outlined here. It is worth noting that formulas relating PCO2 and pH become less accurate at the extremes of PCO2 and pH values and provide only rough guidelines. The human body does not respond to physiologic disturbances with mathematical precision.
Analysis of acid-base status
1.Look at the pH value to determine the net disturbance in acid-base balance. An alkalotic pH (>7.44) indicates the presence of a primary respiratory alkalosis, a metabolic alkalosis, or both. An acidotic pH (<7.36) indicates the presence of a primary respiratory acidosis, a metabolic acidosis, or both. A normal pH (approximately 7.36-7.44) indicates normal acid-base status or a mixed disturbance (of two balancing problems).
2.Look at PCO2. A high PCO2 (>44) indicates that a respiratory acidosis is present. A low PCO2
(<36) indicates that a respiratory alkalosis is present. If the pH value moves in the appropriate direction for the PCO2 change (i.e., ↓ pH with ↑ PCO2; ↑ pH with ↓ PCO2), the respiratory disorder is the primary disturbance. If the pH value does not move in the appropriate direction for the PCO2 change, a metabolic disorder is the primary disorder.
3.When a primary respiratory disorder is present, the pH value should change approximately 0.08 units for each 10 mm Hg change in PCO2 if the process is acute. If the process is chronic, the
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

kidneys compensate (by retaining or losing HCO3−) and blunt the pH change in response to any change in PCO2. The resulting change in pH when the respiratory disorder is chronic is slightly different for acidosis versus alkalosis. With a chronic respiratory acidosis, the expected pH decrease is approximately 0.03 for each 10 mm Hg increase in PCO2. With a chronic respiratory alkalosis, the expected pH increase is approximately 0.02 for each 10 mm Hg decrease in PCO2.
4.If a pH change cannot be explained by an alteration in PCO2, a primary metabolic disturbance is present. A low pH value with a low PCO2 indicates a primary metabolic acidosis with respiratory compensation. A high pH value with a high PCO2 can indicate a primary metabolic
alkalosis with secondary suppression of respiratory drive. However, in many patients the latter pattern of a high pH value with a high PCO2 often represents a complex acid-base disturbance, such as a chronic compensated respiratory acidosis with a superimposed primary metabolic alkalosis (e.g., as a result of diuretics, vomiting, or nasogastric suction).
5.To determine whether there has been appropriate respiratory compensation for a primary metabolic disorder, a rough guideline is that PCO2 should approximate the last two digits of the
pH value. For example, a PCO2 of 25 mm Hg accompanying a pH value of 7.25 indicates appropriate respiratory compensation for a primary metabolic acidosis. However, the degree of compensatory hyperventilation (i.e., lowering of PCO2) for a metabolic acidosis tends to be more predictable than the degree of compensatory hypoventilation (i.e., CO2 retention) accompanying a metabolic alkalosis.
Analysis of oxygenation
1. When analyzing arterial PO2, first calculate alveolar PO2 according to the following equation:
For ambient air (FiO2 = 0.21), the equation can be simplified as follows: PAO2 = 150 − (1.25 × PCO2). Then calculate the alveolar-arterial O2 gradient (AaDO2), which is the difference between the calculated PAO2 and the measured PAO2: AaDO2 = PAO2 − PaO2.
2.If the patient is hypoxemic, PCO2 is elevated, and AaDO2 is normal (<15 mm Hg on ambient air in a young person, although it increases with age), hypoventilation is the cause of the hypoxemia.
3.If the patient is hypoxemic, PCO2 is normal or low, and AaDO2 is increased, either 
mismatch or shunting is present. With  mismatch, the patient’s PaO2 has a good response to administration of supplemental O2. With a true shunt, PaO2 does not rise appropriately with supplemental O2 (even 100% O2).
mismatch, the patient’s PaO2 has a good response to administration of supplemental O2. With a true shunt, PaO2 does not rise appropriately with supplemental O2 (even 100% O2).
4.If the patient is hypoxemic, PCO2 is high, and AaDO2 is increased, the patient has both hypoventilation and either  mismatch or shunt as the cause of the low PaO2.
mismatch or shunt as the cause of the low PaO2.
Sample problems
Determine the acid-base status and calculate the alveolar-arterial oxygen difference (AaDO2) for each numbered problem. All blood gases are drawn with the patient breathing room air (FiO2 = 0.21), except
as otherwise noted.
1. Room air |
Po2 = 45 mm Hg |
Pco2 = 30 mm Hg |
pH = 7.47 |
|
|
|
|
|
|
(100% O2) |
Po2 = 65 mm Hg |
Pco2 = 32 mm Hg |
pH = 7.46 |
|
|
|
|
|
|
2. Room air |
Po2 = 45 mm Hg |
Pco2 = 30 mm Hg |
pH = 7.47 |
|
|
|
|
|
|
(100% O2) |
Po2 = 560 mm Hg |
Pco2 = 32 mm Hg |
pH = 7.46 |
|
|
|
|
|
|
3. |
Po2 = 88 mm Hg |
Pco2 = 20 mm Hg |
pH = 7.55 |
|
|
|
|
|
|
4. |
Po2 = 65 mm Hg |
Pco2 = 60 mm Hg |
pH = 7.35 |
|
|
|
|
|
|
5. |
Po2 |
= 30 mm Hg |
Pco2 = 60 mm Hg |
pH = 7.35 |
|
|
|
|
|
6. |
Po2 |
= 110 mm Hg |
Pco2 = 20 mm Hg |
pH = 7.30 |
|
|
|
|
|
7. |
Po2 |
= 55 mm Hg |
Pco2 = 48 mm Hg |
pH = 7.49 |
|
|
|
|
|
8. |
Po2 |
= 90 mm Hg |
Pco2 = 60 mm Hg |
pH = 7.20 |
|
|
|
|
|
Answers
1.Acute respiratory alkalosis. On room air, the patient’s AaDO2 = 67.5 mm Hg, which is elevated. The minimal elevation in PO2 with 100% O2 indicates that a shunt is the major cause of the
hypoxemia.
2. Identical to Problem 1, except that the dramatic increase in PO2 with 100% O2 indicates that ventilation-perfusion mismatch is the major cause of the hypoxemia.
3. Acute respiratory alkalosis. Even though PO2 appears normal, AaDO2 is elevated to 37 mm Hg, indicating the presence of a disorder impairing normal oxygenation of blood.
4. Chronic respiratory acidosis. AaDO2 = 10 mm Hg, indicating that hypoxemia is due to hypoventilation.
5. Chronic respiratory acidosis, as in Problem 4. However, in contrast to Problem 4, AaDO2 is elevated (to 45 mm Hg), indicating that both hypoventilation and either ventilation-perfusion mismatch or shunting (most likely the former) are responsible for the hypoxemia.
6.Mixed acid-base disorder with a primary metabolic acidosis complicated by a primary respiratory alkalosis. PCO2 is too low to represent only compensation for the metabolic acidosis,
indicating the presence of a respiratory alkalosis as well. AaDO2 = 15 mm Hg, the upper limit of normal for a young adult.
7.The simplest explanation of the acid-base status is a compensated metabolic alkalosis. However, this pattern is probably seen more commonly with a mixed acid-base disorder consisting of a compensated respiratory acidosis complicated by a superimposed primary metabolic alkalosis. AaDO2 = 35 mm Hg. Therefore, hypoxemia is partly due to hypoventilation but mostly due to
ventilation-perfusion mismatch or shunt, probably the former.
8. Something is wrong because AaDO2 is negative (−15 mm Hg). Several possible explanations are
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
(a) the patient was receiving supplemental O2, (b) a laboratory error was made, or (c) the blood was not collected or transported properly under anaerobic conditions.
