
Книги по МРТ КТ на английском языке / The Embryonic Human Brain An Atlas of Developmental Stages. Third Edition. 2006. By Ronan O'Rahilly
.pdf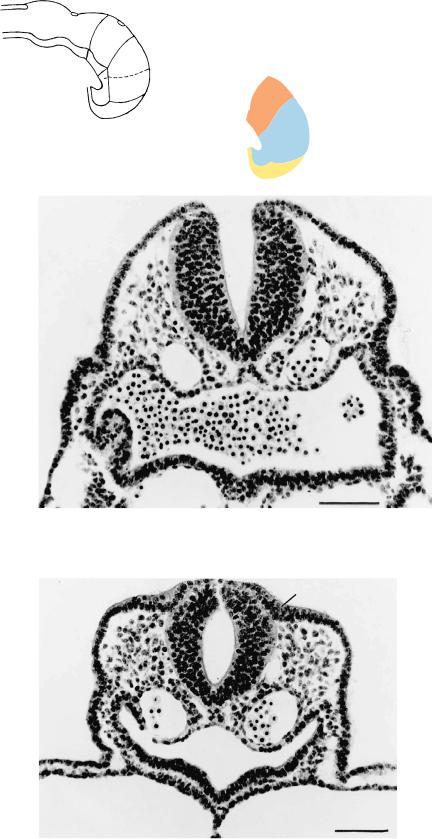
48 |
|
C h a p t e r 1 0 : THE NEURAL TUBE AND THE OPTIC PRIMORDIUM |
|
10 – 12 |
11 |
||
|
|
|
M |
|
|
|
Di. |
|
|
|
|
T
Figure 10–11. The neural groove of rhombomere 2. Ventral to it, the folding notochordal plate is visible and, on each side, a dorsal aorta. Transverse anastomoses between the right and left dorsal aortae have been recorded at stage 11, and fusion has definitely begun at stages 13 and 14.
Ot.
Figure 10–12. The neural tube of rhombomere 5. Mitotic figures are visible near the cavity of the tube. The otic plates appear on each side as ectodermal thickenings. Extramural blood vessels are forming ventrally and laterally, adjacent to the wall of the brain, but they do not yet give off intramural branches. A histological distinction between the perineural arteries and veins becomes apparent by stage 20 and for the intramural vessels during trimester 2.
NEUROTERATOLOGY |
49 |
TABLE 10–3. The Authors’ Interpretation of the Neural Crest and Related Tissues in the Human Embryo
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stage 8 |
|
|
|
|
|
|
|
|
Stage 10 |
|
|
|
|
Stage 12 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Neural |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
Neurosomatic |
|
|
|
|
|
“Islands” in |
|||||||||
|
|
|
ectoderm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
junction |
|
|
|
|
|
somatic |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ectoderm |
|
|
|
|
|
Stage 11 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retina |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Epipharyngeal |
|
|||||||
|
|
|
|
|
|
|
|
|
|
Cranial neural crest |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
discs |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mesencephalic |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
Optic crest |
|
|
|
|
|
|
|
|
|
|
Trigeminal |
|
|
|
|
|
|
5 |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
Otic |
|
|
|
|
Stage 13 |
|
|
|
|
Facial |
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
crest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vestibular |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
Nasal crest |
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Glossophar- |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
yngeal |
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
Vomeronasal |
|
|
|
|
Vagal |
|
|
|
10 |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
crest |
|
|
|
|
Hypoglossal |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
Terminalis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Spinal neural crest |
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
crest |
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Neuroteratology
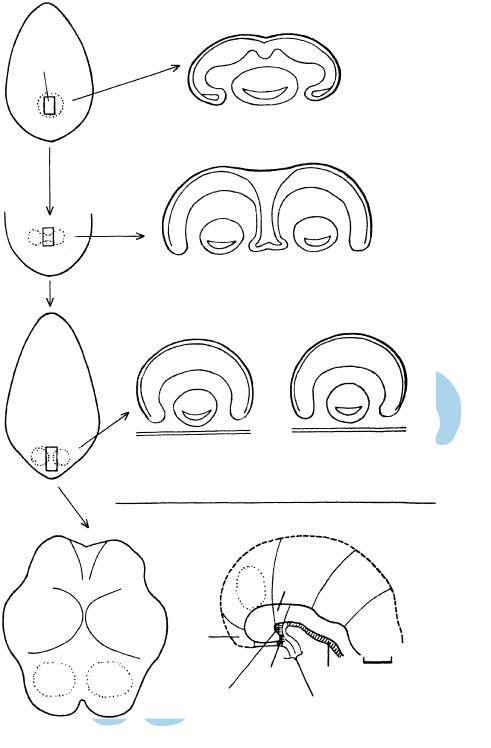
Cyclopia (the term is based on a mythical race of Sicilian giants) is characterized by a single median eye and orbit. The sine qua non is the presence of a single median (bony) orbit. The condition is accompanied by holoprosencephaly, and the nose is usually represented by a proboscis placed above the median eye. The impression of a fusion of formerly paired optic primordia is no longer considered to reflect the origin of the condition. In cyclopia sensu stricto the ocular structures are single rather than paired. The prosencephalon fails to “diverticulate” into the right and left optic evaginations. Such a disturbance could arise as early as stages 6 to 8 if the single retinal field, which is normally converted to paired primordia, is not so transformed because of a lack of inhibition of the median part by the prechordal plate. (Fig. 10–13A). The teratogenetic termination-point is probably 3 weeks for cyclopia sensu stricto (a median eye in a single orbit) and 4 weeks for cyclopia sensu lato (paired ocular structures in a single orbit, Fig. 16–14). A parallel situation in the development of the
limbs (the origin of symmelia) has been noted (O’Rahilly and Muller,¨ 1989b).
Axial cells of the prechordal plate and the notochord express SHH in normal embryos and induce neural development, including the subdivision of the single retinal field into two separate domains. Experimental work with molecular markers indicates that disturbances can be produced during or even before stage 8 (Goodman, 2003).
Synophthalmia is a variety of cyclopia sensu lato in which some or all of the ocular structures are paired within a single globe. Although the prosencephalon “diverticulates” into two optic primordia, these fail to lateralize. Such a disturbance could arise as early as stage 9, when the future optic area is probably changing from a median to bilateral regions (see Fig. 10–13). For an unknown reason, several embryos of stage 16 showing this variety of cyclopia have been available and have been described (Muller¨ and O’Rahilly, 1989c).
50 C h a p t e r 1 0 : THE NEURAL TUBE AND THE
A
|
|
|
CYCLOPIA |
|
|
|
SINGLE |
Prechordal |
RETINAL |
||
plate |
FIELD |
||
|
|
|
|
|
|
|
|
SYNOPHTHALMIA
B |
|
TWO |
|
RETINAL |
|
|
|
|
|
|
FIELDS |
|
|
|
C
NORMAL
OPTIC PRIMORDIUM
Figure 10–13. Early development of the optic primordia and the floor plate.
(A) Dorsal view of an embryonic disc at stage 8 and a projection of the prechordal plate, which occupies about 15–20% of the width and 12% of the length of the embryonic disc. It is maintained that a single retinal field exists at the rostral end of the neural plate (future neuromere D1). Persistence of this single field would lead to cyclopia. (B) Subsequently (although probably still in stage 8), the prechordal plate is believed to be responsible for suppressing the median part, so that bilateral retinal fields are formed. Lack of inhibition of the median component would lead to synopthalmia. (C) The retinal fields are completely separated from each other, which is the normal arrangement. (D) Dorsal view of an embryo of stage 10 in which the retinal fields occupy neuromere D1. (E) Median view at stage 10 showing neuromere D2, distinguishable from its relationship to the neurophypophysial (NH) and adenohypophysial (AH) primordia. The notochordal plate extends as far rostrally as the caudal boundary of NH and it induces the floor plate in the overlying neural ectoderm. The prenotochordal part of the brain comprises neuromeres T, D1, and the rostral portion of D2.
D E
D1 D2
M
|
N-H |
|
|
Ch. |
|
T |
|
|
|
A-H |
Not. |
Prechordal |
|
|
plate |
Oropharyngeal membrane |
|
C H A P T E R 11
STAGE 11: CLOSURE
OF THE ROSTRAL
NEUROPORE
Approximately 2.5–4.5 mm in Greatest Length;
Approximately 30 Postfertilizational Days
The rostral, or cephalic, neuropore closes during stage 11. The closure, in which the neural ectoderm also participates, is basically bidirectional; that is, it
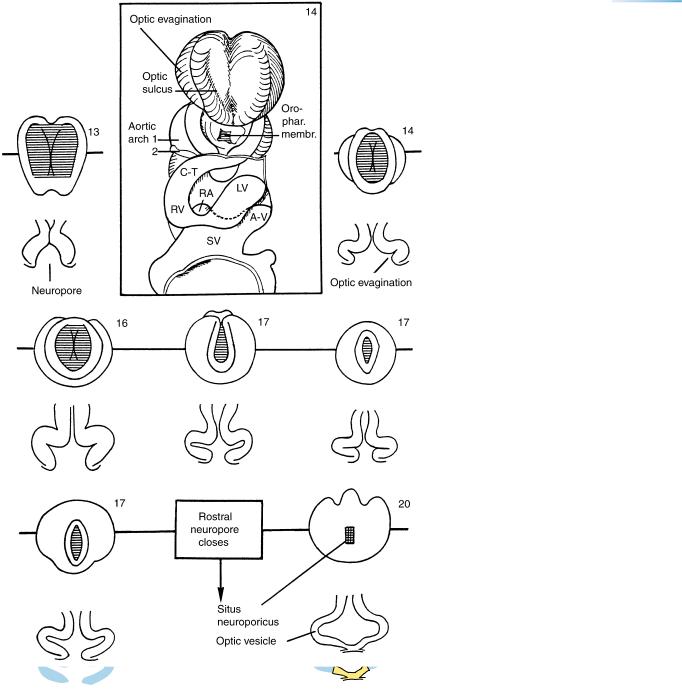
proceeds from the region of D2 (its “dorsal lip”) and simultaneously from the telencephalon (its “terminal lip”). Also during this stage, the floor of the telencephalon medium becomes distinct as the future lamina terminalis and commissural plate. The optic vesicle is being formed from the optic sulcus in Dl, and is providing optic crest. Both adenohypophysial and neurohypophysial primordia can be distinguished, and they are in contact from the beginning of their development; the hypophysis arises as a single organ.
Notochordal and neural axial structures are still closely related.
Precision
The neuropores are rostral (or cranial) and caudal, not anterior and posterior, which have specialized meanings in human anatomy. There is at present no embryological evidence in the human that a specific pattern of multiple sites of closure of the neural tube exists, such as has been described in the mouse.
The Embryonic Human Brain: An Atlas of Developmental Stages, Third Edition. By O’Rahilly and Muller¨ Copyright C 2006 John Wiley & Sons, Inc.
51
52 |
C h a p t e r 1 1 : CLOSURE OF THE ROSTRAL NEUROPORE |
12 |
10 9 Ot. |
8.7 |
5 |
Opt.
Figure 11–1. Right lateral view of an embryo of stage 11 with the brain superimposed (interrupted line), and showing the rostral and caudal neuropores. The number of somitic pairs was 17; the range in number at this stage is 13–20. The umbilical vesicle and the body stalk have been sectioned at the right.
Figure 11–2. Right lateral view of the brain of another embryo. The features shown are the optic vesicle, the trigeminal and faciovestibulocochlear ganglia, the otic vesicle, the glossopharyngeal and vagal ganglia, and the hypoglossal cell cord, which will form the connective tissue of the tongue. Cells given off from the wall of the developing otic vesicle appear to be crest cells that form a ventral sheath; these latter will contribute perhaps more to the otic capsule than to the facio-vestibulocochlear ganglia. Trigeminal and otic arteries are present and later (stages 13–15) form parts of the caroticobasilar anastomosis (Fig. 21–23).
CLOSURE OF THE ROSTRAL NEUROPORE |
53 |
D2
D1
T
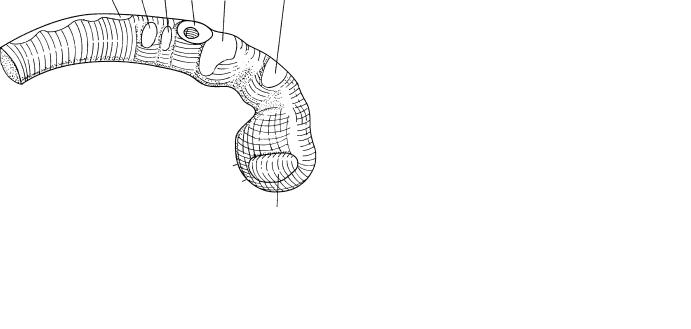
Figure 11–3. Graphic reconstruction prepared from transverse sections showing a median view of the brain. The asterisk indicates the junction with the spinal cord. The rostral, or cephalic, neuropore is indicated by a thick line between two arrowheads. Each of the two neuropores, rostral and caudal, measures about 0.5 mm in length (mean of 24 embryos). The rostral neuropore closes within a few hours during stage 11, when about 20 somitic pairs are present. (The embryo illustrated here has 17.) The thin surface ectoderm overlying the dorsal aspect of the neural tube has not been included in the drawing.
D1 still consists mostly of optic primordium. The chiasmatic plate is first at the rostral end of the neural plate (13 pairs of somites), as in stage 10. When 14 or more somitic pairs have appeared, however, fusion at the “terminal” lip (Fig. 11–7A) of the neuropore results in the formation of a floor for the telencephalon medium. This represents the future lamina terminalis and commissural plate.
In D2, the neural floor adjacent to the adenohypophysial primordium is the neurohypophysial region. Both components of the hypophysis cerebri develop in contact with each other, so that no migration or growth of one to meet the other occurs. The level of the adenohypophysial primordium is caudal to that of the chiasmatic plate, and its site is clearly defined by the oropharyngeal membrane, which has not yet ruptured (in 11 out of 16 embryos).
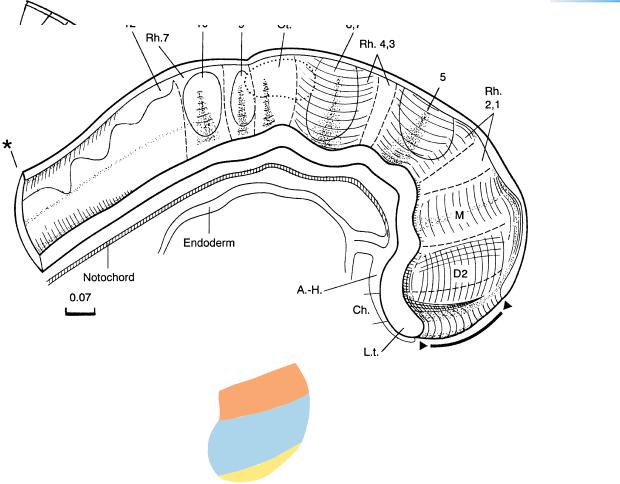
The percentage of length occupied by the mesencephalon in relation to that of the whole brain remains almost constant from stage 9 up to and including stage 11. The rhombencephalon takes up approximately three-quarters of the brain in length. The relative increase in prosencephalic length is slightly less than in stage 10. Cranial ganglia and neural crest are projected onto the median view. Eight rhombomeres are distinguishable (Fig. 10–4B).
The notochord or the notochordal plate is still closely related to the neural tube or the neural plate, so that it is possible that induction of the floor plate could continue along almost the whole length of the neural axis.
54 |
C h a p t e r 1 1 : CLOSURE OF THE ROSTRAL NEUROPORE |
D1 |
D2 |
M |
Rh.
1
2
3
4
5
6
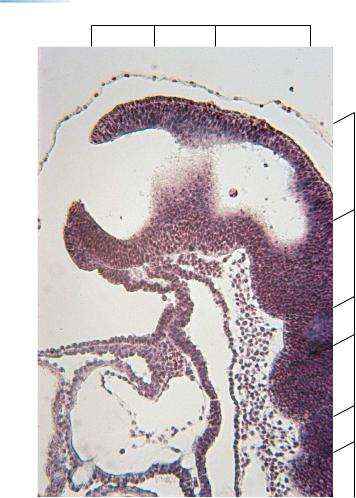
Figure 11–4. Sagittal section through an embryo at stage 11 (17 somites) showing the rostral neuropore, through which the lumen of the neural tube still communicates with the amniotic cavity. (The thin line of the amnion is visible above.) Further caudally, the wall of the neural tube shows portions of several rhombomeres. Also recognizable are the oropharyngeal membrane and foregut, and the heart, which presents myocardial mantle, cardiac jelly (appearing here as an almost clear space), and endocardium.
CLOSURE OF THE ROSTRAL NEUROPORE |
55 |
Figure 11–5. Drawings of the rostral end of the neural tube in seven embryos of stage 11, to show the progressive closure of the rostral neuropore. Under each is shown a horizontal section taken at the level indicated by the horizontal lines. The embryos shown had 13, 14, 16, 17, 17, 17, and 20 somitic pairs, respectively. Modified from Streeter, as reproduced by O’Rahilly and Muller¨ (1987a). The rostral neuropore, which is still open when 19 pairs of somites are present, is closed when 20 pairs have formed (O’Rahilly and Gardner, 1979, Table 2). See also Figure 12–12.
The rostral neuropore is not considered to be the “front” end (das wahre ursprungliche¨
Ende) of the neural tube, which is frequently taken to be the infundibulum or the infundibular recess (Dart, 1924, in agreement with von Baer). There is much to be said, however, for Johnston’s (1909) view that “in all vertebrates the anterior end of the head is the point at which the brain plate meets the general ectoderm at the same time that it comes into contact with the anterior end of the entoderm. This point is marked in the adult by the optic chiasma.”
Abbreviations: A-V, atrioventricular canal; C-T, conotruncus; LV, left ventricle; RA, right atrium; RV, right ventricle; SV, sinus venosus.
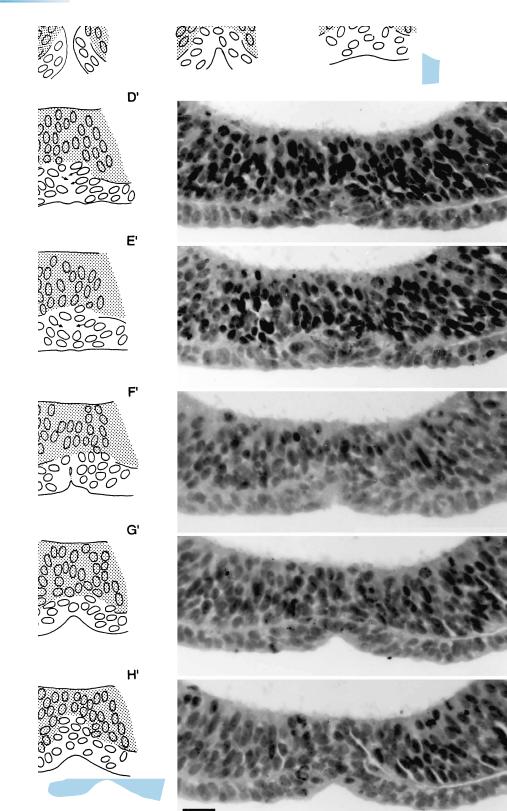
56 |
C h a p t e r 1 1 : CLOSURE OF THE ROSTRAL NEUROPORE |
Figure 11–6. Drawings and photomicrographs to illustrate the closure of the rostral neuropore in an embryo of stage 11. Basically, the neuropore closes bidirectionally, i.e., from (site β) its “dorsal lip” (near D2) and simultaneously from (site α) its “terminal lip” (in the telencephalon, adjacent to the chiasmatic plate). The surface ectoderm participates in the closure at the terminal lip, and the area of fusion of the neural folds lies between the two nasal discs. The right and left components of the neural ectoderm (stippled) and those of the surface ectoderm (in blue) seem to fuse simultaneously across the median plane. The bar represents 0.02 mm. A key drawing showing the lips and the plane of sections A and H is provided in Figure 11–7 A. From O’Rahilly and Muller¨ (1989a).
The phenomenon of closure at the “terminal lip” is ignored in most publications on embryology. In the chick embryo, near the time of fusion of the neural folds, occlusion of the lumen of the neural tube occurs and has been considered to be necessary for the growth of the brain. In the human embryo, however, occlusion is infrequent during stage 11 and has been found only when the rostral neuropore is still open (Muller¨ and O’Rahilly, 1986a), thereby precluding any hydrodynamic function. Moreover, growth of the brain is practically at a standstill during stages 11 and 12 (Muller¨ and O’Rahilly, 1987,
Table 3).
The closure at the dorsal lip is much simpler. It concerns the fusion of both the unilaminar surface ectoderm and the unilaminar neuroectoderm, represented schematically in Figure 10-5E.

CLOSURE OF THE ROSTRAL NEUROPORE |
57 |
Figure 11–7. Views of the rostral neuropore and the beginning optic vesicles at stage 11.
(A) End-on and median views as a key to Figure 11–6. Tel. indicates the surface ectoderm overlying the telencephalon. In the median view, the thin surface ectoderm overlying the dorsal lip has not been included in the drawing. The two horizontal lines show the plane of sections A and H. (B) End-on and right lateral views as a key to the plane of section of the photomicrograph, C. (C) The optic vesicles are being formed by further deepening of the optic sulci in D1. Optic crest is developing from the external layer of the optic vesicle and is met by migrating mesencephalic neural crest. The two form the sheath of the optic vesicle, i.e., of the future optic cup. The basement membrane is interrupted where the cells of the optic crest leave the neural ectoderm. Optic crest is formed by mitotic division in the superficial cells of the optic vesicle, an exception to the general rule that mitosis in the neural tube occurs adjacent to the ventricle. The rostral neuropore is visible at the bottom of the photomicrograph. Bar: 0.15 mm.
Mesencephalon
|
Neuropore |
C |
Optic sulcus |
