
- •Table of Contents
- •Copyright
- •Dedication
- •Introduction to the eighth edition
- •Online contents
- •List of Illustrations
- •List of Tables
- •1. Pulmonary anatomy and physiology: The basics
- •Anatomy
- •Physiology
- •Abnormalities in gas exchange
- •Suggested readings
- •2. Presentation of the patient with pulmonary disease
- •Dyspnea
- •Cough
- •Hemoptysis
- •Chest pain
- •Suggested readings
- •3. Evaluation of the patient with pulmonary disease
- •Evaluation on a macroscopic level
- •Evaluation on a microscopic level
- •Assessment on a functional level
- •Suggested readings
- •4. Anatomic and physiologic aspects of airways
- •Structure
- •Function
- •Suggested readings
- •5. Asthma
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Treatment
- •Suggested readings
- •6. Chronic obstructive pulmonary disease
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach and assessment
- •Treatment
- •Suggested readings
- •7. Miscellaneous airway diseases
- •Bronchiectasis
- •Cystic fibrosis
- •Upper airway disease
- •Suggested readings
- •8. Anatomic and physiologic aspects of the pulmonary parenchyma
- •Anatomy
- •Physiology
- •Suggested readings
- •9. Overview of diffuse parenchymal lung diseases
- •Pathology
- •Pathogenesis
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Suggested readings
- •10. Diffuse parenchymal lung diseases associated with known etiologic agents
- •Diseases caused by inhaled inorganic dusts
- •Hypersensitivity pneumonitis
- •Drug-induced parenchymal lung disease
- •Radiation-induced lung disease
- •Suggested readings
- •11. Diffuse parenchymal lung diseases of unknown etiology
- •Idiopathic pulmonary fibrosis
- •Other idiopathic interstitial pneumonias
- •Pulmonary parenchymal involvement complicating systemic rheumatic disease
- •Sarcoidosis
- •Miscellaneous disorders involving the pulmonary parenchyma
- •Suggested readings
- •12. Anatomic and physiologic aspects of the pulmonary vasculature
- •Anatomy
- •Physiology
- •Suggested readings
- •13. Pulmonary embolism
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic evaluation
- •Treatment
- •Suggested readings
- •14. Pulmonary hypertension
- •Pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic features
- •Specific disorders associated with pulmonary hypertension
- •Suggested readings
- •15. Pleural disease
- •Anatomy
- •Physiology
- •Pleural effusion
- •Pneumothorax
- •Malignant mesothelioma
- •Suggested readings
- •16. Mediastinal disease
- •Anatomic features
- •Mediastinal masses
- •Pneumomediastinum
- •Suggested readings
- •17. Anatomic and physiologic aspects of neural, muscular, and chest wall interactions with the lungs
- •Respiratory control
- •Respiratory muscles
- •Suggested readings
- •18. Disorders of ventilatory control
- •Primary neurologic disease
- •Cheyne-stokes breathing
- •Control abnormalities secondary to lung disease
- •Sleep apnea syndrome
- •Suggested readings
- •19. Disorders of the respiratory pump
- •Neuromuscular disease affecting the muscles of respiration
- •Diaphragmatic disease
- •Disorders affecting the chest wall
- •Suggested readings
- •20. Lung cancer: Etiologic and pathologic aspects
- •Etiology and pathogenesis
- •Pathology
- •Suggested readings
- •21. Lung cancer: Clinical aspects
- •Clinical features
- •Diagnostic approach
- •Principles of therapy
- •Bronchial carcinoid tumors
- •Solitary pulmonary nodule
- •Suggested readings
- •22. Lung defense mechanisms
- •Physical or anatomic factors
- •Antimicrobial peptides
- •Phagocytic and inflammatory cells
- •Adaptive immune responses
- •Failure of respiratory defense mechanisms
- •Augmentation of respiratory defense mechanisms
- •Suggested readings
- •23. Pneumonia
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features and initial diagnosis
- •Therapeutic approach: General principles and antibiotic susceptibility
- •Initial management strategies based on clinical setting of pneumonia
- •Suggested readings
- •24. Bacterial and viral organisms causing pneumonia
- •Bacteria
- •Viruses
- •Intrathoracic complications of pneumonia
- •Respiratory infections associated with bioterrorism
- •Suggested readings
- •25. Tuberculosis and nontuberculous mycobacteria
- •Etiology and pathogenesis
- •Definitions
- •Pathology
- •Pathophysiology
- •Clinical manifestations
- •Diagnostic approach
- •Principles of therapy
- •Nontuberculous mycobacteria
- •Suggested readings
- •26. Miscellaneous infections caused by fungi, including Pneumocystis
- •Fungal infections
- •Pneumocystis infection
- •Suggested readings
- •27. Pulmonary complications in the immunocompromised host
- •Acquired immunodeficiency syndrome
- •Pulmonary complications in non–HIV immunocompromised patients
- •Suggested readings
- •28. Classification and pathophysiologic aspects of respiratory failure
- •Definition of respiratory failure
- •Classification of acute respiratory failure
- •Presentation of gas exchange failure
- •Pathogenesis of gas exchange abnormalities
- •Clinical and therapeutic aspects of hypercapnic/hypoxemic respiratory failure
- •Suggested readings
- •29. Acute respiratory distress syndrome
- •Physiology of fluid movement in alveolar interstitium
- •Etiology
- •Pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Treatment
- •Suggested readings
- •30. Management of respiratory failure
- •Goals and principles underlying supportive therapy
- •Mechanical ventilation
- •Selected aspects of therapy for chronic respiratory failure
- •Suggested readings
- •Index

Berylliosis, which resembles sarcoidosis in many respects, represents a cellular immune response to beryllium.
Berylliosis is now known to represent a cellular immune (delayed hypersensitivity) response to beryllium. Lymphocytes harvested from blood or bronchoalveolar lavage (BAL) fluid of patients with berylliosis demonstrate proliferation and transformation when exposed to beryllium salts in vitro. This “beryllium lymphocyte proliferation test” not only suggests the pathogenesis of the disease but also serves as a useful diagnostic test in individuals with a clinical picture consistent with berylliosis. In addition, sensitization to beryllium can be demonstrated in some workers before the onset of clinical disease, a finding that may be important for prevention or early intervention to arrest progression from subclinical to clinical disease.
Aspects of the pathogenesis of beryllium lung disease are still being elucidated. According to current understanding, after being inhaled, beryllium reaches the alveoli, associates with human leukocyte antigens on the surface of antigen-presenting cells (APCs), and sensitizes CD4+ lymphocytes. In the lymph nodes. APCs promote the expansion of beryllium-specific CD4+ cells, which release several cytokines, including IL-2, IFN-γ, and TNF-α. The secretion of IFN-γ and TNF-α is important in the recruitment of macrophages and the formation of granulomas. Only a minority of people exposed to beryllium develop disease. Innate variability in major histocompatibility complexes affects the response of APCs to beryllium. One form of this susceptibility is identified by the presence of glutamate in position 69 of the human leukocyte antigen DPB1 molecule.
Clinically and radiographically, the disease closely mimics sarcoidosis (see Chapter 11). Specifically, patients with berylliosis demonstrate granulomatous inflammation in the pulmonary parenchyma and intrathoracic lymph nodes. Although extrathoracic involvement can occur, it is less common than in sarcoidosis. Unlike CWP and asbestosis, in which fibrosis is the primary pathology and immunosuppression is not effective, berylliosis represents delayed hypersensitivity to beryllium. Thus, patients with symptoms and pulmonary function abnormalities due to berylliosis are often treated with systemic corticosteroids, typically oral prednisone, to suppress the immune response.
Hypersensitivity pneumonitis
In hypersensitivity pneumonitis, immunologic phenomena directed against an antigen are responsible for the production of diffuse parenchymal lung disease. This disorder is sometimes referred to as extrinsic allergic alveolitis.
The antigens that induce the series of immunologic events are inhaled particulates and aerosolized antigens from a variety of sources. Almost all the antigens are derived from microorganisms, plant proteins, and animal proteins. Exposure often is related either to the patient’s occupation or to some avocation. The first of the hypersensitivity pneumonitides to be described was farmer’s lung, which is due to antigens from microorganisms (thermophilic actinomycetes) that may be present on moldy hay. The list of antigens and types of exposure is quite extensive and includes entities such as air conditioner or humidifier lung (caused by antigens from microorganisms contaminating a forced air system) and bird breeder’s or bird fancier’s lung (attributable to avian proteins). In an entity called “hot tub lung,” the responsible antigens are from nontuberculous mycobacteria, most commonly organisms classified as Mycobacterium avium complex, that are contaminating the water in the hot tub.
Hypersensitivity pneumonitis represents an immunologic response to an inhaled organic antigen.

Interestingly, even when a large number of individuals are exposed to a given antigen by virtue of their occupation or avocation, disease develops in only a small percentage. Current understanding of the pathogenesis of hypersensitivity pneumonitis indicates that, in genetically susceptible individuals, repeated exposure to a specific environmental antigen (an “inducer”) triggers a cascade of immunologically mediated events which result in the clinical manifestations. Most of the genetic polymorphisms associated with hypersensitivity pneumonitis involve pathways associated with processing or presenting antigens.
Despite much research, we do not yet have a complete understanding of the pathogenesis of hypersensitivity pneumonitis. A first step appears to be that, in genetically susceptible individuals, repeated antigen recognition by pattern recognition receptors on APCs of the innate immune system leads to transcription of proinflammatory cytokines and interferons. A type IV immune reaction (cell-mediated or delayed hypersensitivity, mediated by T lymphocytes) causing a lymphocytic alveolitis is known to be of prime importance in producing the disease. A type III (immune complex disease) mechanism plays a contributory role, especially in acute disease. Evidence suggests that T lymphocytes in the lower respiratory tract become sensitized to the particular organic antigen. They may then release soluble cytokines that attract macrophages and possibly induce them to form granulomas in the lung. Antigenantibody immune complexes also may be involved, with binding of complement and the resulting production of chemotactic factors and activation of macrophages.
Pathologic examination of the lung in patients with hypersensitivity pneumonitis reveals an alveolitis composed primarily of lymphocytes and macrophages, as well as the presence of granulomas (Fig. 10.6). The granulomas often are loosely formed, unlike the well-defined granulomas characteristic of berylliosis and sarcoidosis (see Chapter 11). Often the pathologic changes have a peribronchiolar prominence, thus accounting for the frequent physiologic evidence for obstruction of small airways.
FIGURE 10.6 Pathology of hypersensitivity pneumonitis, showing a chronic
inflammatory process with small lymphocytes, macrophages, and poorly formed
granulomas (arrows).
Clinically, hypersensitivity pneumonitis manifests in different ways, ranging from acute episodes of
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

dyspnea, cough, fever, and infiltrates on chest radiograph (occurring approximately 4-6 hours after exposure to the offending antigen) to a chronic form of diffuse parenchymal lung disease. The latter presentation is more insidious. The patient often reports gradual onset of shortness of breath and cough, along with systemic symptoms of fatigue, loss of appetite, and weight loss. Long-term antigen exposure has been occurring in these circumstances, and, because acute episodes are not necessarily an important feature, the patient does not associate the symptoms with any particular exposure.
Hypersensitivity pneumonitis occurs either with acute episodes 4 to 6 hours after exposure to the offending antigen or with the more insidious course of chronic diffuse parenchymal lung disease.
Unlike the acute form, the chronic form of hypersensitivity pneumonitis can behave like other forms of fibrotic diffuse parenchymal lung disease, resembling and mimicking idiopathic pulmonary fibrosis. Unless the physician is attuned to the possibility that hypersensitivity to an antigen in the environment might be responsible for the patient’s lung disease, the entity may easily be missed, and exposure to the antigen may continue.
With an acute episode of hypersensitivity pneumonitis, the chest radiograph shows patchy or diffuse infiltrates. As the disease becomes chronic, the abnormality may take on a more nodular quality, eventually appearing as the reticulonodular pattern characteristic of the other chronic diffuse parenchymal lung diseases. In the chronic form of disease, an upper lobe predominance to the radiographic changes is often seen. High-resolution chest computed tomography (CT) scanning may be particularly helpful in suggesting the diagnosis, often demonstrating a mosaic ground-glass pattern (see Fig. 3.9). An important distinction, which may be suggested by the radiographic appearance, is whether or not fibrosis is present. This has led to a currently used framework that characterizes hypersensitivity pneumonitis as either fibrotic or nonfibrotic. The distinction between fibrotic and nonfibrotic hypersensitivity pneumonitis has implications relating both to prognosis and to the likelihood of improvement with removal of the antigen and response to therapy.
The diagnosis is more likely to be considered if the patient gives a history of acute episodes that either occur by themselves or punctuate a more chronic illness. Historic features concerning the patient’s occupation, hobbies, and other environmental exposures may provide valuable clues for detecting the responsible factor. One diagnostic test is a search for precipitating antibodies to the common organic antigens known to cause hypersensitivity pneumonitis. Unfortunately, the presence of antibodies indicates exposure but not necessarily disease. For example, the finding of precipitins to thermophilic actinomycetes, the agent responsible for farmer’s lung, is relatively common in healthy farmers without any evidence of the disease. In addition, supporting a diagnosis of hypersensitivity pneumonitis by the finding of precipitins requires that the responsible antigen be included in the panel of antigens tested, which is not always possible.
If a lung biopsy is performed for diagnosis of diffuse parenchymal lung disease, findings on microscopic examination typically include chronic inflammation and small, indistinct non-necrotizing granulomas, as described above.
The best treatment is avoidance of exposure. Unfortunately, the chronic form of the disease often leads to irreversible fibrotic changes in the lung that persist after exposure is terminated. For patients with severe disease or for those in whom antigen avoidance does not lead to resolution, corticosteroid administration is considered, but the results are variable. Trials with antifibrotic agents are underway in patients who develop chronic fibrotic changes.
Drug-induced parenchymal lung disease
As the list of available pharmacologic agents expands every year, so does the list of potential complications. The lung is certainly one of the target organs for these adverse effects, and diffuse parenchymal lung disease is a particularly important manifestation of drug toxicity. It is imperative that drug toxicity be considered in all patients who develop diffuse parenchymal lung disease. Each drug cannot be considered in detail here, nor can a complete list of the growing number of drugs that have been implicated be provided. However, this chapter briefly discusses the general principles of drug-induced parenchymal lung disease and the major agents responsible.
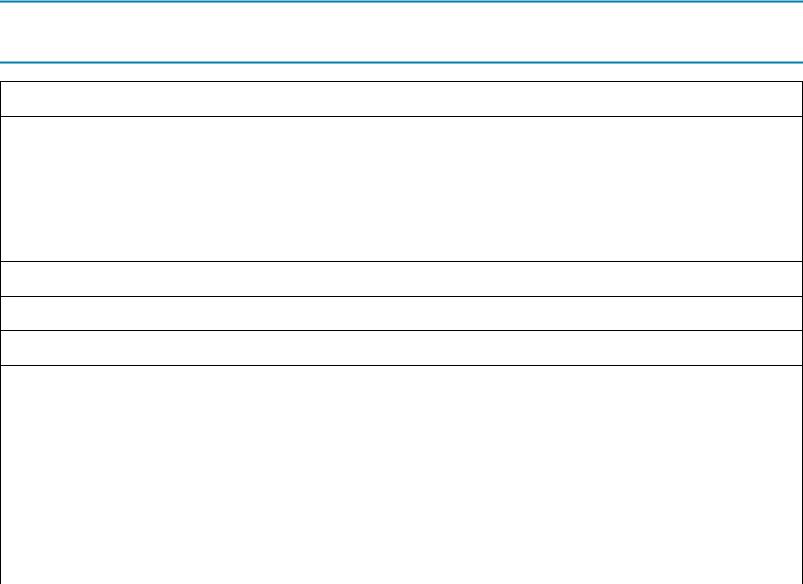
The major categories of drugs associated with disease of the alveolar wall, along with examples of each, are shown in Table 10.1. A large category includes the traditional chemotherapeutic or cytotoxic agents, drugs designed primarily as antitumor agents. Individual drugs that have been commonly implicated in the development of lung disease include bleomycin, mitomycin, busulfan, cyclophosphamide, gemcitabine, taxanes, and the nitrosoureas, although several others have been described in smaller numbers of cases. Recently, severe and potentially fatal pneumonitis has been recognized due to checkpoint inhibitor immunotherapy that targets cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) or proteins inhibiting programmed cell death (PD-1 and PD-L1). In general, the risk of developing diffuse parenchymal lung disease increases with higher cumulative doses of a particular agent, but occasional cases with relatively low cumulative doses are described. In most cases, diffuse parenchymal lung disease develops in a period ranging from 1 month to several years after use of the agent, but some agents can also be associated with the development of more acute disease. Busulfan is particularly notable for late development of complications, often several years after onset of therapy.
TABLE 10.1
Selected Drugs Potentially Causing Diffuse Parenchymal Lung Disease
Cytotoxic Chemotherapy
Bleomycin
Busulfan
Cyclophosphamide
Gemcitabine
Nitrosoureas
Taxanes (e.g., paclitaxel, docetaxel)
Antimetabolites
Methotrexate
Targeted Biologic Agents
Tumor necrosis factor (TNF)-α inhibitors or soluble receptors
Infliximab
Adalimumab
Certolizumab pegol
Etanercept
Tyrosine kinase inhibitors
Afatinib
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

Dasatinib
Erlotinib
Gefitinib
Idelalisib
Imatinib
Osimertinib
Trametinib
Immune checkpoint inhibitors (antibodies against PD-1, PD-1 ligand, or CTLA-4)
Ipilimumab
Nivolumab
Pembrolizumab
Inhibitors of anaplastic lymphoma kinase (ALK)
Alectinib
Ceritinib
Crizotinib
Miscellaneous
Trastuzumab (monoclonal antibody against HER2)
Rituximab (monoclonal antibody against CD20)
Inhibitors of mechanistic target of rapamycin (mTOR, e.g., everolimus)
Miscellaneous Other Drugs
Nitrofurantoin
Amiodarone
Drug-Induced Syndromes
Drug-induced lupus (e.g., procainamide, hydralazine)
Drug-induced pulmonary infiltrates with eosinophilia (e.g., sulfa-containing drugs)
A wide variety of different drug classes are associated with diffuse parenchymal lung disease.
The pathogenesis of chemotherapy-induced diffuse parenchymal lung disease often appears to involve either direct toxicity to normal lung parenchymal cells, especially epithelial cells, or oxidant injury induced by generation of toxic oxygen radicals. When oxidant damage is involved, as with bleomycin, other agents that promote formation of oxygen free radicals (e.g., radiation therapy, high concentrations of inhaled oxygen) can augment the injury caused by the chemotherapeutic agent.
The pathologic appearance of diffuse parenchymal lung disease caused by cytotoxic agents frequently is notable for the presence of atypical bizarre-appearing type II alveolar epithelial cells with large hyperchromatic nuclei. When this feature is associated with the other usual findings of diffuse parenchymal lung disease, the pathologist should suspect that a chemotherapeutic agent may be responsible.

Cytotoxic drug–induced diffuse parenchymal lung disease shows atypical bizarre-appearing alveolar type II epithelial cells.
Methotrexate, an antimetabolite affecting folic acid metabolism, is used in low doses for treatment of rheumatoid arthritis and other rheumatologic diseases, and in higher doses as an antineoplastic agent, especially for treatment of hematologic malignancies. A hypersensitivity mechanism appears to play an important role in the pathogenesis of methotrexate pneumonitis, as evidenced by the frequent presence of granulomas on pathology.
Biologic agents, a large and rapidly increasing category of drugs developed from biologic sources and often involving use of recombinant gene technology, are commonly monoclonal antibodies or other inhibitors targeted against cytokines and a variety of signaling pathways. In addition to treatment of cancer, some of these agents are used for the treatment of systemic inflammatory or immune-related diseases. Although the frequency of pulmonary toxicity with most of these agents is quite low, the possibility of a drug-related complication should be considered in any patient on one of these agents who develops parenchymal lung disease.
Several drugs that are not chemotherapeutic or biologic agents have been implicated in the development of parenchymal lung disease. Nitrofurantoin, an antibiotic, has been associated with both acute and chronic reactions. The acute problem, which presumably is a hypersensitivity phenomenon, often is characterized by pulmonary infiltrates, pleural effusions, fever, and eosinophilia in peripheral blood. The chronic problem, which does not appear to be related to prior acute episodes, is characterized by a nonspecific interstitial pneumonitis and fibrosis akin to that of the other diffuse parenchymal lung diseases.
The commonly used antiarrhythmic agent amiodarone is associated with clinically significant parenchymal lung disease in approximately 1% to 5% of treated patients. Amiodarone pulmonary toxicity is dose-related and may be fatal. In addition to nonspecific inflammation and fibrosis, the pathologic appearance of amiodarone-induced diffuse parenchymal lung disease is notable for macrophages that appear foamy because of cytoplasmic phospholipid inclusions. However, similar foamy macrophages with cytoplasmic inclusions may be seen in lung tissue from amiodarone-treated patients without interstitial inflammation and fibrosis. This finding suggests that the phospholipid inclusions are a marker of amiodarone use but are not necessarily directly responsible for the other pathologic and clinically important pulmonary consequences of amiodarone. Radiographically, patients with amiodarone-induced lung disease can develop either focal or diffuse infiltrates. CT scanning commonly shows a relatively high density of the infiltrates, resulting from a high iodine content within the amiodarone molecule.
Amiodarone-induced lung disease is an important cause of either focal or diffuse pulmonary infiltrates.
A large number of drugs have been linked with development of an illness that resembles systemic lupus erythematosus, and patients with this “drug-induced lupus” may have parenchymal lung disease as one manifestation. In addition, a variety of drugs have been associated with pulmonary infiltrates and peripheral blood eosinophilia. This constellation of pulmonary infiltrates with eosinophilia, of which drugs are just one of several possible causes, is often abbreviated as the PIE syndrome.
Clinically, fever is a common accompaniment to the respiratory symptoms associated with druginduced diffuse parenchymal lung disease. An increase in eosinophils in peripheral blood is often noted in patients with methotrexate-induced lung disease and is characteristic of patients with the PIE syndrome.
When pulmonary infiltrates develop in patients with malignancy or anyone receiving a drug associated with suppression of the immune response, several diagnostic considerations arise, especially when the
clinical presentation is accompanied by fever. In addition to the possibility of drug toxicity, there is
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
