
Книги по МРТ КТ на английском языке / The Embryonic Human Brain An Atlas of Developmental Stages. Third Edition. 2006. By Ronan O'Rahilly
.pdf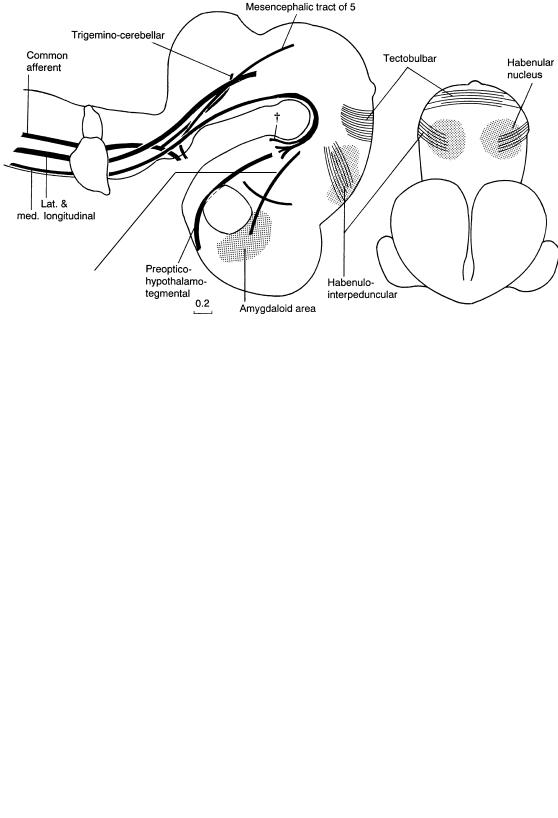
Med. forebrain bundle
Figure 15–5. A number of tracts have appeared in the following order: the lateral longitudinal fasciculus, the spinal tract of the trigeminal nerve (which is a component of the common afferent tract), the medial longitudinal fasciculus, the mesencephalic tract of the trigeminal nerve, and the mamillotegmental tract (dagger). Fibers of the preoptico-hypothalamic tract are present, and precursor fibers of the habenulointerpedunclar and hypothalamo-thalamic tracts are forming. Fibers of the decussation of the superior colliculi are the precursors of the tectobulbar tract. Fibers to the amygdaloid part of the medial ventricular eminence (future striatosubthalamic tract) constitute a portion of the medial prosencephalic fasciculus. The shifting of the amygdaloid area into a clearly telencephalic position is comparable to the relocation of the globus pallidus from the diencephalon into the corpus striatum.
Figure 15–6. Right lateral view showing some peripheral nerves. All cranial nerves are present except 1, 2, and 8c. (The optic nerve is represented merely by the optic stalk until nerve fibers appear at stage 18.) The least advanced is the abducent, which is found in only one-third of embryos of this stage. Intramural trochlear fibers are indicated by two rows of dots. The vestibular nerve possesses sensory fibers that join the common afferent tract. The hypoglossal nerve has many connections with cervical nerves 1 to 3. The various ganglia of the cranial nerves are identifiable.
Pax, En, and Wnt genes are involved in the patterning of the mesencephalon and metencephalon (Song et al., 1996), and the mesrhombencephalic boundary shows Pax-5 gene expression (Gerard´ et al., 1995).
LONGITUDINAL ZONING IN THE DIENCEPHALON |
99 |
Nasal pit
Fig. 15 – 7 |
V3 |
Sulcus limitans
V4 |
Cerebellum |
|
Figure 15–7. Section through the diencephalon and rhombencephalon showing the optic cup and the cerebellar plate on each side. The lens vesicles are closed from the surface. The optic stalk on the right-hand side of the photomicrograph shows the continuity between the third ventricle and the temporary optic ventricle.
A B C
|
|
|
Torus |
|
|
|
|
Lat. |
hemisphericus |
Isthmus |
|
|
|
ventricle |
|
|
|
|
|
|
|
M2 |
Rh. |
|
|
V3 |
|
|
|
|
Tel. |
D1 |
|
|
|
|
|
|
|
|
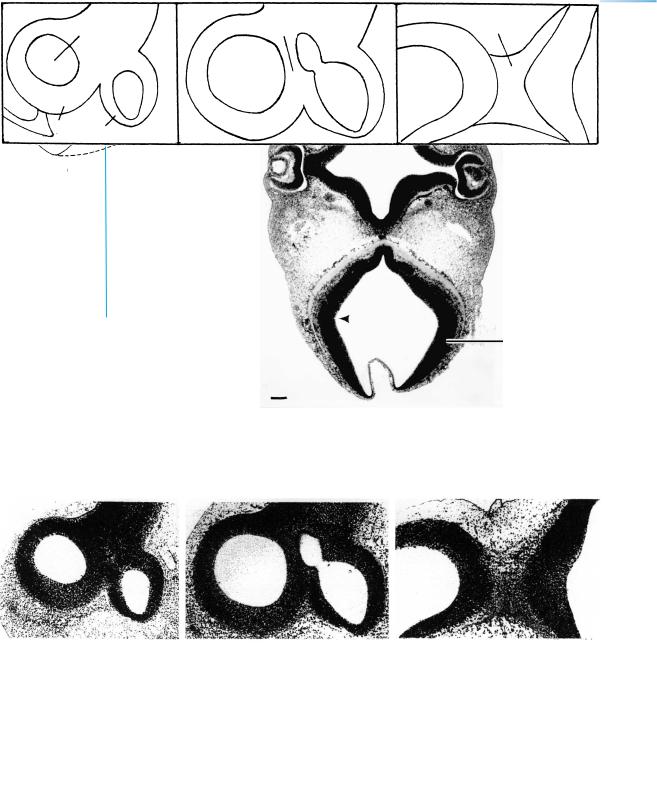
Figure 15–8. Sagittal sections in lateromedial sequence. The cellular strands in the lower left-hand corner of A are neural crest (asterisk in key).
Figures 15–7, 15–9, and 15–10. These photomicrographs are from the same embryo and their planes of section are shown on the next page. Bars: 0.15 mm.
100 |
C h a p t e r 1 5 : LONGITUDINAL ZONING IN THE DIENCEPHALON |
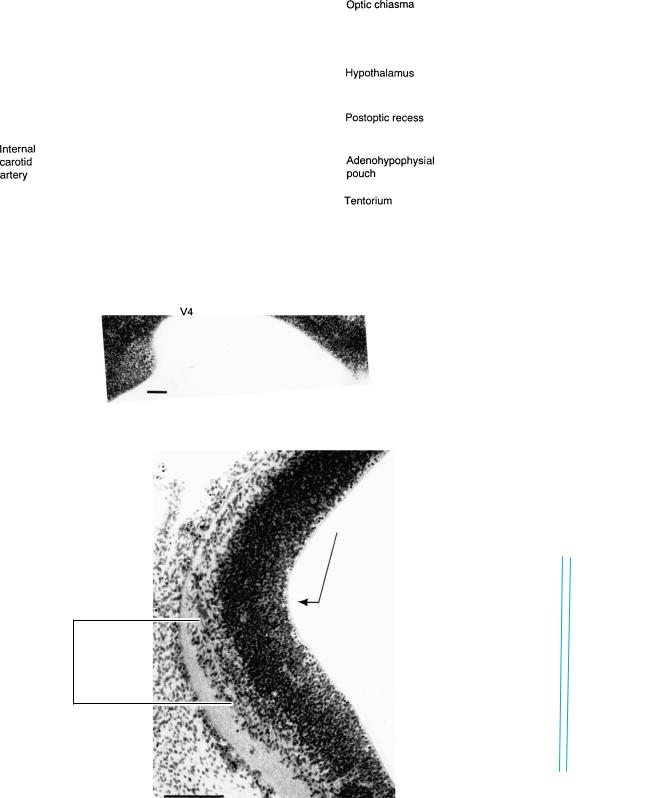
Figure 15–9. Section through the hypothalamus and adenohypophysial pouch, showing a part of the optic chiasma.
Sulcus
limitans Fig.
15 – 9 10
Locus caeruleus
Figure 15–10. Section through the cerebellar region at Rh.1 showing the locus caeruleus.
The cells of the future locus caeruleus are present early (stage 13) and are situated in the intermediate layer of rhombomeres 1 and 2, ventral to the sulcus limitans. Later (at about stages 19 and 20), brightly fluorescent processes have been traced into the telencephalic wall (Zecevic and Verney, 1995), and dopaminergic and adrenergic fibers reach the tectum.
LONGITUDINAL ZONING IN THE DIENCEPHALON |
101 |
A |
B |
Cbl |
|
|
Aq.
Rh. 2 1
C
Cer.
Is.
Rh. 8 |
7 |
6 |
1 |
|
|
|
5 4 3 2
D
|
5 |
4 |
3 |
2 |
1 |
Is. |
|
|
|
|
|||
7 |
6 |
|
|
|
M |
|
|
|
|
|
|||
|
|
|
|
|
|
11
10 |
9 |
7 |
5 |
|
|||
|
|
8v. |
|
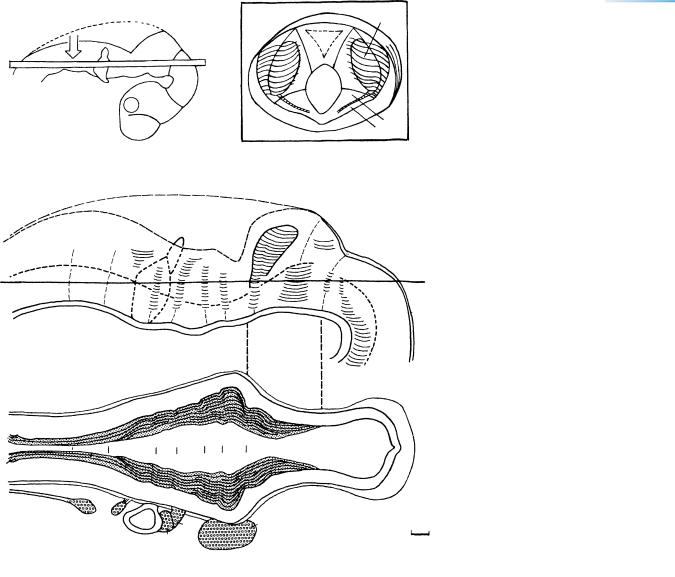
Figure 15–11. Reconstructions of the rhombencephalon and isthmus rhombencephali. (A) Right lateral view to clarify (arrow) view D. (B) A block showing the isthmus and aqueduct seen from the caudal aspect (“from behind”). Rhombomere 1 is separated from rhombomere 2 by a ridge. The interrupted vertical lines between reconstructions C and D indicate the caudorostral extent of the block. (C) The ventricular surface of the left half of the brain. The roof of the isthmus represents the future superior medullary velum. Rh. 1 to 5 are clearly delineated by crests; Rh. 7 and 8 have to be distinguished by their relationship to cranial nerves 9, 10, and 12 respectively. (D) The internal surface of the rhombencephalon ventral to the horizontal lines in A and C. The trigeminal ganglion is related mainly to Rh.2, the facial (geniculate) to Rh.4, the otic vesicle to Rh.5 and 6, the superior glossopharyngeal ganglion to Rh.6, and the superior vagal ganglion to Rh.7. Bar, 0.2 mm.
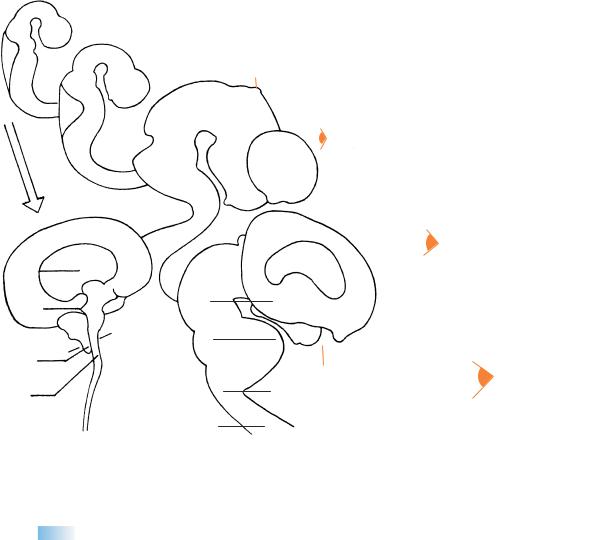
102 |
C h a p t e r 1 5 : LONGITUDINAL ZONING IN THE DIENCEPHALON |
A
B
C
A D
Hydrocephaly
"Beaking" of inf. colliculi
Level of foramen
magnum
Herniated vermis
Descent of medulla
Figure 15–12. The pontine flexure, barely discernible at (A) stage 15, becomes progressively accentuated at (B, C) stages 16 and 18 and at
(D) 33 mm. Its failure to develop (A′ ) is one of several characteristic features of the (Cleveland–) Arnold–Chiari malformation.
NEUROTERATOLOGY:
ARNOLD–CHIARI
MALFORMATION
Arnold-Chiari Malformation (Chiari Type 2). This obscure condition was first described by Cleland in 1883. Hypoplasia of the tentorium cerebelli is found, and hydrocephaly and myelomeningocele are usual but not invariable accompaniments. Many features are probably secondary rather than primary (Gardner et al., 1975). A
failure of the pontine flexure (stage 14) to form is believed to be an important factor in the abnormal elongation of the hindbrain, but attempts to formulate a unified theory have been singularly unconvincing. The time of origin of the condition lies in the embryonic period proper, perhaps between 3 and 5 weeks. An example at about stage 16 has been described (Ruano Gil, 1965). Irregularity of the future vertebral column noted in vivo by ultrasound at 7 12 postfertilizational weeks was followed at 10 weeks by signs of the Arnold–Chiari malformation (Blaas et al., 2000a).

C H A P T E R 16
STAGE 16: EVAGINATION
OF THE
NEUROHYPOPHYSIS
Approximately 8–11 mm in Greatest Length;
Approximately 37–39 Postfertilizational Days
he presence of the hippocampal thickening and |
vestibulocerebellar. The cochlear nerve has suddenly ap- |
Tvarious histological features makes possible a dis- |
peared. Asymmetry of the cerebral hemispheres was found |
tinction among the main, future cortical areas: archipal- |
in one embryo. Embryonic movements can be detected ul- |
lium, paleopallium, and neopallium. The most advanced |
trasonically at 5–6 weeks. |
differentiation is in the cortex of the future temporal pole |
Cerebral Asymmetry. A slight asymmetry of the left |
at the periphery of the amygdaloid area, in which the pri- |
and right cerebral hemispheres is well documented in the |
mordial plexiform layer can be discerned. Olfactory fibers |
adult and dates back to at least the fetal period (Bossy et al., |
enter the wall of the brain at the site of the future olfac- |
1976; Sun et al., 2005). It is entirely possible, if not indeed |
tory bulb, and the future olfactory tubercle is detectable. |
likely, that the asymmetry begins during the embryonic pe- |
The olfactory tubercle is the earliest component of the |
riod proper, perhaps even as early as stage 16. Hence some |
prosencephalic septum to appear. The marginal ridge sep- |
of the problems in lateralization discussed by Geschwind |
arates the dorsal from the ventral thalamus. The neurohy- |
and Galaburda (1985) may have a very early origin. |
pophysial outgrowth is now becoming distinct. A number |
Table 16–1 is useful in interpreting the important vas- |
of important pathways are beginning, e.g., olfactory and |
cular studies of Padget (1948, 1957). |
The Embryonic Human Brain: An Atlas of Developmental Stages, Third Edition. By O’Rahilly and Muller¨
Copyright C 2006 John Wiley & Sons, Inc.
103
104 |
C h a p t e r 1 6 : EVAGINATION OF THE NEUROHYPOPHYSIS |
M
Di.
T
a
b
c
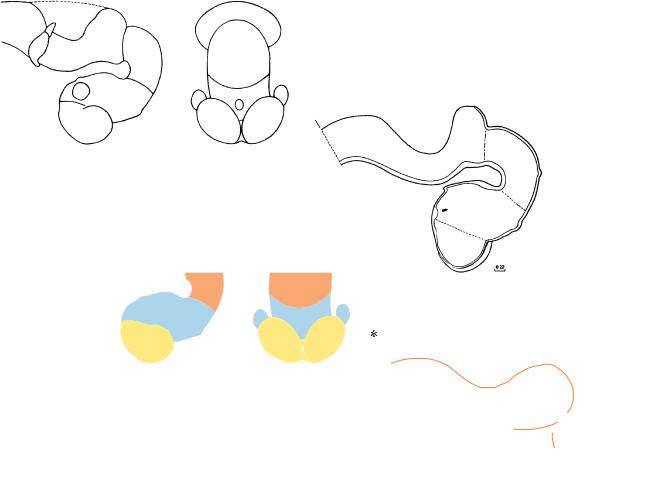
Figure 16–1. Right lateral view of the brain. The asterisk indicates the junction with the spinal cord. The inset includes an end-on view showing the cerebellar plates, mesencephalon, epiphysis cerebri, diencephalon and optic cups, and cerebral hemispheres. In one embryo the cerebral hemispheres were strikingly asymmetrical, the left hemisphere being smaller than the right (as confirmed by a Born reconstruction). Such an early cerebral asymmetry does not seem to have been recorded previously.
In the eye, the retinal (so-called choroid) fissure closes during stages 16 and 17. The polygonal outline of the (future iridial) rim of the optic cup is characteristic. Photomicrographs of the embryonic eye at each stage have been published (O’Rahilly, 1966). A comparison of the wall of the neural tube and that of the optic cup is shown in Figure 14–8. In addition, the main events of optic development have been summarized (O’Rahilly, 1983b). The right-hand key drawing shows (a) the sulcus limitans, (b) the hypothalamic sulcus, and (c) the marginal ridge.
EVAGINATION OF THE NEUROHYPOPHYSIS |
105 |
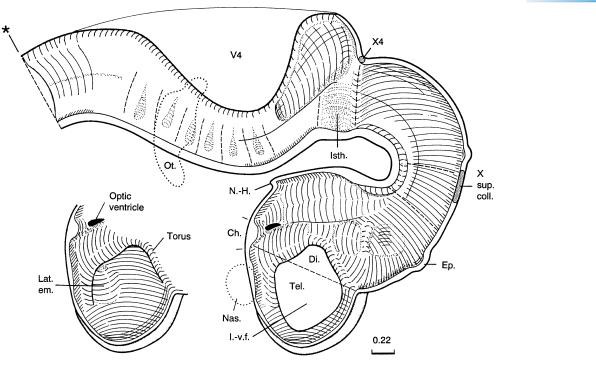
Figure 16–2. Graphic reconstruction prepared from transverse sections to show a median view of the brain. The outgrowth of the infundibulum is now becoming marked; i.e., the evagination of the neurohypophysis is becoming distinct (Table 17–2). The rhombencephalon has become flatter and broader, and the isthmus, which has become clearer, now shows an enlargement ventrally. The change in shape of the isthmus was appreciated by Hochstetter (1919), who described this region as funnel-shaped (at stage 15). The pontine flexure is deeper. The rhombomeres are no longer clearly visible on the external surface. Amoeboid cells that may be precursors of microglial cells have been detected histochemically in the cerebral wall at about stage 16 (Fujimoto et al., 1989). Furthermore, glial cells can be distinguished immunologically from neurons at about this time.
106 |
C h a p t e r 1 6 : EVAGINATION OF THE NEUROHYPOPHYSIS |
Syn.
Syn.
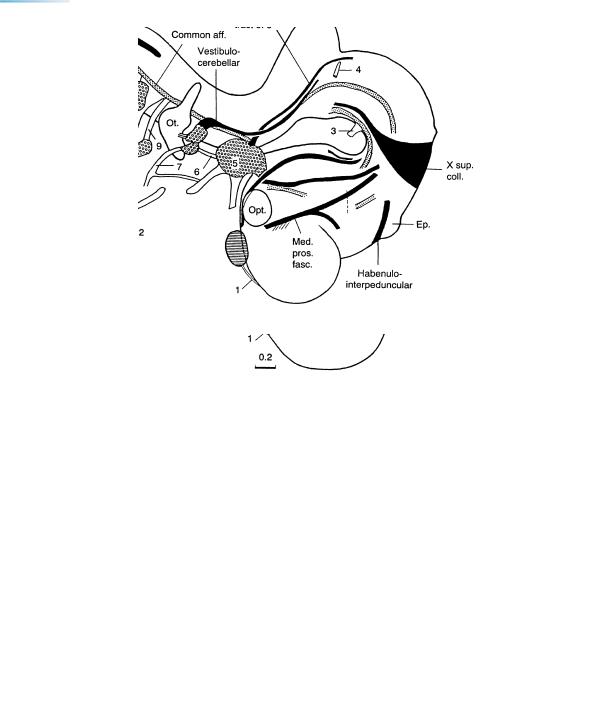
Figures 16–3 and 16–4. Cranial nerves and tracts. The cochlear nerve appears suddenly, and all the cranial nerves are now visible. The hypoglossal nerve, which now reaches the apex of the tongue, is joined by fibers of cervical nerves 2 and 3, presumably the precursors of the ansa cervicalis and its roots (O’Rahilly and Muller,¨ 1984b). Most motor nuclei of the cranial nerves have developed during stages 12 to 14. An examination of first-class material shows that it is not possible to distinguish the nucleus ambiguus at stage 16 or earlier, as has been claimed (Brown, 1990). Fibers ascending to the cerebellum are situated mainly along the sulcus limitans and are not clearly seen to penetrate that organ. Vestibulocerebellar and trigeminocerebellar fibers proceed towards the basal part of the cerebellar plate. The diencephalic subthalamic nucleus is present and includes fibers proceeding to the supramamillary commissure. The preoptico-hypothalamotegmental tract is marked by a dagger (Fig. 16–4).
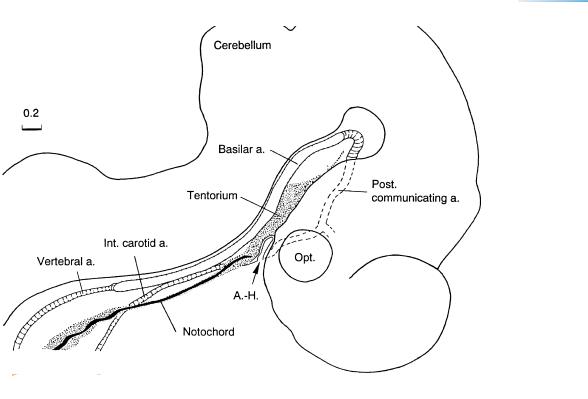
EVAGINATION OF THE NEUROHYPOPHYSIS |
107 |
Figure 16–5. Arterial development and the tentorium cerebelli. The cellular sheath of the notochord, which is anchored to the adenohypophysis (Fig. 15–9), is continuous with the medial part of the future tentorium cerebelli. The space between the hypothalamus and the rhombencephalon becomes increasingly compressed, resulting in a mesenchymal condensation (the Mittelhirnpolster of Hochstetter) between the diencephalic floor and the basilar artery. The notochord lies either ventral to or, in the more advanced embryos, within the notochordal cellular sheath. The latter and the sclerotomic material will form the basioccipital part of the skull.
Most of the components of the future circulus arteriosus (of Willis) are now present: the basilar and the paired posterior communicating arteries, as well as the internal carotid vessels on both sides. An anterior communicating artery has not yet developed but will do so during stage 19 (Padget, 1948, Fig. 7b). The posterior communicating represents the original caudal division of the internal carotid artery (Fig. 21–23). The adult stem of origin of the posterior cerebral is at first the distal end of the original posterior communicating artery (Padget, 1948). Penetrating capillaries can be observed in most parts of the surface of the brain, except in the future archipallial and neopallial components of the telencephalon. They enter as deeply as the ventricular layer.
The posterior communicating artery, a very important embryonic vessel that develops from stages 14 to 16, was also reconstructed by Padget (1948, Fig. 4). The posterior communicating is the vessel through which the internal carotid artery supplies the embryonic hindbrain. Although present and illustrated, the posterior cerebral artery was not named in Padget’s figure.
