
Книги по МРТ КТ на английском языке / The Embryonic Human Brain An Atlas of Developmental Stages. Third Edition. 2006. By Ronan O'Rahilly
.pdf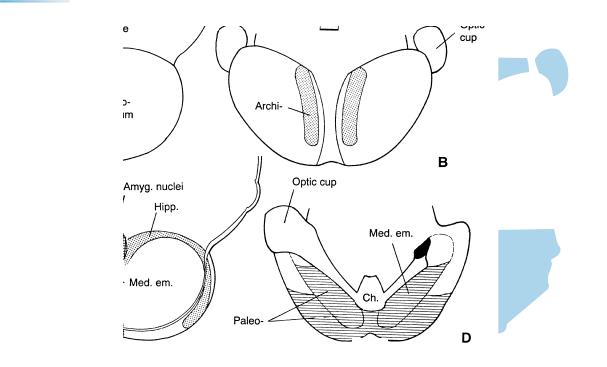
108 |
C h a p t e r 1 6 : EVAGINATION OF THE NEUROHYPOPHYSIS |
Archipallium
Paleopallium
Figure 16–6. (A) lateral, (B) dorsal, (C) medial, and (D) ventral views of the telencephalon. The archipallium (stippled), paleopallium (horizontal lines), and neopallium (unshaded) are distinguishable on the basis of histological differences. The archistriatum (amygdaloid nuclei, Fig. 19–6) is indicated by cross-hatching, and the paleostriatum by interrupted horizontal lines at the interior surface in C. This is the area to which the globus pallidus externus moves during stages 22 and 23 (Figs. 23–17 and 23–25). The most advanced differentiation is in the future temporal pole (gamma) at the periphery of the amygdaloid nuclei. Here presumed Cajal–Retzius cells and fibers were already present in stage 15. These horizontally arranged cells in the “marginal” (Fig. 21–7) layer are more developed than the cells of the ventricular layer (Fig. 17–11). The “marginal” layer in this region constitutes the primordial plexiform layer, the “pallial Anlage,” which is believed to be functional by stage 20 (Mar´ın-Padilla and Mar´ın-Padilla, 1982). Olfactory fibers enter the wall of the brain at the site of the future olfactory bulb, and cellular islands indicate the future olfactory tubercle.
Up to stage 16 the amygdaloid region, which is derived almost exclusively from the medial ventricular eminence, is recognizable by the advanced differentiation of its primordial plexiform layer. Neurogenesis in the amygdaloid complex of the rhesus monkey begins at a comparable time, and the cells have been considered to be the earliest postmitotic neurons in the telencephalon.
Whatever the pros and cons of the tripartite brain hypothesis (so-called reptilian, paleomammalian, and neomammalian regions), scant support for it is apparent in human embryology, any more than for the outmoded “biogenetic law” of “recapitulation.” It would be very difficult (with present methods) to attempt to distinguish archi-, paleo-, and neopallial regions before histological differentiation has begun. The recorded appearance of synapses in the neopallium at stage 17 and later, however, serves to emphasize that that region is clearly more advanced than either the hippocampus or the olfactory bulb at the same stage. In other words, there seems to be no evidence that the development of the (human) brain follows a phylogenetic pattern from archito paleoto neocortical structures.

EVAGINATION OF THE NEUROHYPOPHYSIS |
109 |
Figures 16–7A to 16–7F. Six sections |
F E D C B A |
of the brain. From Bartelmez and Dekaban |
|
(1962). |
|
A
L.t. Olf.
D
Di.
A.-H.
Ot.
10
11
Rh.
B
Spinal
cord
E
Thal.d.
Thal.v.
5
C
Hem.
Med. em.
Di.
|
12 |
|
Rh.8 |
Hyp.-th. |
F |
sulcus |
V4
Rh.
Hypothal
cord
Marginal |
Figure 16–7. (A) The olfactory |
ridge |
area and the lamina terminalis. |
|
(B) The medial ventricular |
Ep. |
eminence forms the basal part of |
|
the torus hemisphericus. (C) The |
|
optic stalk and the hypothalamic |
|
cell cord of the two sides of the |
|
brain. (D) Section at the level of |
|
the adenohypophysis. (E) The |
5 |
dorsal and ventral thalami, and |
|
the hypothalamic sulcus. Both |
V4 |
ventral and dorsal thalami possess |
|
a thick ventricular layer and a |
|
beginning intermediate layer. |
|
(F) Section at the level of the |
|
epiphysis cerebri (pineal body, |
Rh. |
Table 17–1). The marginal ridge |
|
(future zona limitans |
|
intrathalamica) can be discerned |
|
and separates the ventral from the |
|
doral thalamus. |
110 |
C h a p t e r 1 6 : EVAGINATION OF THE NEUROHYPOPHYSIS |
Fig.16 – 8,
10 9 11
Di.
A.-H.
Int. carotid a.
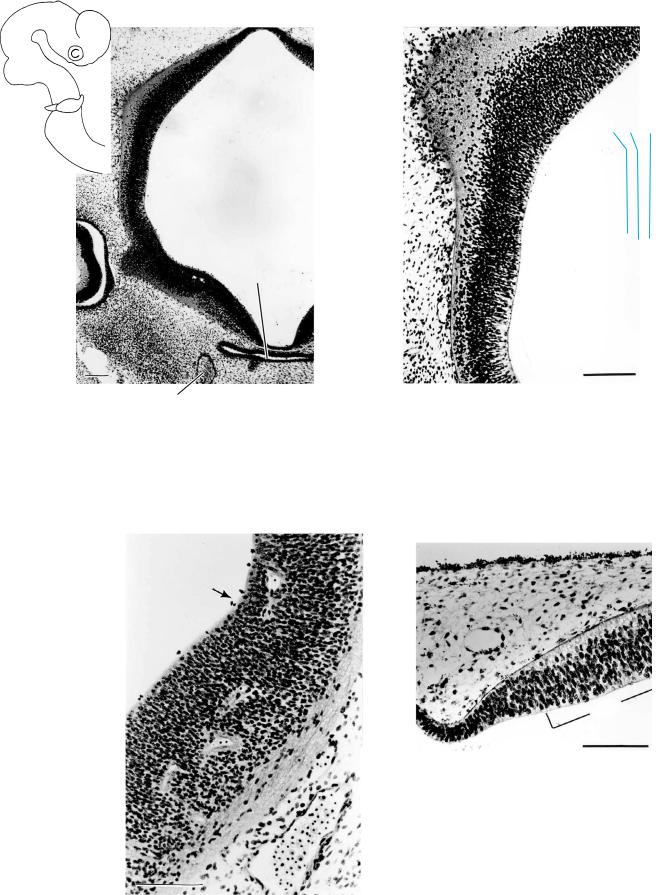
Figure 16–8. Cerebral hemisphere showing the loose distribution of the superficial cells at the periphery. Up to and including stage 16 the neocortical part of the cerebral hemispheres is avascular (Larroche and Jardin, 1985), although it is already surrounded by a prominent “perineural” vascular process (Streeter, 1919; Padget, 1948; Mar´ın-Padilla, 1978, 1988a,b). Bar: 0.2 mm.
Hyp.-th.
sulcus
Figure 16–9. Some two sections more deeply, the surface of the cerebral hemisphere presents widely dispersed cells, indicating progressive differentiation. Bar: 0.2 mm.
The cell columns of the ventricular layer of the diencephalon in Figures 16–9 and 16–10 are high and are penetrated by blood vessels. The cells of the intermediate layer are not as tightly packed as in the telencephalic area shown in Figure 16–11, and this is a clear difference. Peripheral to the intermediate layer, nerve fibers and scattered cells give an impression of a primordial plexiform layer.
. Hipp
Figure 16–11. The hippocampal area, characterized by a marginal layer and a discrete ventricular thickening. Bar: 0.1 mm.
Figure 16–10. The subthalamus, ventral to (below) the hypothalamic sulcus, showing fibers in the marginal layer and capillaries that have penetrated the cerebral wall. Such diencephalic blood vessels are present in most embryos of this stage. Bar: 0.1 mm.
EVAGINATION OF THE NEUROHYPOPHYSIS
Is.
A |
Cel. |
|
S.I. |
||
|
M2
M1
8 |
7 |
6 |
|
|
|
2 |
|
5 |
4 |
3 |
|||
|
|
|||||
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
Ot. |
|
|
|
B
Cel. M2
Is.
C
B
C
Rh. 6 5 4 3 2
111
Figure 16–12. Three-dimensional representation of the rhombencephalon and part of the mesencephalon. The orientation of views B and C is shown in the key drawing. (A) The left half seen from the median plane. Intramural fibers of the trochlear nerve are indicated by two, closely set interrupted lines which run in the rostralmost part of the isthmic segment inside a ventricular crest. (B) Block with the roof of the mesencephalon removed, so that part of the floor of the isthmic segment becomes visible. At the left, the cerebellum (mostly Rh. 1) is seen to be much wider than the mesencephalon. (C) Block showing the ventral part of the rhombencephalon, which is composed of rhombomeres 2 to 8.
112
A
1
TA
B C
Longitudinal
C h a p t e r 1 6 : EVAGINATION OF THE NEUROHYPOPHYSIS
23
4
Posterior communicating
D
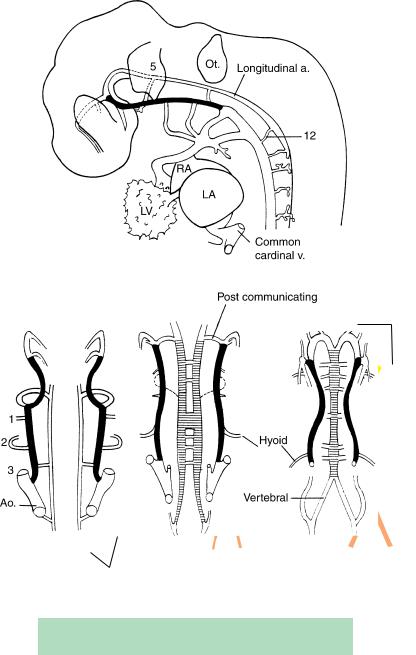
Figure 16–13. The arterial system at about 4 to 5 postfertilizational weeks. The internal carotid artery is shown in black, and the basilar by horizontal hatching. These four drawings are simplified versions of the graphic reconstructions made by Padget (1948), whose work should be studied for further details. The venous system at stages 14 and 16 has been illustrated by Padget (1957, Figs. 2 and 3). (A) Lateral view at stage 13. The longitudinal artery (which is present bilaterally) is connected to the internal carotid (black) by temporary trigeminal, otic, and hypoglossal arteries (green). Aortic arches 1 to 4 are proceeding from the truncus arteriosus (TA). (B) Stage 13. The bilateral longitudinal arteries will unite to constitute the basilar.
(C) Stage 14. The posterior communicating artery can be identified and at this time is the major supply of carotid blood to the hindbrain. Anastomotic channels unite the two longitudinal arteries, thereby initiating the formation of the basilar artery. Aortic arch 2 supplies the hyoid artery, which in turn will give rise to the stapedial artery. The interrupted lines indicate the caroticobasilar anastomosis. (D) Stage 16. The two longitudinal vessels have now united to form the basilar artery, and the vertebral arteries are evident. The posterior cerebral arteries (yellow) have begun their development.
TABLE 16–1. Relationship Between Padget’s Vascular
Phases and Carnegie Stages
Arterial Phases |
Venous Phases |
Carnegie Stages |
|
|
|
— |
1 |
13 |
1 |
— |
13–14 |
2 |
2 |
14 |
— |
3 |
15–16 |
3 |
— |
16 |
4 |
— |
17 |
— |
4 |
17–18 |
5 |
— |
18–19 |
— |
5 |
19 |
6 |
6 |
20–21 |
7 |
7 |
40 mm GL |
— |
7a |
60–80 mm GL |
|
|
|

NEUROTERATOLOGY |
113 |
NEUROTERATOLOGY: HOLOPROSENCEPHALY
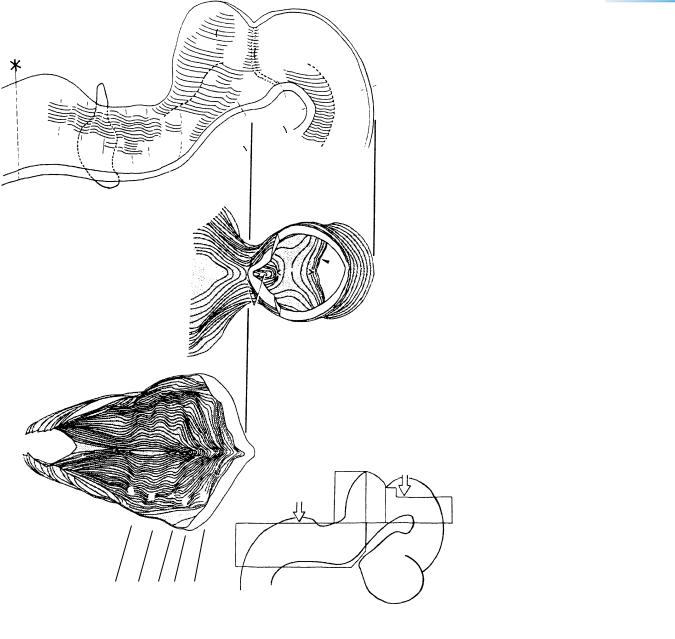
Holoprosencephaly is a term used for a graded series of cerebral and facial anomalies that range from cyclopia to the almost normal. The “entire prosencephalon remains holospheric rather than becoming hemispheric” (DeMyer, 1987); i.e., the prosencephalon remains as a whole, a single, more or less undifferentiated sphere, incompletely cleft into complex halves. In the telencephalon the future septal area and the commissural plate (future anterior commissure and corpus callosum) are missing, and the telencephalic portion of the corpus striatum is not formed (Muller¨ and O’Rahilly, 1989c). At the diencephalic level, disturbances are less evident, except at the level of the optic primordia. The adenohypophysis may be absent or may merely become detached. Its absence may perhaps be related to a disturbance of the prechordal plate.
It is possible that holoprosencephaly involves a fundamental event, such as induction, and has an early origin (probably in stage 8). Absence of the rostral part of the notochord (with consequent cranial defects) could result from faulty interaction with the prechordal plate. At stage 8 a mesenchymal disturbance could already be active. Faulty development of the paraxial mesenchyme from the primitive streak or node could interfere with the elevation of the neural folds. Defective prechordal mesenchyme may fail to produce the normal facial structures and to induce adequate morphogenesis of optic primordia and of the brain. In stage 10 production of the neural crest is beginning. The ganglia of the cranial nerves derived
from rhombencephalic neural crest are smaller in holoprosencephaly. Many basal areas of the forebrain that become distinct in stage 14 are absent in holoprosencephaly, e.g., the olfactory region (paleocortex), the optic stalks, and the neurohypophysial area. Hence, holoprosencephaly is more than a mere failure to “diverticulate” into cerebral hemispheres. In those rare instances where holoprosencephaly is found independently of synophthalmia and arhinencephaly, the origin is possibly at a later time, but nervertheless not later than stage 13 or 14. Such conditions, although variable in their manifestation, would arise during the first 4 or 5 weeks (Fig. 16–14E, F).
Holoprosencephaly is more than 60 times more frequent prenatally than in live births. Examples from stages 13–23 have been described (Yamada et al., 2004). The condition may be autosomal dominant, autosomal recessive, or X-linked recessive, and about half of affected newborns have chromosomal disorders (chiefly chromosome 13, 18, or 21; deletions in chromosome 7 may be implicated). Association with trisomy 13 and triploidy may also occur.
Holoprosencephaly is commonly graded into (1) alobar (a holotelencephalon that lacks division into hemispheres; it can be detected ultrasonically by the demonstration of a single ventricle), (2) semilobar (incomplete cerebral hemispheres), and (3) lobar (well-formed cerebral hemispheres and longitudinal fissure). Alobar holoprosencephaly has been visualized in vivo by 3D ultrasound at 7 12 postfertilizational weeks (Blaas et al., 2000b). The clinical manifestations of holoprosencephaly, together with a survey of holoprosencephalic syndromes and an atlas of 35 cases, can be found in a useful book by Siebert et al. (1990).
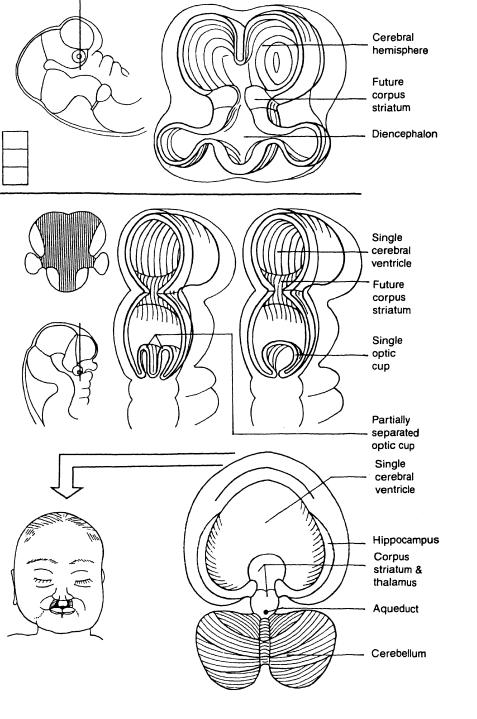
114
A
Telencephalon
Diencephalon
Medial ventricular eminence
D E
C
G
C h a p t e r 1 6 : EVAGINATION OF THE NEUROHYPOPHYSIS
B |
|
Figure 16–14. Holoprosencephaly. (A) |
|
and (B) The normal telencephalon and |
|
|
|
|
|
|
diencephalon at 5 weeks (stage 16). (C–F) |
|
|
The development of holoprosencephaly. |
|
|
(D) A ventral view in which shading |
|
|
indicates the basal part of the brain that is |
|
|
missing. (E) and (F) Dorsal views that |
|
|
show a single telencephalic ventricle. In E |
|
|
the optic cups are partially separated, |
|
|
whereas F has only a single optic cup. |
|
|
(G) Alobar holoprosencephaly in an infant |
|
|
presenting cebocephaly, slight |
|
|
hypotelorism, and median cleft lip and |
|
|
palate. (H) Dorsal view of another example |
|
|
of alobar prosencephaly after removal of |
|
|
the scalp and skull. The single |
|
F |
telencephalic ventricle is evident. A to F |
|
are based on personal reconstructions, G |
|
|
|
|
|
|
and H on photographs in Siebert et al. |
|
|
(1990) and in DeMyer (1975), respectively. |
H

C H A P T E R 17
STAGE 17: THE FUTURE
OLFACTORY BULBS AND
THE FIRST AMYGDALOID
NUCLEI
Approximately 11–14 mm in Greatest Length;
Approximately 40 Postfertilizational Days
Rostral and caudodorsal growth of the cerebral hemispheres results in deepening of the longitudinal fissure, in which the vessels of the future choroid
plexus develop. The olfactory bulb and tubercle become outlined. The amygdaloid area, which is related to the medial ventricular eminence, contains three to four nuclei.
The first dopamine-producing areas are present. The first indication of a septal nucleus is recognizable. The hemispheric stalk unites the cerebral hemispheres with the ventral thalamus and with the diencephalic part of the medial ventricular eminence. The di-mesencephalic boundary passes between the posterior commissure and the commissure of the superior colliculi (Table 15–2). Gustatory fibers are beginning to separate from the common afferent tract.
The Embryonic Human Brain: An Atlas of Developmental Stages, Third Edition. By O’Rahilly and Muller¨ Copyright C 2006 John Wiley & Sons, Inc.
115
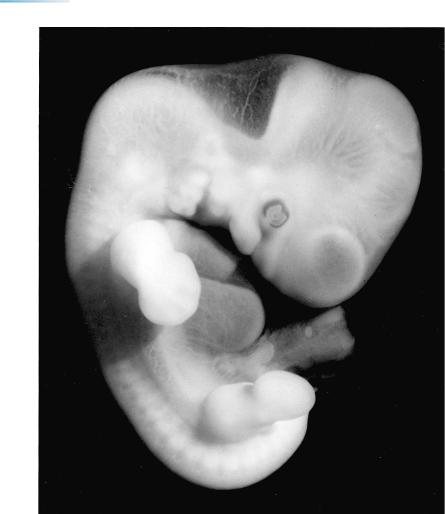
116 |
C h a p t e r 1 7 : THE FUTURE OLFACTORY BULBS AND THE FIRST AMYGDALOID NUCLEI |
Figure 17–1. The various parts of the brain are clearly visible not only in reconstructions but even through the translucent tissues of the intact embryo. The pontine flexure is now deeper, and the interval between the hypothalamus and the rhombencephalon has become narrow. The future frontal and temporal poles are marked α and γ in the key drawing of Figure 2. A three-dimensional reconstruction, prepared ultrasonically, of the ventricles in vivo in an embryo of 13 mm is illustrated by Blaas et al. (1995b).
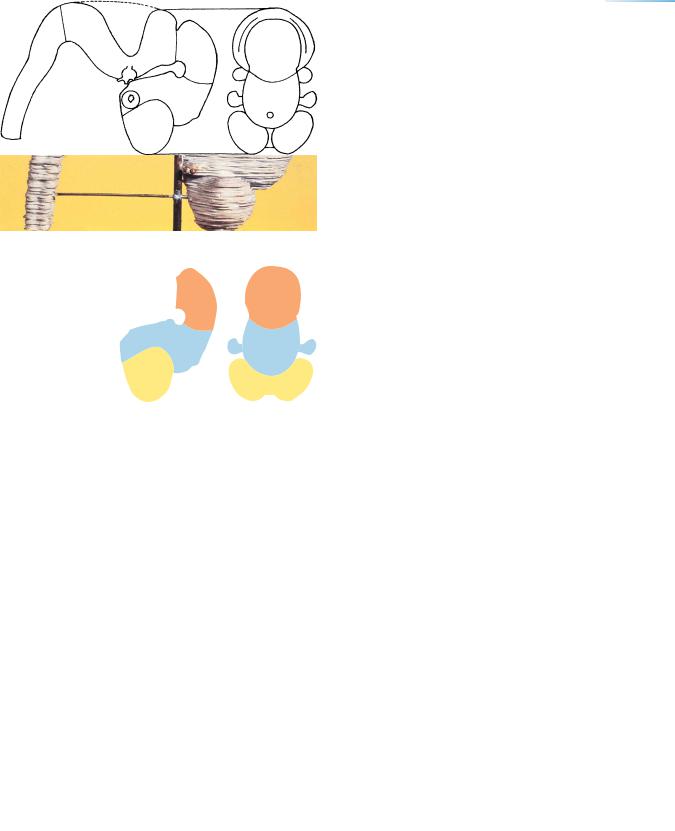
THE FUTURE OLFACTORY BULBS AND THE FIRST AMYGDALOID NUCLEI |
117 |
γ
α
Figure 17–2. Solid (Born) reconstruction and key. The growth of the cerebral hemispheres initiates the appearance of the longitudinal fissure. The telencephalon begins to overlap the diencephalon.
Figure 17–3. Lateral view (reversed) of the brain. The cerebellar plate is as wide as the combined width of the two cerebral hemispheres (Fig. 17–2). The inferior cerebellar peduncle is present already, the superior is distinguishable at the next stage, and the middle peduncle develops during the fetal period. The posterolateral fissure delimits the external cerebellar swelling. Included in this figure are the oculomotor and trochlear nerves, the trigeminal ganglion, the three divisions of the trigeminal nerve, and the optic cup. Drawn by Mary M. Cope from a reconstruction.
