
Книги по МРТ КТ на английском языке / PLUM AND POSNER S DIAGNOSIS OF STUPOR AND COM-1
.pdf
126 Plum and Posner’s Diagnosis of Stupor and Coma
difficult to distinguish from brain tissue, particularly if the hematomas are bilaterally symmetric and do not cause the brain to shift. The lack of definable sulci in the area of the hematoma and a ‘‘supraphysiologic’’-appearing brain in an elderly individual (i.e., a brain that lacks atrophy and deep sulci, usually seen with aging) are clues to the presence of bilateral isodense subdural hematomas. Chronic subdurals may become hypodense. A CT scan with contrast clearly defines the hematoma as the membranes, with a luxuriant, leaky vascular supply, enhance profusely. MRI scanning can also define the hematoma, but the density is a complex function of the sequence used and age of the hemorrhage.
Lumbar puncture is potentially dangerous in a patient with a subdural hematoma. If the brain is balanced on the edge of herniation, the sudden relief of subarachnoid pressure from below may further enhance the pressure cone and lead to frank herniation. In such patients, the CSF pressure may be low, due to the blockage at the foramen magnum, leading to a false sense of security. Hence, all patients who have an impaired level of consciousness require an imaging study of the brain prior to lumbar puncture, even if meningitis is a consideration.33 This issue is discussed further in the section on meningitis on page 133.
The treatment of subdural hematomas has traditionally been surgical.34 Three surgical procedures, twist drill drainage, burr hole drainage,
and craniotomy with excision of membranes, are used.34,35 The procedure chosen depends
on whether the subdural hematoma has developed membranes, requiring more extensive drainage, or is complex and compartmentalized, requiring excision of the membranes. The outcome of treatment varies in different series and probably reflects differences in the patient population.34
Although there have been no randomized clinical trials of medical treatment of subdural hematomas, many patients who have modestsized subdural hematomas with minimal symptoms (typically only a headache) and considerable ventricular and cisternal space, so there is no danger of herniation, can be treated conser-
vatively with corticosteroids for several months until the hematoma resorbs.31,36 However, sub-
dural hematomas have a tendency to recur after both medical and surgical therapy, and patients must be followed carefully for the first several months after apparently successful treatment.
Patient 4–1
A 73-year-old professor of art history developed chronic bifrontal, dull headache. He had no history of head trauma, but was taking 81 mg of aspirin daily for cardiovascular prophylaxis. He felt mentally dulled, but his neurologic examination was normal. CT scan of the brain disclosed bilateral chronic (low density) subdural hematomas of 8 mm depth on the left and 11.5 mm on the right. He was started on 20 mg/day of prednisone with immediate resolution of the headaches, and over a period of 2 months serial CT scans showed that the hematoma resolved spontaneously (see Figure 4–2). Repeat scan 3 months later showed no recurrence.
Epidural Abscess/Empyema
In developing countries, epidural infections are a feared complication of mastoid or sinus infection.37 In developed countries, neurosurgical procedures,38 particularly second or third craniotomies in the same area, and trauma are more likely causes.39 Sinusitis and otitis, if inadequately treated, may extend into the epidural space, either along the base of the temporal lobe or along the surface of the frontal lobe. The causative organisms are usually aerobic and anaerobic streptococci if the lesion originates from the ear or the sinuses, and Staphylococcus aureus if from trauma or surgery. The patient typically has local pain and fever. Vomiting is common37; focal skull tenderness and meningism suggest infection rather than hemorrhage. The pathophysiology of impairment of consciousness is similar to that of an epidural hematoma, except that epidural empyema typically has a much slower course and is not associated with acute trauma. CT scan is characterized by a crescentic or lentiform mass between the skull and the brain with an enhanced rim. Diffusion is restricted on diffusion-weighted
MRI, distinguishing it from hematomas or effusions where diffusion is normal or increased.40,41
Antibiotics and surgical drainage are effective treatments.38 The causal organisms can usually be cultured to allow appropriate selection of antibiotics. Some children whose epidural abscess originates from the sinuses can be treated
conservatively with antibiotics and drainage of the sinus rather than the epidural mass.42
Dural and Subdural Tumors
A number of tumors and other mass lesions may invade the dura and compress the brain. These lesions include dural metastases,43 primary tumors such as hemangiopericytoma,44 hematopoietic neoplasms (plasmacytoma, leukemia, lymphoma), and inflammatory diseases such as sarcoidosis.44 These are often mistaken for the most common dural tumor, meningioma.45
Meningiomas can occur anywhere along the dural lining of the anterior and middle cranial fossas. The most common locations are over the convexities, along the falx, or along the base of the skull at the sphenoid wing or olfactory tubercle. The tumors typically present by compression of local structures. In some cases, this produces seizures, but over the convexity there may be hemiparesis. Falcine meningiomas may present with hemiparesis and upper motor neuron signs in the contralateral lower extremity; the ‘‘textbook presentation’’ of paraparesis is quite rare. If the tumor occurs near the frontal pole, it may compress the medial prefrontal cortex, causing lapses in judgment, inconsistent behavior, and, in some cases, an apathetic, abulic state. Meningioma underlying the orbitofrontal cortex may similarly compress both frontal lobes and present with behavioral and cognitive dysfunction. When the tumor arises from the olfactory tubercle, ipsilateral loss of smell is a clue to the nature of the problem. Meningiomas of the sphenoid wing may invade the cavernous sinus and cause impairment of the oculomotor (III), trochlear (IV), and abducens nerves (VI) as well as the first division of the trigeminal nerve (V1).
On rare occasions, a meningioma may first present symptoms of increased intracranial pressure or even impaired level of consciousness. Acute presentation with impairment of consciousness may also occur with hemorrhage into a meningioma. Fortunately, this condition is rare, involving only 1% to 2% of meningiomas, and may suggest a more malignant phenotype.46 In such cases, the tumor typically has reached sufficient size to cause diencephalic compression or herniation. There is often considerable edema of the adjacent brain, which may be due in part to the leakage of blood ves-
Specific Causes of Structural Coma |
127 |
sels in the tumor or to production by the tumor of angiogenic factors.47 Treatment with corticosteroids reduces the edema and may be lifesaving while awaiting a definitive surgical procedure.
On CT scanning, meningiomas are typically isodense with brain, although they may have areas of calcification. On MRI scan, a typical meningioma is hypointense or isointense on T1-weighted MRI and usually hypointense on T2. In either imaging mode, the tumor uniformly and intensely enhances with contrast unless it is heavily calcified, a situation where the CT scan may give more accurate information. The CT scan may also help in identifying bone erosion or hyperostosis, the latter rather characteristic of meningiomas. Meningiomas typically have an enhancing dural tail that spreads from the body of the tumor along the dura, a finding less common in other dural tumors. The dural tail is not tumor, but a hypervascular response of the dura to the tumor.48
Dural malignant metastases and hematopoietic tumors grow more rapidly than meningiomas and cause more underlying brain edema. Thus, they are more likely to cause alterations of consciousness and, if not detected and treated early enough, cerebral herniation. Breast and prostate cancer and M4-type acute myelomonocytic leukemia have a particular predilection for the dura, and that may be the only site of metastasis in an otherwise successfully treated patient. CT and MRI scans may be similar to those of meningioma, the diagnosis being established only by surgery.
PITUITARY TUMORS
Tumors of the pituitary fossa are outside the brain and its coverings, separated from the subarachnoid space by the diaphragma sellae, a portion of the dura that covers the pituitary fossa but that contains an opening for the pituitary stalk. Pituitary tumors may cause alterations of consciousness, either by causing endocrine failure (see Chapter 5) or by hemorrhage into the pituitary tumor, so-called pituitary apoplexy.49
Pituitary adenomas typically cause symptoms by growing out of the pituitary fossa. Because the optic chiasm overlies the pituitary fossa, the most common finding is bitemporal hemianopsia. If the tumor extends laterally through the wall of the sella turcica into the cavernous sinus, there may be impairment of cranial
128 Plum and Posner’s Diagnosis of Stupor and Coma
nerves III, IV, VI, or V1. In some cases, pituitary tumors may achieve a very large size by suprasellar extension. These tumors compress the overlying hypothalamus and basal forebrain and may extend up between the frontal lobes or backward down the clivus. Such tumors may present primarily with prefrontal signs, or signs of increased ICP, but they occasionally present with impairment of consciousness.
The most common endocrine presentation in women is amenorrhea and in some galactorrhea due to high prolactin secretion. Prolactin is the only pituitary hormone under inhibitory control; if a pituitary tumor damages the pituitary stalk, other pituitary hormones fall to basal levels, but prolactin levels rise. Most pituitary adenomas are nonsecreting tumors, but some pituitary tumors may secrete anterior pituitary hormones, resulting in Cushing’s syndrome (if the tumor secretes adrenocorticotropic hormone [ACTH]), hyperthyroidism (if it secretes thyroid-stimulating hormone [TSH]), galactorrhea/amenorrhea (if it secretes prolactin), or acromegaly (if it secretes growth hormone).
Pituitary adenomas may outgrow their blood supply and undergo spontaneous infarction or hemorrhage. Pituitary apoplexy49 presents with the sudden onset of severe headache, signs of
local compression of the optic chiasm, and sometimes the nerves of the cavernous sinus.50,51
There may be subarachnoid blood and there often is impairment of consciousness. It is not clear if the depressed level of consciousness is due to the compression of the overlying hypothalamus, the release of subarachnoid blood (see below), or the increase in intracranial pressure. If there are cranial nerve signs, pituitary apoplexy is often sufficiently characteristic to be diagnosed clinically, but if the main symptoms are due to subarachnoid hemorrhage, it may be confused with meningitis or meningoencephalitis52; the correct diagnosis is easily confirmed by MRI or CT scan (Figure 4–3). If the tumor is large, it typically requires surgical intervention. However, subarachnoid hemorrhage can be treated conservatively. The hemorrhage may destroy the tumor; careful follow-up will determine whether there is remaining tumor that continues to endanger the patient.
Craniopharyngiomas are epithelial neoplasms that are thought to arise from a remnant of Rathke’s pouch, the embryologic origin of the anterior pituitary gland.53 The typical presentation is similar to that of a pituitary tumor, but craniopharyngiomas are often cystic and may rupture, releasing thick fluid into the subarachnoid space that may cause a chemical meningitis (see below). Craniopharyngiomas are more common in childhood, but there is a second peak in the seventh decade of life.54
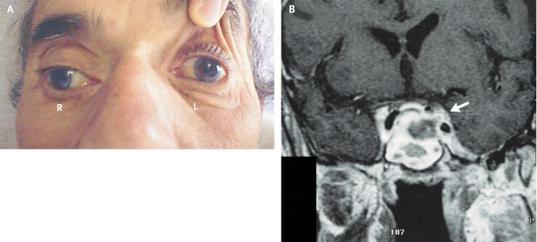
Figure 4–3. Images from a patient with pituitary apoplexy. This 63-year-old man had a severe headache with sudden onset of left III and IV nerve palsies. In A, the examiner is holding the left eye open because of ptosis, and the patient is trying to look to his right. An MRI scan, B, shows a hemorrhage (bright white on T1 imaging) into a large pituitary tumor that is invading the left cavernous sinus (arrow). The tumor abuts the optic chiasm. In pituitary apoplexy, there may be sudden visual loss in either or both eyes if the optic nerves are compressed, or in a bitemporal pattern if the chiasm is compressed, as well as impairment of some combination of cranial nerves III, IV, VI, and V1.
PINEAL TUMORS
The pineal gland is technically outside the brain, sitting in the subdural space overlying the pretectal area and rostral midbrain. Tumors of the pineal gland commonly compress the dorsal surface of the midbrain, causing Parinaud’s syndrome (loss of upward gaze, large poorly reactive pupils, and retractory convergence nystagmus), which points to the diagnosis. The tumor may also compress the cerebral aqueduct, causing hydrocephalus; typically this only alters consciousness when increased intracranial pressure from hydrocephalus causes plateau waves (see page 93) or if there is sudden hemorrhage into the pineal tumor (pineal apoplexy).55 CT or MRI will demonstrate both the tumor and the hydrocephalus and can detect hemorrhage into the tumor.
SUBARACHNOID LESIONS
Like epidural, dural, and subdural lesions, subarachnoid lesions are outside of the brain itself. Unlike epidural or dural lesions, alterations of consciousness resulting from subarachnoid lesions are not usually the result of a mass effect, but occur when hemorrhage, tumor, or infection either compress, infiltrate, or cause inflammation of blood vessels in the subarachnoid space that supply the brain, or alter CSF absorptive pathways, thus causing hydrocephalus. Thus, strictly speaking, in some cases the damage done by these lesions may be more ‘‘metabolic’’ than structural. On the other hand, subarachnoid hemorrhage and bacterial meningitis are among the most acute emergencies encountered in evaluating comatose patients, and for that reason this class of disorders is considered here.
Subarachnoid Hemorrhage
Subarachnoid hemorrhage, in which there is little if any intraparenchymal component, is usually due to a rupture of a saccular aneurysm, although it can also occur when a superficial arteriovenous malformation ruptures. Saccular aneurysms occur throughout life, generally at branch points of large cerebral arteries, such as the origin of the anterior communicating artery from the anterior cerebral artery; the origin of the posterior communicating artery from
Specific Causes of Structural Coma |
129 |
the posterior cerebral artery; the origin of the posterior cerebral artery from the basilar artery; or the origin of the middle cerebral artery from the internal carotid artery. Microscopic examination discloses an incomplete elastic media, which results in an aneurysmal dilation that may enlarge with time. Aneurysms are found with increasing frequency with age.
Aneurysms are typically silent until they hemorrhage. Some ruptures are presaged by a severe headache, a so-called sentinel headache,56,57 presumably resulting from sudden dilation or leakage of blood from the aneurysm. The frequency of sentinel headaches varies in different series from 0% to 40%. Giant aneurysms of the internal carotid artery sometimes occur in the region of the cavernous sinus, and these may present as a mass lesion causing impairment of the cranial nerves of the cavernous sinus (III, IV, VI, and V1) or by compressing the frontal lobes. Occasionally an aneurysm of the posterior communicating artery compresses the adjacent third nerve causing ipsilateral pupillary dilation. For this reason, new onset of anisocoria even in an awake patient is considered a medical emergency until the possibility of a posterior communicating artery aneurysm is eliminated.
Unfortunately, most aneurysms are not apparent until they bleed. The classic presentation of a subarachnoid hemorrhage is the sudden onset of the worst headache of the patient’s life. However, many other types of headaches may present in this way (e.g., ‘‘thunderclap headache’’),58 so it is often necessary to rule out subarachnoid hemorrhage in the emergency department. If the hemorrhage is sufficiently large, the sudden pressure wave, as intracranial pressure approximates arterial pressure, may result in impaired cerebral blood flow and loss of consciousness. About 12% of patients with subarachnoid hemorrhage die before reaching medical care.59 At the other end of the spectrum, if the leak is small or seals rapidly, there may be little in the way of neurologic signs. The most important finding is impairment of consciousness. The symptoms may vary from mild dullness to confusion to stupor or coma. The cause of the behavioral impairment after subarachnoid hemorrhage is not well understood. It is believed that the blood excites an inflammatory response with cytokine expression that may diffusely impair brain metabolism as well as cause brain edema. Parenchymal signs are
130 Plum and Posner’s Diagnosis of Stupor and Coma
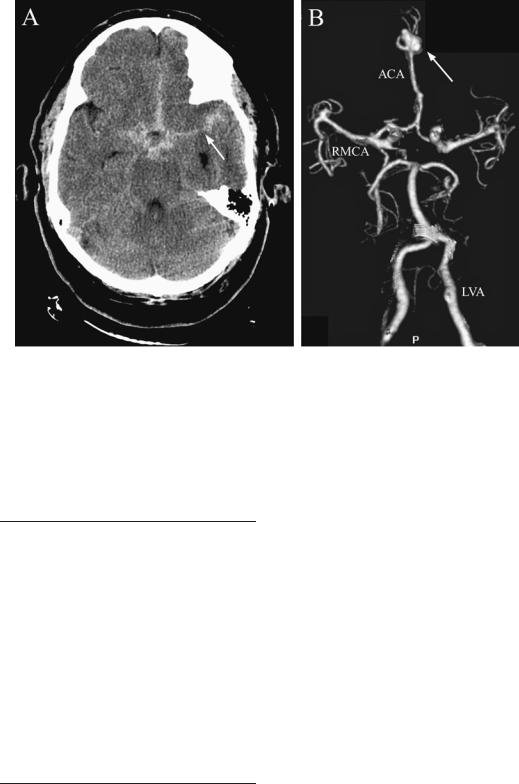
Figure 4–4. A 66-year-old man was brought to the Emergency Department after sudden onset of a severe global headache with nausea and vomiting. His legs collapsed under him. CT scan (A) showed blood in the cisterns surrounding the circle of Willis at the base of the brain, with blood extending into the interhemispheric fissure at the midline, and the right Sylvian fissure (arrow). A CT angiogram (B) showed that the anterior cerebral arteries were fused from the anterior communicating artery up to a bifurcation point, at which a large saccular aneurysm was noted (arrow). ACA, anterior cerebral artery; LVA, left vertebral artery; RMCA, right middle cerebral artery.
often lacking unless a jet of blood from the ruptured aneurysm has damaged the brain.
Patient 4–2
An 18-year-old woman was brought to the emergency department by her sister because she had been confused and forgetful for 2 days. She did not offer a history of headache, but upon being asked, the patient did admit that she had one. On examination the neck was stiff, but the neurologic examination showed only lethargy and inattention. A CT scan disclosed a subarachnoid hemorrhage, with blood collection around the circle of Willis on the right side. Lumbar puncture yielded bloody fluid, with 23,000 red blood cells and 500 white blood cells. Cultures were negative. A cerebral angiogram demonstrated a saccular aneurysm at the junction of the internal carotid and middle cerebral arteries on the right.
CT scans are highly sensitive to subarachnoid blood, making the diagnosis in more than 95% of cases if done within 12 hours60 (Figure 4–4). MRI fluid-attenuated inversion recovery (FLAIR) sequences may be more sensitive,61,62 but in a patient with a suspected subarachnoid
hemorrhage if the CT is negative, a lumbar puncture is mandatory.57,62,63 As lumbar punc-
ture itself may introduce blood into the CSF, the analysis of blood in the CSF is of great importance. Signs that suggest that the blood was present before the tap include the persistence of the same number of red cells in tubes 1 and 4, or the presence of crenated red blood cells and/or xanthochromia if the hemorrhage is at least several hours old. Spectrophotometry of CSF is available in some institutions.64 Another alternative is to centrifuge the CSF and test the supernate with a urine dipstick for blood. If the bleeding preceded the tap by at least 6 hours, it is likely that there will be blood breakdown products in the CSF, which can be visualized on the dipstick.
Even in those patients who are not comatose on admission, alterations of consciousness may develop in the ensuing days. Deterioration may occur due to rebleeding, which is particularly common in the first 24 to 48 hours. About 3 to 7 days after the hemorrhage, cerebral vasospasm may occur.65 Vasospasm typically develops first and is most intense in the area of the greatest amount of extracerebral clot. This delayed cerebral ischemia may result in brain infarction and further edema, thus exacerbating the impairment of consciousness. Acutely developing hydrocephalus66 from obstruction of spinal fluid pathways may also impair consciousness. The patient should be observed carefully for these complications and appropriate treatment applied.65,66
Subarachnoid Tumors
Both benign and malignant tumors may invade the subarachnoid space, infiltrating the leptomeninges either diffusely or focally and sometimes invading roots, or growing down the Virchow-Robin spaces to invade the brain. Leptomeningeal tumors include lymphomas and leukemias and solid tumors such as breast, renal cell, and lung cancers, as well as medulloblastomas and glial tumors.67–69 The hallmark of meningeal neoplasms is multilevel dysfunction of the nervous system, including signs of damage to cranial or spinal nerves, spinal cord, brainstem, or cerebral hemispheres. Many patients with meningeal carcinoma have impairment of consciousness that is difficult to explain on the basis of the distribution of the tumor cells. The cause of the depressed level of consciousness in these patients is not clear. Explanations have included hydrocephalus from obstruction of spinal fluid pathways,70,71 invasion of the brain along the Virchow-Robin spaces of penetrating pial vessels (the so-called encephalitic form of metastatic carcinoma),72
nonconvulsive status epilepticus,73 interference by the tumor with cortical metabolism,74,75 or
an immunologic response to the tumor76 with production of cytokines and prostaglandins; most patients also have some white blood cells in their CSF as well as tumor cells.
The diagnosis of subarachnoid tumor is challenging, particularly when the multilevel dysfunctions of the nervous system are the first signs of the tumor. The MRI scan may show
Specific Causes of Structural Coma |
131 |
tumor implants in the leptomeninges or on the surface of the brain, or it may demonstrate thickening of cranial nerve or spinal roots (Figure 4–5). If the scan is negative, the diagnosis is established by the presence of tumor cells77 or tumor markers78 in the spinal fluid. However, the clinician must think of the diagnosis to perform these tests. Fortunately, there are nearly always other abnormalities in the CSF (lymphocytes, low glucose, elevated protein), which may lead to repeat examination if the first cytology is negative, as CSF cytology has a low degree of sensitivity. Wasserstrom and colleagues found that in patients with pathologically demonstrated meningeal carcinoma or lymphoma, only 40% of the first CSF samples contained malignant cells.79
Although the diagnosis of meningeal cancer generally indicates a poor prognosis, there are occasional patients with leukemia, lymphoma, or breast cancer in whom vigorous treatment of the meningeal tumor may result in marked improvement or even complete remission. Treatment usually includes high-dose intravenous80 or intraventricular chemotherapy, as well as irradiation of areas of focal central nervous system (CNS) dysfunction (but not the entire neuraxis).81
Subarachnoid Infection
Subarachnoid infection (i.e., meningitis) is a common cause of impaired consciousness. Meningitis can be either acute or chronic and can be caused by a variety of different organisms including bacteria, fungi, rickettsiae, and viruses. Neurologic signs and symptoms caused by meningitis vary depending on the acuity of the infection and the nature of the infecting organisms, but certain aspects are common to all. For organisms to cause meningitis, they must first invade the meninges. This is usually done via the bloodstream, and for this reason blood cultures will often identify the organism. Less commonly, meningitis is a result of spread of organisms from structures adjacent to the brain (sinusitis, otitis). Meningitis can also occur in the absence of sepsis if there is communication between the meninges and the surface (CSF fistula, head injury, neurosurgery). Once in the meninges, organisms multiply, inducing the macrophage system that lines the meninges and superficial blood vessels in the brain

132 Plum and Posner’s Diagnosis of Stupor and Coma
Figure 4–5. A pair of images from a magnetic resonance imaging (MRI) scan with contrast in a patient with meningeal lymphoma. This 52-year-old man presented with bilateral visual distortion and some left leg weakness. Both chronic lymphocytic leukemia and a non-Hodgkin’s lymphoma had recently been diagnosed. The MRI scan showed superficial enhancement outlining the cortical sulci (arrows).
to produce a variety of cytokines and other proinflammatory molecules that in turn attract other white cells to the meninges. The inflammatory reaction can disrupt the blood-brain barrier; obstruct spinal fluid absorptive pathways, causing hydrocephalus and cellular swelling; or cause a vasculitis of subarachnoid or penetrating cortical blood vessels with resulting cerebral ischemia or infarction. Inflammatory reactions also cause metabolic disturbances that lower the pH, promoting vasodilation and increasing cerebral blood volume, leading to increased ICP.82 Thus, although the infection itself does not cause a supratentorial mass, the combination of vasogenic and cytotoxic edema caused by the inflammatory response may produce enough diffuse mass effect to cause herniation. Both transtentorial and tonsillar herniation may occur, although both are rare.
The major causes of community-acquired bacterial meningitis include Streptococcus pneumoniae (51%) and Neisseria meninigitis
(37%).83 In immunocompromised patients,
Listeria monocytogenes meningitis accounts for about 4% of cases.84–86 Listeria meningitis may be noticeably slower in its course but has a tendency to cause brainstem abscesses. Staphylococcus aureus and, since a vaccine became
available, Haemophilus influenzae are uncommon causes of community-acquired meningitis.83
Acute bacterial meningitis is a medical emergency, as treated patients can die within hours of onset. Viral meningitis may clinically mimic bacterial meningitis, but in most cases are selflimiting. The clinical signs of acute bacterial meningitis are headache, fever, stiff neck, photophobia, and an alteration of mental status. Focal neurologic signs can occur either from ischemia of underlying brain or from damage to cranial nerves as they pass through the subarachnoid space. In a series of adults with acute bacterial meningitis,87 97% of patients had fever, 87% nuchal rigidity, and 84% headache. Nausea or vomiting was present in 55%, confusion in 56%, and a decreased level of consciousness in 51%. Papilledema was identified in only 2% of patients, although it was not tested in almost half. Seizure activity occurred in 25% of patients, but was always within 24 hours of the clinical diagnosis of acute meningitis. Over 40% of the patients had been partially treated before the diagnosis was established, so that in 30% of patients neither Gram stain nor cultures were positive. Eighteen percent of the patients died (Table 4–3).

Table 4–3 Clinical Findings in
103 Patients With Acute Bacterial
Meningitis
Symptom |
% |
|
|
Fever |
97* |
Nuchal rigidity |
87 |
Headache |
66 |
Nausea/vomiting |
55 |
Confusion |
56 |
Altered consciousness |
51 |
Seizures |
25 |
Focal signs |
23 |
Papilledema |
2 |
|
|
*Not all patients were examined for each finding.
Data from Hussein and Shafran.87
In a series of 62 adults with communityacquired acute bacterial meningitis admitted to an intensive care unit, 95% had impaired consciousness.
However, the classic triad of fever, nuchal rigidity, and alteration of mental status was present in only 44% of patients in a large series ofcommunity-acquiredmeningitis.83 Focalneurologic signs were present in one-third and included cranial nerve palsies, aphasia, and hemiparesis; papilledema was found in only 3%.
Subacute or chronic meningitis runs an indolent course and may be accompanied by the same symptoms, but also may occur in the absence of fever in debilitated or immunesuppressed patients. Both acute and chronic meningitis may be characterized only by lethargy, stupor, or coma in the absence of the other common signs. Chronic meningitis (e.g., with tuberculosis or cryptococcus) can also cause a local arteritis, resulting in cranial nerve dysfunction and focal areas of CNS infarction.88 Aspergillus meningitis, which is typically seen only in patients who have been immune suppressed, causes a hemorrhagic arteritis, which may produce a combination of focal findings and impaired consciousness. However, the impairment of consciousness in each of these cases is primarily due to the immunologic processes concerned with the infection rather than structural causes (see Chapter 5).
The examination should include careful evaluation of nuchal rigidity even in patients who are stuporous. Attempting to flex the neck in a patient with meningitis may lead to gri-
Specific Causes of Structural Coma |
133 |
macing and a rapid flexion of knees and hips (Brudzinski sign). Lateral movement of the neck, such as in eliciting the doll’s head/eye signs, is not resisted. If one flexes the thigh to the right angle with the axis of the trunk, the patient grimaces and resists extension of the leg on the thigh (Kernig sign). Pain with jolt accentuation (the patient turns the head horizontally at two to three cycles per second) is a very sensitive sign of meningismus (positive in 97% of patients with meningitis) if the patient is sufficiently awake to cooperate, but is nonspecific (positive in 40% of patients with suspected meningitis, but no pleocytosis in the CSF).89 Examination of the nose and ears for CSF discharge, and of the back for a CSF- to-skin sinus tract, may aid in the diagnosis. CSF can be distinguished from other clear fluid discharges at the bedside by its containing glucose. Measurement of beta-trace protein in the blood and discharge fluid is more accurate.90
Meningitis, particularly in children, can cause acute brain edema with transtentorial herniation as the initial sign. Clinically, such children rapidly lose consciousness and develop hyperpnea disproportionate to the degree of fever. The pupils dilate, at first moderately and then widely, then fix, and the child develops decerebrate motor signs. Urea, mannitol, or other hyperosmotic agents, if used properly, can prevent or reverse the full development of the ominous changes that are otherwise rapidly fatal.
In elderly patients, bacterial meningitis sometimes presents as insidiously developing stupor or coma in which there may be focal neurologic signs but little evidence of severe systemic illness or stiff neck. In one series, 50% of such patients with meningitis were admitted to the hospital with another and incorrect diagnosis.91,92 Such patients can be regarded incorrectly as having suffered a stroke, but this error is readily avoided by accurate spinal fluid examinations.
If meningitis is suspected, a lumbar puncture is essential. Whether it should be per-
formed before or after a CT scan is controversial.33,93,94 Some observers believe that the
diagnostic value warrants the small but definite risk. Many physicians believe that a CT scan cannot determine the safety of a lumbar puncture. Many patients with either supratentorial or infratentorial mass lesions tolerate lumbar puncture without complication; conversely, some patients with apparently normal CT may

134 Plum and Posner’s Diagnosis of Stupor and Coma
herniate. Most who want to perform CT first argue that when there is a strong suspicion of acute bacterial meningitis, one can begin antibiotics before the CT scan if the tap is done promptly after an emergent CT; Gram stain and cultures may still be positive. They further argue that the presence of a mass lesion suggests that the neurologic signs are not a result of meningitis alone and that lumbar puncture is probably unnecessary. Finally, even in the absence of a mass lesion, obliteration of the perimesencephalic cisterns or descent of the tonsils below the foramen magnum is a major risk factor for the development of herniation after a lumbar puncture. In such cases, lumbar puncture should be deferred until hyperosmolar agents (see Chapter 7) decrease the ICP. Regardless of which approach is taken, it is critical for the diagnostic evaluation not to prevent the immediate drawing of blood cultures, followed by administration of appropriate antibiotics.
In acute bacterial meningitis, CSF pressure at lumbar puncture is usually elevated. A normal or low pressure raises the question of whether there has already been partial herniation of the cerebellar tonsils. The cell count and protein are elevated, and glucose may be depressed or normal. Examination for bacte-
rial antigens sometimes is diagnostic in the absence of a positive culture. Examination of the spinal fluid helps one differentiate acute bacterial meningitis from acute aseptic meningitis (Table 4–4). Because S. pneumoniae and N. meningitidis are the most common causal organisms, empiric therapy in adults should include either ceftriaxone (4 g/day in divided doses every 12 hours), cefotaxime (up to 8 to 12 g/day in divided doses every 4 to 6 hours), or cefepime (4 to 6 g/day in divided doses every 8 to 12 hours); vancomycin should be added until the results of antimicrobial susceptibility testing are known. In elderly patients and those who are immune suppressed,
L. monocytogenes and H. influenzae play a role, and ampicillin should be added to those drugs. Meropenem may turn out to be an attractive candidate for monotherapy in elderly patients. In a setting where Rocky Mountain spotted fever or ehrlichiosis are possible infectious organisms, the addition of doxycycline is prudent.
Whether corticosteroids should be used is controversial. Adjuvant dexamethasone is recommended for children and adults with haemophilus meningitis or pneumococcal meningitis but is not currently recommended for the treatment of Gram-negative meningitis.
Table 4–4 Typical Cerebrospinal Fluid (CSF) Findings in Bacterial Versus Aseptic Meningitis
CSF Parameter |
Bacterial Meningitis |
Aseptic Meningitis |
|
|
|
Opening pressure |
>180 mm H2O |
Normal or slightly elevated |
Glucose |
<40 mg/dL |
<45 mg/dL |
CSF-to-serum glucose ratio |
<0.31 |
>0.6 |
Protein |
>50 mg/dL |
Normal or elevated |
White blood cells |
>10 to <10,000/mm3—neutrophils |
50–2,000/mm3—lymphocytes |
|
predominate |
predominate |
Gram stain |
Positive in 70%–90% of untreated |
Negative |
|
cases |
|
Lactate |
3.8 mmol/L |
Normal |
C-reactive protein |
>100 ng/mL |
Minimal |
Limulus lysate assay |
Positive indicates Gram-negative |
Negative |
|
meningitis |
|
Latex agglutination |
Specific for antigens of Streptococcus |
Negative |
|
pneumoniae, Neisseria meningitidis |
|
|
(not serogroup B), and Hib |
|
Coagglutination |
Same as above |
Negative |
Counterimmunoelectrophoresis |
Same as above |
Negative |
From Roos et al.,95 with permission

Nevertheless, if prompt antibiotic therapy is begun and the patient shows any signs of increased ICP, it is probably wise to use dexamethasone.96 CT scans may show pus in the subarachnoid space as hypodense CSF with enlargement of sulci, but in the absence of prior scans in the same patient, this is often difficult to interpret. Meningeal enhancement usually does not occur until several days after the onset of infection. Cortical infarction, which may be due to inflammation and occlusion either of penetrating arteries or cortical veins, also tends to occur late. The MRI scan is much more sensitive for showing the changes indicated above but may be entirely normal in patients with
acute meningitis (Table 4–5).97
Specific Causes of Structural Coma |
135 |
INTRACEREBRAL MASSES
Intracerebral masses by nature tend to include both destructive and compressive elements. However, in many cases, the damage from the mass effect far exceeds the damage from disruption of local neurons and white matter. Hence, we have included this class of lesions with compressive processes.
Intracerebral Hemorrhage
Intracerebral hemorrhage may result from a variety of pathologic processes that affect the blood vessels. These include rupture of deep
Table 4–5 Imaging Findings in Acute Meningitis
Finding |
CT* |
MR* |
Sensitivity |
|
|
|
|
Sulcal dilation |
Hypodense CSF; |
T1WI: Hypointense |
MR>CT |
|
enlargement of sulci |
CSF in sulci |
|
|
|
T2WI: Hyperintense |
|
|
|
CSF in sulci |
|
Leptomeningeal |
CE: Increase in density |
T1WI, CE: Marked increase |
MR>CT |
enhancement |
of subarachnoid space |
in signal intensity |
|
Ischemic cortical |
Hypodense cortical mass |
T1WI: Hypointense cortex; |
MR>CT |
infarction |
effect |
mass effect |
|
secondary to |
CE: Subacute increase |
T2WI: Hyperintense cortex, |
|
vasculitis |
in density (enhancement) |
mass effect |
|
|
|
FLAIR: Hyperintense cortex, |
|
|
|
mass effect |
|
|
|
CE: Subacute enhancement; |
|
|
|
hyperintense on T1WI |
|
|
|
DWI: Bright (white) |
|
|
|
ADC: Dark (black) |
|
Subdural collections |
Hypodense peripheral CSF |
T1WI: Hypointense |
MR>CT |
|
plus density collection |
peripheral collection |
|
|
CE: Hygroma, no; empyema, |
T2WI: Hyperintense |
|
|
yes |
peripheral collection |
|
|
|
FLAIR: hygroma, hypointense; |
|
|
|
empyema, variable |
|
|
|
CE: Hygroma, no; empyema, yes |
|
|
|
DWI: Hydroma, dark; |
|
|
|
empyema, bright |
|
|
|
ADC: Hygroma, bright; |
|
|
|
empyema, dark |
|
|
|
|
|
ADC, apparent diffusion coefficient map; CE, contrast enhanced; CSF, cerebrospinal fluid; CT, computed tomography; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; MR, magnetic resonance; T1WI, T1weighted image; T2WI, T2-weighted image.
*Intensity relative to normal brain ±.
From Zimmerman et al.,98 with permission.
