
- •Table of Contents
- •Copyright
- •Dedication
- •Introduction to the eighth edition
- •Online contents
- •List of Illustrations
- •List of Tables
- •1. Pulmonary anatomy and physiology: The basics
- •Anatomy
- •Physiology
- •Abnormalities in gas exchange
- •Suggested readings
- •2. Presentation of the patient with pulmonary disease
- •Dyspnea
- •Cough
- •Hemoptysis
- •Chest pain
- •Suggested readings
- •3. Evaluation of the patient with pulmonary disease
- •Evaluation on a macroscopic level
- •Evaluation on a microscopic level
- •Assessment on a functional level
- •Suggested readings
- •4. Anatomic and physiologic aspects of airways
- •Structure
- •Function
- •Suggested readings
- •5. Asthma
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Treatment
- •Suggested readings
- •6. Chronic obstructive pulmonary disease
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach and assessment
- •Treatment
- •Suggested readings
- •7. Miscellaneous airway diseases
- •Bronchiectasis
- •Cystic fibrosis
- •Upper airway disease
- •Suggested readings
- •8. Anatomic and physiologic aspects of the pulmonary parenchyma
- •Anatomy
- •Physiology
- •Suggested readings
- •9. Overview of diffuse parenchymal lung diseases
- •Pathology
- •Pathogenesis
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Suggested readings
- •10. Diffuse parenchymal lung diseases associated with known etiologic agents
- •Diseases caused by inhaled inorganic dusts
- •Hypersensitivity pneumonitis
- •Drug-induced parenchymal lung disease
- •Radiation-induced lung disease
- •Suggested readings
- •11. Diffuse parenchymal lung diseases of unknown etiology
- •Idiopathic pulmonary fibrosis
- •Other idiopathic interstitial pneumonias
- •Pulmonary parenchymal involvement complicating systemic rheumatic disease
- •Sarcoidosis
- •Miscellaneous disorders involving the pulmonary parenchyma
- •Suggested readings
- •12. Anatomic and physiologic aspects of the pulmonary vasculature
- •Anatomy
- •Physiology
- •Suggested readings
- •13. Pulmonary embolism
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic evaluation
- •Treatment
- •Suggested readings
- •14. Pulmonary hypertension
- •Pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic features
- •Specific disorders associated with pulmonary hypertension
- •Suggested readings
- •15. Pleural disease
- •Anatomy
- •Physiology
- •Pleural effusion
- •Pneumothorax
- •Malignant mesothelioma
- •Suggested readings
- •16. Mediastinal disease
- •Anatomic features
- •Mediastinal masses
- •Pneumomediastinum
- •Suggested readings
- •17. Anatomic and physiologic aspects of neural, muscular, and chest wall interactions with the lungs
- •Respiratory control
- •Respiratory muscles
- •Suggested readings
- •18. Disorders of ventilatory control
- •Primary neurologic disease
- •Cheyne-stokes breathing
- •Control abnormalities secondary to lung disease
- •Sleep apnea syndrome
- •Suggested readings
- •19. Disorders of the respiratory pump
- •Neuromuscular disease affecting the muscles of respiration
- •Diaphragmatic disease
- •Disorders affecting the chest wall
- •Suggested readings
- •20. Lung cancer: Etiologic and pathologic aspects
- •Etiology and pathogenesis
- •Pathology
- •Suggested readings
- •21. Lung cancer: Clinical aspects
- •Clinical features
- •Diagnostic approach
- •Principles of therapy
- •Bronchial carcinoid tumors
- •Solitary pulmonary nodule
- •Suggested readings
- •22. Lung defense mechanisms
- •Physical or anatomic factors
- •Antimicrobial peptides
- •Phagocytic and inflammatory cells
- •Adaptive immune responses
- •Failure of respiratory defense mechanisms
- •Augmentation of respiratory defense mechanisms
- •Suggested readings
- •23. Pneumonia
- •Etiology and pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features and initial diagnosis
- •Therapeutic approach: General principles and antibiotic susceptibility
- •Initial management strategies based on clinical setting of pneumonia
- •Suggested readings
- •24. Bacterial and viral organisms causing pneumonia
- •Bacteria
- •Viruses
- •Intrathoracic complications of pneumonia
- •Respiratory infections associated with bioterrorism
- •Suggested readings
- •25. Tuberculosis and nontuberculous mycobacteria
- •Etiology and pathogenesis
- •Definitions
- •Pathology
- •Pathophysiology
- •Clinical manifestations
- •Diagnostic approach
- •Principles of therapy
- •Nontuberculous mycobacteria
- •Suggested readings
- •26. Miscellaneous infections caused by fungi, including Pneumocystis
- •Fungal infections
- •Pneumocystis infection
- •Suggested readings
- •27. Pulmonary complications in the immunocompromised host
- •Acquired immunodeficiency syndrome
- •Pulmonary complications in non–HIV immunocompromised patients
- •Suggested readings
- •28. Classification and pathophysiologic aspects of respiratory failure
- •Definition of respiratory failure
- •Classification of acute respiratory failure
- •Presentation of gas exchange failure
- •Pathogenesis of gas exchange abnormalities
- •Clinical and therapeutic aspects of hypercapnic/hypoxemic respiratory failure
- •Suggested readings
- •29. Acute respiratory distress syndrome
- •Physiology of fluid movement in alveolar interstitium
- •Etiology
- •Pathogenesis
- •Pathology
- •Pathophysiology
- •Clinical features
- •Diagnostic approach
- •Treatment
- •Suggested readings
- •30. Management of respiratory failure
- •Goals and principles underlying supportive therapy
- •Mechanical ventilation
- •Selected aspects of therapy for chronic respiratory failure
- •Suggested readings
- •Index

surgically. However, when surgery is performed, usually a lobe and sometimes an entire lung must be removed. Because patients are generally smokers, they are at high risk for having significant underlying chronic obstructive pulmonary disease, and they may not tolerate removal of a substantial amount of lung tissue. Spirometry is helpful as the initial step in determining whether a patient is a suitable surgical candidate. If the results of spirometry are marginal and do not clearly define whether the patient will be able to tolerate lung resection, other tests are used to determine exercise tolerance or the relative amount of function contributed by the area of lung to be removed.
Assessment of pulmonary function helps to determine whether surgical resection can be tolerated by the functionally compromised patient.
Screening for lung cancer
Given that the likelihood of curing lung cancer through surgical resection is greatest when the lesion has not yet metastasized to lymph nodes or distant sites, it seems logical that screening high-risk individuals for early, small lesions that have not yet caused symptoms would improve overall survival. In the past, controlled studies of sputum cytology and chest radiography in a high-risk population of smokers were not able to demonstrate improved mortality from lung cancer with use of these screening techniques. However, in a major change from previous recommendations, based on the results of the National Lung Screening Trial (NSLT), lung cancer screening with low-dose computed tomography (LDCT) of the chest is now recommended for high-risk individuals. NSLT randomized over 50,000 patients at high risk for lung cancer to screening with either LDCT or standard chest radiographs. The trial was stopped early when a 20% mortality reduction was detected in the LDCT group. Screening with LDCT is currently recommended by the US Preventive Services Task Force and other organizations for adults aged 50 to 80 years with a greater than 20 pack-year history of smoking who are still smoking or have quit less than 15 years prior.
However, there are additional considerations that are essential for both clinicians and patients to understand. It is important to note that almost 40% of patients in the LDCT group had a false-positive finding—that is, a lesion found on screening that proved not to be a lung cancer. Of all lesions found, 96% were not lung cancers. The diagnosis of lung cancer requires microscopic evaluation of tissue samples; thus, many patients were subjected to psychological distress and invasive procedures only to discover the lesion was benign. Another potential consequence is overdiagnosis—this refers to the possibility that a lung cancer is discovered that is slow-growing and would otherwise not influence mortality. It is hoped that ongoing studies to refine the criteria for screening and improve the diagnostic techniques will minimize any disadvantages of lung cancer screening.
Principles of therapy
The choice of treatment for lung cancer depends on functional status, cell type, staging, and the molecular characteristics of the tumor. In general, treatment algorithms start with the distinction between SCLC and NSCLC. With recent advances in understanding of the molecular biology of lung cancer, the development of targeted therapy for some tumors, and the use of immunotherapy with ICI, the approach to treatment of patients with lung cancer is continuously evolving.
The five major forms of treatment available for lung cancer are surgery, radiation therapy, chemotherapy, molecularly targeted therapy directed toward driver mutations, and immunotherapy. In addition to the patient’s functional status and the presence or absence of comorbid disease, four primary tumor-related factors determine how a particular tumor should be treated: its staging (i.e., size, location,
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
and extent of spread), its cell type, the presence of “driver mutations” (genetic mutations within tumor cells that drive cell growth and can be targeted by specific inhibitors), and the extent of PD-L1 expression (which correlates with the expected response to treatment with ICI).
Treatment of non–small-cell lung cancer
As long as a patient has sufficient pulmonary function reserve, surgery remains the treatment of choice for localized NSCLC regardless of the presence or absence of activating mutations such as EGFR. Complete resection of the entire tumor, usually by removing the entire lobe with the tumor (lobectomy), affords the best chance for cure. Thus, patients with stages I, II, and IIIA are considered for surgery with curative intent. For patients with stage I or II disease who decline surgery or are considered inoperable because of comorbid disease or limited pulmonary function, stereotactic body radiation therapy (SBRT) is an increasingly used alternative. SBRT delivers high doses of radiation that are precisely targeted to the tumor while attempting to minimize delivery of radiation to adjacent normal tissue.
Depending on the stage, chemotherapy may be used as adjunctive therapy before or after surgery as well. When the tumor has extended directly to the pleura (with malignant cells found in the pleural fluid) or to lymph nodes outside of the hemithorax, it usually is considered unresectable, and chemotherapy, radiation, molecularly targeted therapy, immunotherapy, or a combination of modalities is used. There is great interest in trying to determine the best modes of therapy for patients in whom the benefit of surgery is debatable, such as those with ipsilateral mediastinal node involvement or chest wall involvement.
If metastasis to distant tissues or organs has occurred, surgery usually is not an appropriate form of therapy; however, in carefully chosen patients with a solitary metastasis, both the primary tumor and the metastatic lesion may be resected. For example, surgical removal of an isolated brain metastasis in selected cases may be associated with improved survival. In virtually all patients with stage IV (metastatic) disease, the management options include chemotherapy, molecularly targeted therapy, and immunotherapy.
Previously, the typical chemotherapy for all types of NSCLC was a cytotoxic platinum-based regimen, which was often difficult for patients to tolerate. With recognition of the importance of the tumor’s molecular characteristics, the recommended treatment for inoperable or recurrent NSCLC has changed dramatically in recent years. Now, when available, molecular subtyping is performed to evaluate for driver mutations that can be targeted by specific drugs to inhibit their function. The first and best characterized is the EGFR mutation, although many others are clinically targeted or currently being studied. Mutations in EGFR occur in approximately 15% of patients with adenocarcinoma in the United States and up to 60% of such patients from regions in Asia. EGFR mutations also appear to be more common in women and in lung cancer patients who have not smoked.
EGFR is a normally occurring, highly regulated cell surface receptor; when activated, it triggers an intracellular tyrosine kinase, resulting in cellular proliferation. The mutated EGFR receptor does not respond to normal inhibitory signals, resulting in continuous activation of tyrosine kinase and uncontrolled cell growth. When an EGFR mutation is present, treatment with specific tyrosine kinase inhibitors (e.g., osimertinib) is associated with longer progression-free survival.
The newest therapeutic approach for patients with advanced NSCLC involves immunotherapy targeted to the programmed death (PD) pathway. Many lung tumors express PD-L1, which interacts with the programmed death receptor (PD-1) on immune effector cells to serve as a checkpoint limiting the immune response to tumor cells. A number of monoclonal antibodies have now been developed that block the binding of PD-L1 to PD-1, thus upregulating the immune response and allowing T lymphocytes to recognize and kill tumor cells. These antibodies, called ICI, have become a mainstay of therapy for advanced NSCLC, either alone or in combination with standard chemotherapy. For patients with
advanced NSCLC whose tumors do not demonstrate an activating mutation, treatment options include standard cytotoxic therapy with consideration of the addition of immunotherapy with an ICI. Finally, bevacizumab, a monoclonal antibody directed at vascular endothelial growth factor (VEGF), is another agent that has been used in combination with cytotoxic chemotherapy in a subset of NSCLC patients.
When NSCLC is unresectable on the basis of any criteria and contains no targetable mutations or viable immunotherapy options, the clinician is faced with a choice of no treatment, radiotherapy, or chemotherapy. The final strategy often is highly influenced not only by the particular tumor but also by the physician’s judgment and the patient’s preferences. In some cases, radiation therapy treatments are instituted early in an attempt to shrink the tumor and delay local complications. In other circumstances, therapy is withheld until a complication ensues, such as bleeding or airway obstruction. Radiation treatments then are given with the goal of tumor size reduction for temporary alleviation of the acute problem. Unfortunately, this is not curative, and further problems with the tumor are certain to develop.
A general overview of treatment of NSCLC has been presented here. Because the details of treatment for NSCLC are nuanced, constantly evolving, and beyond the scope of this text, the reader is referred to detailed Suggested Readings at the end of the chapter.
Treatment of small-cell lung cancer
Because SCLC has already disseminated at the time of diagnosis in almost all patients, surgery is not considered the treatment of choice unless the particular small-cell tumor is a solitary peripheral nodule without any evidence of mediastinal or distant spread. In the typical presentation of SCLC as a central mass, unresectable disease is virtually assured, and chemotherapy (with or without radiotherapy) is considered the primary mode of therapy. For limited stage disease, a platinum-based regimen of combination chemotherapy is typically used, supplemented by thoracic radiation. Patients who respond to this initial treatment will also often receive prophylactic cranial radiation. Patients with extensive stage disease are treated with a combination chemotherapeutic regimen, often supplemented by immunotherapy with an ICI. As with limited stage disease, selected patients with a good response may sometimes receive thoracic or prophylactic cranial radiation.
For all patients with lung cancer, the formal principles of palliative care should be incorporated early as part of their management. These include a focus on symptom management, psychosocial health, and goals of care, and are associated with improved quality of life.
Restoration of airway patency
For both NSCLC and SCLC, other forms of therapy are being used to reestablish patency of an airway that has been partially or completely occluded or compressed by tumor. The treatment typically is accomplished with either flexible or rigid bronchoscopy, using techniques such as laser, argon plasma coagulation, photodynamic therapy, cryotherapy, or electrocautery to diminish the size of the endobronchial tumor and reestablish an effective lumen. Alternatively or as a combined approach used in addition to those techniques, an endobronchial stent (i.e., a hollow and relatively rigid plastic or metal sheath) can be positioned within the airway lumen to help maintain airway patency.
Bronchial carcinoid tumors
Bronchial carcinoid tumors, which are classified as a type of bronchial neuroendocrine tumor, constitute approximately 5% of primary lung tumors. Previously thought to be benign lesions, bronchial carcinoids now are recognized to be a low-grade malignancy. Lung carcinoids are classified as either typical, with a low mitotic rate and indolent growth, or atypical, with an intermediate mitotic rate and clinical behavior.
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

As noted above, SCLC and large-cell neuroendocrine carcinomas are also tumors with neuroendocrine features; however, based on molecular, genetic analysis and clinical behavior, bronchial carcinoids are considered by many authorities to be in a different family of neoplasms. Most patients with these tumors have an excellent prognosis and are cured by surgical removal.
Two important epidemiologic features distinguish bronchial carcinoid tumors from the other pulmonary neoplasms discussed here. First, smoking does not appear to be a risk factor. Second, as a group, patients with bronchial carcinoid tumors are younger than those with other pulmonary malignancies; frequently, young adults are affected.
Bronchial carcinoid tumors arise most commonly in central airways of the tracheobronchial tree and are often discovered on either an abnormal chest radiograph or during episodes of hemoptysis or pneumonia distal to an obstructing airway tumor. Ectopic hormone production may be found, relating to the presumed neurosecretory origin of the neoplastic cells. The carcinoid syndrome, generally involving episodic flushing, diarrhea, and wheezing that results from the effects of serotonin produced by the tumor, is uncommon, being found in less than 5% of all cases of bronchial carcinoid tumors.
Treatment of these tumors is surgical resection if at all possible. For many patients, the prognosis is excellent, and recurrent or distant disease does not occur after surgical removal. However, metastatic disease is found more commonly in patients whose tumors have atypical histology, and the prognosis is worse for these patients.
Common features of bronchial carcinoid tumors:
1.Often found in young adults
2.Hemoptysis
3.Pneumonia distal to an obstructing endobronchial mass
Solitary pulmonary nodule
A solitary pulmonary nodule on chest radiograph or CT scan (defined as a single, rounded lesion 3 cm or less in diameter) is a common presentation of lung cancer (Fig. 21.5). However, there is actually a broad differential diagnosis for this radiographic abnormality, and only approximately 5% of incidentally identified nodules turn out to be malignant. The physician is faced with judging the likelihood that a nodule is malignant and choosing the appropriate pathway for diagnosis and management. Because lung cancer that manifests as a pulmonary nodule may be curable by surgical resection, management of such a lesion should not be neglected until the lesion is no longer curable. On the other hand, to subject a patient to thoracotomy, which is a major surgical procedure, for removal of a benign lesion requiring no therapy is likewise undesirable.

FIGURE 21.5 Chest CT cross-sectional (axial) scan image showing a right lower
lobe adenocarcinoma presenting as a solitary pulmonary nodule.
The diagnostic possibilities for the solitary pulmonary nodule are listed in Table 21.3. Besides primary lung cancer, the major alternative diagnoses are benign pulmonary neoplasms, solitary metastases to the lung from a distant primary carcinoma, and infections (especially healed granulomatous lesions from tuberculosis or fungal disease). Estimating the likelihood of a malignant versus a benign lesion from its radiographic appearance on CT scan is based on six major factors:
1.Size. The larger the nodule, the more likely it is to be malignant. Nodules less than 5 mm in diameter are malignant in less than 1%, whereas nodules greater than 20 mm represent a malignancy over 50% of the time.
2.Growth. Perhaps the most helpful piece of information a physician can have is a prior chest radiograph or CT scan. Comparison of old and new images shows whether a lesion is stable and gives an approximation of the rate of growth. Although it is difficult to say with certainty whether a lesion is benign or malignant based on the rate of growth, the absence of any increase in size for at least 2 years is an extremely good (but not infallible) indication that a lesion is benign.
3.Attenuation. Nodules are classified as solid or subsolid, and some lesions are mixed solid and subsolid (also called part solid). A nodule that is part solid has a higher risk of being malignant.
4.Calcification. The presence of calcification within a pulmonary nodule, best demonstrated on CT scan, may favor the diagnosis of a benign lesion, especially a granuloma or hamartoma. If certain patterns of calcification are found—diffuse speckling, dense calcification, laminated (onion-skin) calcification, or “popcorn” calcification—then the lesion almost assuredly is benign. On the other hand, calcification at the periphery of a lesion or amorphous calcification within the lesion may be suggestive of malignancy. For example, a peripheral area of calcification is entirely consistent with a scar carcinoma arising in the region of an old, calcified parenchymal scar (e.g., an old calcified granuloma).
5.Border appearance. An irregular or spiculated margin is suggestive of a malignant lesion,
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
whereas a benign lesion commonly has a smooth and discrete border.
6.Location. A nodule in the upper lobes has a higher likelihood of malignancy than nodules in the lower lobes.
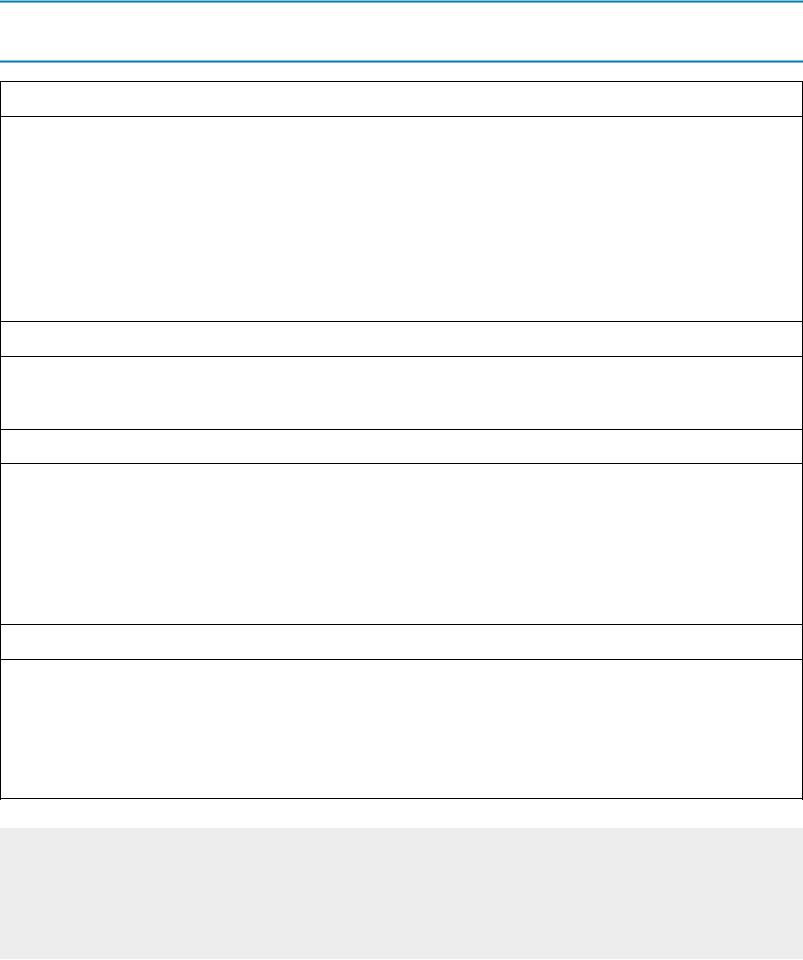
TABLE 21.3
Differential Diagnosis of the Solitary Pulmonary Nodule
Neoplasms
Malignant
Primary lung cancer
Solitary pulmonary metastasis from distant carcinoma
Bronchial carcinoid (bronchial adenoma)
Benign
Hamartoma
Miscellaneous (e.g., fibroma, lipoma)
Vascular Abnormality
Arteriovenous malformation
Infection
Infectious granuloma
Tuberculosis (“tuberculoma”)
Histoplasmosis (“histoplasmoma”)
Bacterial abscess
Miscellaneous (e.g., hydatid cyst, canine heartworm)
Miscellaneous
Rounded atelectasis
Hematoma
Intrapulmonary lymph node
Pseudotumor (fluid loculated in a fissure)
Criteria for assessing the likelihood that a solitary pulmonary nodule is malignant are as follows:
1.Size
2.Stability or change in size of the lesion
3.Attenuation—solid, subsolid, or part solid
4.Presence or absence of calcification; pattern of calcification
