
- •Table of Contents
- •Copyright
- •Dedication
- •Introduction to the eighth edition
- •Online contents
- •List of Illustrations
- •List of Tables
- •1. Pulmonary anatomy and physiology: The basics
- •Anatomy
- •Physiology
- •Abnormalities in gas exchange
- •Suggested readings
- •2. Presentation of the patient with pulmonary disease
- •Dyspnea
- •Cough
- •Hemoptysis
- •Chest pain
- •Suggested readings
- •3. Evaluation of the patient with pulmonary disease
- •Evaluation on a macroscopic level
- •Evaluation on a microscopic level
- •Assessment on a functional level
- •Suggested readings
- •4. Anatomic and physiologic aspects of airways
- •Structure
- •Function
- •Suggested readings
- •5. Asthma
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Treatment
- •Suggested readings
- •6. Chronic obstructive pulmonary disease
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach and assessment
- •Treatment
- •Suggested readings
- •7. Miscellaneous airway diseases
- •Bronchiectasis
- •Cystic fibrosis
- •Upper airway disease
- •Suggested readings
- •8. Anatomic and physiologic aspects of the pulmonary parenchyma
- •Anatomy
- •Physiology
- •Suggested readings
- •9. Overview of diffuse parenchymal lung diseases
- •Pathology
- •Pathogenesis
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Suggested readings
- •10. Diffuse parenchymal lung diseases associated with known etiologic agents
- •Diseases caused by inhaled inorganic dusts
- •Hypersensitivity pneumonitis
- •Drug-induced parenchymal lung disease
- •Radiation-induced lung disease
- •Suggested readings
- •11. Diffuse parenchymal lung diseases of unknown etiology
- •Idiopathic pulmonary fibrosis
- •Other idiopathic interstitial pneumonias
- •Pulmonary parenchymal involvement complicating systemic rheumatic disease
- •Sarcoidosis
- •Miscellaneous disorders involving the pulmonary parenchyma
- •Suggested readings
- •12. Anatomic and physiologic aspects of the pulmonary vasculature
- •Anatomy
- •Physiology
- •Suggested readings
- •13. Pulmonary embolism
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic evaluation
- •Treatment
- •Suggested readings
- •14. Pulmonary hypertension
- •Pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic features
- •Specific disorders associated with pulmonary hypertension
- •Suggested readings
- •15. Pleural disease
- •Anatomy
- •Physiology
- •Pleural effusion
- •Pneumothorax
- •Malignant mesothelioma
- •Suggested readings
- •16. Mediastinal disease
- •Anatomic features
- •Mediastinal masses
- •Pneumomediastinum
- •Suggested readings
- •17. Anatomic and physiologic aspects of neural, muscular, and chest wall interactions with the lungs
- •Respiratory control
- •Respiratory muscles
- •Suggested readings
- •18. Disorders of ventilatory control
- •Primary neurologic disease
- •Cheyne-stokes breathing
- •Control abnormalities secondary to lung disease
- •Sleep apnea syndrome
- •Suggested readings
- •19. Disorders of the respiratory pump
- •Neuromuscular disease affecting the muscles of respiration
- •Diaphragmatic disease
- •Disorders affecting the chest wall
- •Suggested readings
- •20. Lung cancer: Etiologic and pathologic aspects
- •Etiology and pathogenesis
- •Pathology
- •Suggested readings
- •21. Lung cancer: Clinical aspects
- •Clinical features
- •Diagnostic approach
- •Principles of therapy
- •Bronchial carcinoid tumors
- •Solitary pulmonary nodule
- •Suggested readings
- •22. Lung defense mechanisms
- •Physical or anatomic factors
- •Antimicrobial peptides
- •Phagocytic and inflammatory cells
- •Adaptive immune responses
- •Failure of respiratory defense mechanisms
- •Augmentation of respiratory defense mechanisms
- •Suggested readings
- •23. Pneumonia
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features and initial diagnosis
- •Therapeutic approach: General principles and antibiotic susceptibility
- •Initial management strategies based on clinical setting of pneumonia
- •Suggested readings
- •24. Bacterial and viral organisms causing pneumonia
- •Bacteria
- •Viruses
- •Intrathoracic complications of pneumonia
- •Respiratory infections associated with bioterrorism
- •Suggested readings
- •25. Tuberculosis and nontuberculous mycobacteria
- •Etiology and pathogenesis
- •Definitions
- •Pathology
- •Pathophysiology
- •Clinical manifestations
- •Diagnostic approach
- •Principles of therapy
- •Nontuberculous mycobacteria
- •Suggested readings
- •26. Miscellaneous infections caused by fungi, including Pneumocystis
- •Fungal infections
- •Pneumocystis infection
- •Suggested readings
- •27. Pulmonary complications in the immunocompromised host
- •Acquired immunodeficiency syndrome
- •Pulmonary complications in non–HIV immunocompromised patients
- •Suggested readings
- •28. Classification and pathophysiologic aspects of respiratory failure
- •Definition of respiratory failure
- •Classification of acute respiratory failure
- •Presentation of gas exchange failure
- •Pathogenesis of gas exchange abnormalities
- •Clinical and therapeutic aspects of hypercapnic/hypoxemic respiratory failure
- •Suggested readings
- •29. Acute respiratory distress syndrome
- •Physiology of fluid movement in alveolar interstitium
- •Etiology
- •Pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Treatment
- •Suggested readings
- •30. Management of respiratory failure
- •Goals and principles underlying supportive therapy
- •Mechanical ventilation
- •Selected aspects of therapy for chronic respiratory failure
- •Suggested readings
- •Index

stimulation of the irritant receptors.
Respiratory tract infection
Respiratory tract infection is an exacerbating factor for patients with either nonallergic or allergic asthma. Viral infections are the most common causes in this category, but bacterial infections of the tracheobronchial tree also can be implicated. The mechanism by which respiratory infections precipitate bronchoconstriction in persons with asthma is not entirely clear but is likely related to epithelial damage and airway inflammation. Potential consequences of epithelial injury include release of mediators from inflammatory cells, stimulation of irritant receptors, and nonspecific bronchial hyperresponsiveness.
Exercise
Exercise can frequently provoke bronchoconstriction in patients with hyperreactive airways. The crucial factor in the pathogenesis appears to be heat movement from the airway wall, resulting in cooling and drying of the airway. During exercise, individuals have a high minute ventilation, and the large amounts of relatively cool and dry inspired air must be warmed and humidified by the tracheobronchial mucosa. When the air is warmed and humidified, water evaporates from the epithelial surface, resulting in cooling and drying of the airway epithelium. The phenomenon of exercise-induced bronchoconstriction can be reproduced by having a person with asthma voluntarily breathe cold dry air at a high minute ventilation. Inhalation of warm saturated air at the same minute ventilation does not produce a similar effect. The mechanism that links airway cooling and drying with bronchoconstriction is less clear. Alteration of the ionic environment after drying of the mucosa, mediator release, hyperemia of the mucosa following airway rewarming, and stimulation of irritant receptors have all been proposed as mechanisms, but none is universally accepted.
Airway cooling and drying are important in exercise-induced bronchoconstriction.
As might be expected from the description of exercise-induced bronchoconstriction, inhalation of cold air during the winter months can cause asthma exacerbations or worsening of symptoms in selected patients. The mechanism of airway narrowing in these patients following inhalation of cold air is also believed to be due to airway cooling and drying and is therefore analogous to the mechanism of exerciseinduced bronchoconstriction.
Pathology
Pathologic findings in asthma traditionally have been described from autopsy studies and thus represent the consequences of particularly severe disease. In these cases, marked overdistention of the lungs is seen, and the airways are occluded by thick, tenacious mucous plugs. However, information regarding the histologic appearance of airways in patients with stable mild disease more recently has become available. Examination of the airways by microscopy demonstrates the following findings of variable severity that are apparent in both mild and more severe disease:
1.Edema and cellular infiltrates within the bronchial wall, especially with eosinophils and lymphocytes
2.Epithelial damage, with a “fragile” appearance of the epithelium and detachment of surface epithelial cells from basal cells
3.Hypertrophy and hyperplasia of the smooth muscle layer
4.Increased deposition of collagen in a layer beneath the epithelium (referred to in the past as

basement membrane thickening)
5.Enlargement of the mucus-secreting apparatus, with hypertrophy of mucous glands and an increased number of goblet cells
As described earlier in this chapter, the presence of histologic abnormalities presumably contributes to the nonspecific bronchial hyperresponsiveness in patients, even when they are free of an acute attack. In addition to the bronchial hyperresponsiveness that results from airway inflammation and remodeling, the more long-standing structural changes that characterize airway remodeling contribute to the component of persistent airflow obstruction that can be seen in some patients with longstanding asthma.
Pathophysiology
The pathophysiologic features of asthma largely follow from the pathologic abnormalities. Contraction of smooth muscle in the bronchial walls, mucosal and submucosal inflammation and edema, and secretions within the airway lumen all contribute to decreased airway diameter, which increases airway resistance. Pathologic changes are present at many levels of the tracheobronchial tree, from large airways down to peripheral airways less than 2 mm in diameter.
As a result of narrowed airways with increased resistance, patients have difficulty with airflow during both inspiration and expiration. However, because intrathoracic airways are subjected to relatively negative external pressure (transmitted from negative pleural pressure) during inspiration, lumen size is larger during the inspiratory phase of the respiratory cycle. During expiration, relatively positive pleural pressure is transmitted to intrathoracic airways, thus decreasing their diameter. Therefore, greater difficulty with airflow on expiration than on inspiration is characteristic of asthma, as it is of any of the diseases that cause obstruction or narrowing of airways within the thorax. The greatest difficulty with expiration occurs when the patient is asked to perform a forced expiration (i.e., breathe out as hard and fast as possible). With forced expiration, pleural pressure becomes much more positive, thereby promoting airway narrowing, closure, and air trapping.
In asthma and other diseases associated with obstruction of intrathoracic airways, airflow is most compromised during expiration.
The effects of increased airway resistance are readily seen by measuring pulmonary function in persons with asthma. Pulmonary function studies performed during an attack show decreases in forced expiratory flow rates and evidence of air trapping. On the forced expiratory spirogram, patients typically exhibit a decrease in both forced vital capacity (FVC) and FEV1, with the decrease in FEV1 more pronounced than the decrease in FVC. Hence, the ratio FEV1/FVC, which reflects the proportion of FVC that can be exhaled during the first second, is decreased. In addition, the maximal midexpiratory flow rate (also called the forced expiratory flow between 25% and 75% of the vital capacity [FEF25%–75%]) is diminished.
Pulmonary function tests in patients with asthma generally demonstrate decreased FEV1, FVC, and FEV1/FVC ratio. Air trapping is demonstrated by increases in RV and measured FRC.
Measurement of lung volumes during an exacerbation shows evidence of air trapping, with an increase in residual volume (RV) and functional residual capacity (FRC) as determined by plethysmography. The most impressive increase is seen in RV, the volume left in the lungs at the end of a maximal exhalation,
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
which may be greater than 200% of the predicted value. The increase in RV is believed to be due to premature small airway closure with expiration as a result of smooth muscle constriction, mucous plugs, and inflammatory changes of the mucosa.
FRC, the resting point of the lungs at the end of expiration, is increased in the setting of an asthma flare due to air trapping. In addition, patients may experience dynamic hyperinflation because more time is required for expiration when airways are obstructed, and patients may not have sufficient time before the next breath to fully exhale the volume from the previous breath. Dynamic hyperinflation is a particular problem when the person with asthma is breathing at a rapid respiratory rate. During an asthma exacerbation, it is also hypothesized that patients have persistent activity of the inspiratory muscles during expiration, maintaining lung volume at a level higher than expected throughout expiration. A physiologic advantage to breathing at higher-than-normal lung volumes is having airways held open at a greater diameter. A disadvantage is increased work of breathing due to reduced compliance of the respiratory system at higher lung volumes (see Fig. 1.3C) and a mechanical disadvantage for diaphragmatic function when the diaphragm is lower and flatter (see Mechanisms of Abnormal Gas Exchange in Chapter 6).
The focus so far has been on pulmonary function and physiologic abnormalities seen with a typical asthmatic attack. Between attacks, pulmonary function, as measured by FEV1 and FVC, often returns to normal. However, even when a person is not having an acute attack, subtle abnormalities in pulmonary function may be present, such as a decrease in maximal midexpiratory flow rate and a mild increase in RV. These abnormalities may reflect some residual disease in the small airways of the lung, frequently the last region to become normal after an attack.
A subgroup of persons with asthma, generally those with long-standing disease, have pulmonary function that does not return fully to normal. Instead, they have demonstrable physiologic abnormalities (e.g., abnormal FEV1 and FVC) that persist between attacks. Even though asthma is generally characterized by reversible episodes of airflow obstruction, these individuals also appear to have a component of irreversible disease, particularly after decades with asthma. Nevertheless, they still generally experience episodes of reversible airflow obstruction and worsening of expiratory flow rates superimposed upon whatever irreversible disease is present.
The increased resistance to airflow in asthma exerts a toll on gas exchange, which is generally disturbed during acute attacks. The most common pattern of arterial blood gases consists of a low PO2 accompanied by a low PCO2 (respiratory alkalosis). The mechanism for the hypoxemia is ventilationperfusion mismatch. The increased airway resistance in asthma is not evenly distributed, such that some airways are affected more than others. Therefore, inspired air is not distributed evenly but tends to go to less diseased areas. However, blood flow remains relatively preserved in the regions that are ventilating poorly due to the suppression of normal hypoxic vasoconstriction by inflammatory mediators. The regions
of low ventilation-perfusion (  ) ratio contribute blood with a low PO2 that cannot be compensated for
) ratio contribute blood with a low PO2 that cannot be compensated for
by increases in the  ratio from other regions of the lung (see Chapter 1).
ratio from other regions of the lung (see Chapter 1).
Patients are typically able to hyperventilate if an acute asthma attack is not too severe, and PCO2 is usually low. The stimulus or mechanism for the hyperventilation is not clear. During an acute attack, activation of irritant receptors may stimulate ventilation, or other reflexes originating in the airways, lung, or chest wall may stimulate ventilation. PCO2 that increases to either a normal or a frankly elevated level often indicates worsening airflow obstruction or fatigue of the respiratory muscles in a tiring individual who is no longer able to maintain normal or high minute ventilation in the face of significant airflow obstruction. Thus, the clinician should view a normal or high PCO2 as a potentially serious warning sign of progressive respiratory failure.

The most common pattern of arterial blood gases in asthma is low PO2 (due primarily to  mismatch) and low PCO2.
mismatch) and low PCO2.
Clinical features
Asthma is clinically recognized most frequently during childhood and young adulthood, although asthma can develop for the first time in older patients. In many patients, particularly those in whom asthma started before age 16 years, the disease eventually regresses, and patients are no longer subject to repeated episodes of reversible airway obstruction.
The symptoms most commonly noted by patients during an exacerbation of asthma are cough, dyspnea, wheezing, and chest tightness. However, some patients may only have unexplained cough or breathlessness on exertion. At times, patients can clearly identify a precipitating factor for an attack, such as exposure to an allergen, respiratory tract infection, exercise, exposure to cold air, emotional stress, or exposure to irritating dusts, fumes, or odors. In other circumstances, no precipitant can be identified. Exposures in the workplace, related to proteins or other chemicals to which the patient may be sensitized, are important precipitants in a subgroup of patients who are said to have occupational asthma. Some persons with asthma are particularly sensitive to ingestion of aspirin, which is believed to favor production of leukotrienes from arachidonic acid. Some patients with aspirin sensitivity also have nasal polyps, leading to a well-recognized triad of asthma, aspirin sensitivity, and nasal polyposis (sometimes referred to as triad asthma, Samter syndrome, or aspirin-exacerbated respiratory disease). Other NSAIDs (which also inhibit the cyclooxygenase enzyme) can also produce bronchoconstriction in patients who are aspirin sensitive.
Major symptoms during an acute asthma attack are as follows:
1.Cough
2.Dyspnea
3.Wheezing
4.Chest tightness
On physical examination, patients experiencing an asthma attack usually have tachypnea and, on chest auscultation, prolonged expiration and evidence of wheezing. Wheezing is more prominent during expiration than during inspiration and may be triggered by having the patient exhale forcefully. Although the tendency is to equate wheezing and asthma, the presence of wheezing does not necessarily indicate a diagnosis of asthma. Wheezing only reflects airflow through narrowed airways and is not synonymous with asthma; it can also be seen in such diverse disorders as congestive heart failure and COPD or in the case of a foreign body in the airway. On the other hand, not all persons with asthma wheeze. It is a common observation that severe asthma may be associated with no wheeze at all if airflow is too impaired to generate an audible wheeze.
Despite its prominence, the presence of wheezing is not synonymous with asthma and merely reflects turbulent airflow through narrowed airways.
During a particularly severe attack that is refractory to initial treatment with bronchodilators, persons with asthma are said to be in status asthmaticus. These patients present difficult therapeutic challenges,
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

may require assisted ventilation, and may even die as a result of the acute attack.
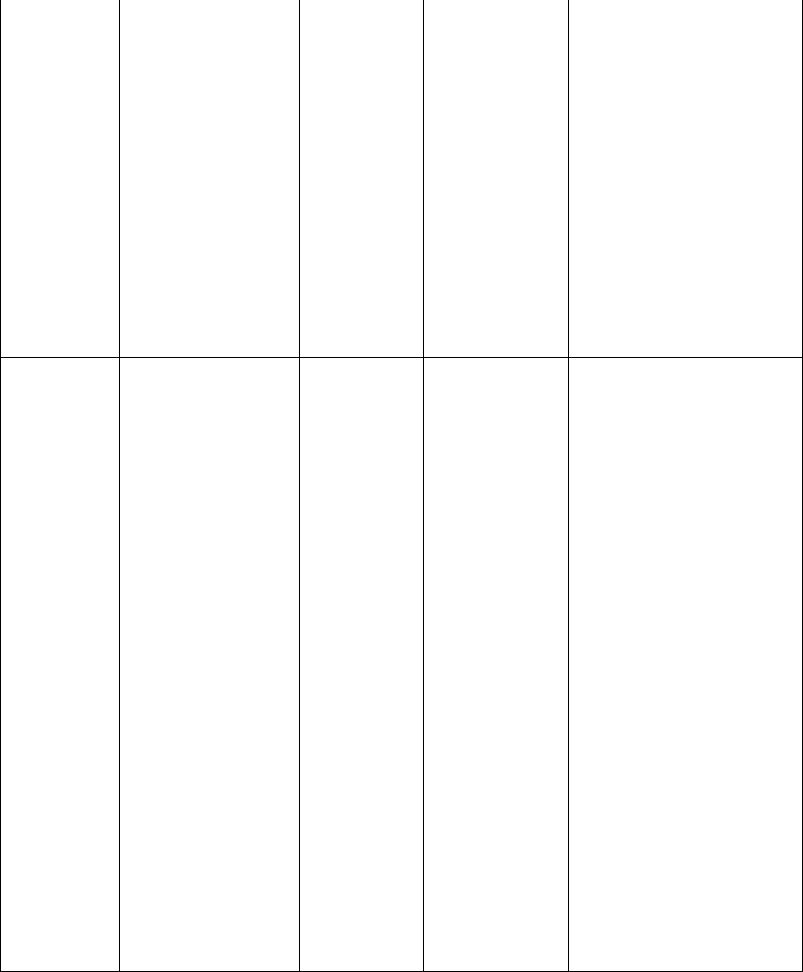
The overall severity of an individual’s asthma can be characterized based on the frequency of exacerbations, nocturnal symptoms, and magnitude of abnormality and variability in pulmonary function. The features used to define four categories of severity (intermittent asthma, mild persistent asthma, moderate persistent asthma, and severe persistent asthma) are listed in Table 5.3.
TABLE 5.3
Classification of Asthma by Severity: Clinical Aspects and Treatment
Asthma |
Clinical Features |
Nighttime |
Lung Function |
Treatment |
||
Severity |
Before Treatment |
Symptoms |
||||
|
|
|
||||
Intermittent |
• Symptoms no |
• No |
• FEV1 > |
|
• Combination |
|
|
|
|||||
|
more than |
more |
80% |
|
glucocorticoid/β2- |
|
|
twice per |
than |
predicted |
|
agonist inhaler as |
|
|
week |
twice |
• |
|
needed |
|
|
• No |
per |
FEV1/FVC |
|
Alternative: Inhaled |
|
|
interference |
month |
normal |
|
short-acting β2- |
|
|
with normal |
|
|
|
agonist as needed |
|
|
activity |
|
|
|
|
|
|
• Exacerbations |
|
|
|
|
|
|
brief |
|
|
|
|
|
|
|
|
|
|
|
|
Mild |
• Symptoms > |
• 3 or 4 |
• FEV1 or |
|
• As needed use of a |
|
persistent |
|
|||||
|
twice per |
times |
PEFR > |
|
combination inhaled |
|
|
week but < |
per |
80% |
|
corticosteroid/long- |
|
|
once per day |
month |
predicted |
|
acting β2-agonist |
|
|
• Minor |
|
• |
|
Alternative: Regular |
|
|
limitation |
|
FEV1/FVC |
|
use of an inhaled |
|
|
with normal |
|
normal |
|
corticosteroid |
|
|
activity |
|
|
|
Alternative: |
|
|
|
|
|
|
Leukotriene |
|
|
|
|
|
|
modifier, |
|
|
|
|
|
|
cromoglycate, or |
|
|
|
|
|
|
sustained-release |
|
|
|
|
|
|
theophylline |
|
|
|
|
|
|
As needed inhaled |
|
|
|
|
|
|
combination |
|
|
|
|
|
|
glucocorticoid/β2- |
|
|
|
|
|
|
agonist or short- |
|
|
|
|
|
|
acting β2-agonist |
|
|
|
|
|
|
if an alternative is |
|
|
|
|
|
|
used |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Moderate |
• Daily |
• More |
• FEV1 > |
• Low-dose inhaled |
persistent |
symptoms |
than |
60% but |
corticosteroid plus |
|
• Daily use of |
once |
< 80% |
long-acting β2- |
|
inhaled short- |
per |
predicted |
agonist |
|
acting β2- |
week |
• |
In addition, inhaled |
|
agonist |
but not |
FEV1/FVC |
combination |
|
• Some |
nightly |
reduced |
glucocorticoid/β2- |
|
limitation |
|
5% |
agonist or short- |
|
with normal |
|
|
acting β2-agonist |
|
activity |
|
|
as needed |
|
• Exacerbations |
|
|
|
|
at least twice |
|
|
|
|
per week; |
|
|
|
|
may last days |
|
|
|
Severe |
• Continual |
• Often |
• FEV1 < |
• Mediumor high- |
persistent |
||||
|
symptoms |
nightly |
60% |
dose inhaled |
|
• Extremely |
|
predicted |
corticosteroid plus |
|
limited with |
|
• |
long-acting β2- |
|
normal |
|
FEV1/FVC |
agonist |
|
activity |
|
reduced |
• Consider addition of |
|
• Frequent |
|
> 5% |
leukotriene modifier, |
|
exacerbations |
|
|
cromoglycate, |
|
|
|
|
sustained-release |
|
|
|
|
theophylline, inhaled |
|
|
|
|
long-acting |
|
|
|
|
anticholinergic |
|
|
|
|
agent, or macrolide |
|
|
|
|
antibiotic |
|
|
|
|
• Consider use of a |
|
|
|
|
biologic agent |
|
|
|
|
• Consider short |
|
|
|
|
course of oral |
|
|
|
|
corticosteroid |
|
|
|
|
• In addition, inhaled |
|
|
|
|
combination |
|
|
|
|
glucocorticoid/β2- |
|
|
|
|
agonist inhaler or |
|
|
|
|
short-acting β2- |
|
|
|
|
agonist as needed |
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PEFR, peak expiratory flow rate.
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
