
Книги по МРТ КТ на английском языке / PLUM AND POSNER S DIAGNOSIS OF STUPOR AND COM-1
.pdf116 Plum and Posner’s Diagnosis of Stupor and Coma
descending motor systems that they are locked in (i.e., have quadriplegia and supranuclear impairment of facial and oropharyngeal motor function).85 Motor responses may be limited to vertical eye movements and blinking.
Destructive lesions of the brainstem may occur as a result of vascular disease, tumor, infection, or trauma. The most common cause of brainstem destructive lesions is the occlusion of the vertebral or basilar arteries. Such occlusions typically produce signs that pinpoint the level of the infarction. Hemorrhagic lesions of the brainstem are most commonly intraparenchymal hemorrhages into the base of the pons, although arteriovenous malformations may occur at any level. Infections that have a predilection for the brainstem include Listeria monocytogenes, which tends to cause rhombencephalic abscesses86 (see Figure 4–13). Trauma that penetrates the brainstem is usually not a problem diagnostically, as it is almost always immediately fatal.
REFERENCES
1.Ropper AH. A preliminary MRI study of the geometry of brain displacement and level of consciousness with acute intracranial masses. Neurology 39 (5), 622–627, 1989.
2.Baloh RW, Furman JM, Yee RD. Dorsal midbrain syndrome: clinical and oculographic findings. Neurology 35 (1), 54–60, 1985.
3.Cuneo RA, Caronna JJ, Pitts L, et al. Upward transtentorial herniation: seven cases and a literature review. Arch Neurol 36, 618–623, 1979.
4.van Loon J, Van Calenbergh F, Goffin J, et al. Controversies in the management of spontaneous cerebellar haemorrhage. A consecutive series of 49 cases and review of the literature. Acta Neurochir (Wien) 122, 187–193, 1993.
5.Hayreh SS The sheath of the optic nerve. Ophthalmologica 189 (1–2), 54–63, 1984.
6.Jacks AS, Miller NR. Spontaneous retinal venous pulsation: aetiology and significance. J Neurol Neurosurg Psychiatry 74, 7–9, 2003.
7.Van Uitert RL, Eisenstadt ML. Venous pulsations not always indicative of normal intracranial pressure. Letter to the editor. Arch Neurol 35, 550–550, 1978.
8.Hayreh SS. Optic disc edema in raised intracranial pressure. V. Pathogenesis. Arch Ophthalmol 95 (9), 1553–1565, 1977.
9.Hayreh SS. Anterior ischemic optic neuropathy. V. Optic disc edema an early sign. Arch Ophthalmol 99 (6), 1030–1040, 1981.
10.d’Avella D, Baroni A, Mingrino S, et al. An electron microscope study of human arachnoid villi. Surg Neurol 14(1), 41–47, 1980.
11.Browder J, Meyers R. Behavior of the systemic blood pressure, pulse rate and spinal fluid pressure associated with acute changes in intracranial pressure artificially produced. Arch Surg 36, 1–19, 1938.
12.Schumacher GA, Wolfe HG. Experimental studies on headache of. Arch Neurol Psychiat 45, 199–214, 1941.
13.Galvin JA, Van Stavern GP. Clinical characterization of idiopathic intracranial hypertension at the Detroit Medical Center. J Neurol Sci 223 (2), 157– 160, 2004.
14.Goodwin J. Recent developments in idiopathic intracranial hypertension (IIH). Semin Ophthalmol 18 (4), 181–189, 2003.
15.Ranjan P, Mishra AM, Kale R, et al. Cytotoxic edema is responsible for raised intracranial pressure in fulminant hepatic failure: in vivo demonstration using diffusion-weighted MRI in human subjects. Metab Brain Dis 20 (3), 181–192, 2005.
16.Yanagihara T, Klass DW, Piepgras DG, et al. Brief loss of consciousness in bilateral carotid occlusive disease. Arch Neurol 46 (8), 858–861, 1989.
17.Magnaes B. Body position and cerebrospinal fluid pressure. Part I: clinical studies on the effect of rapid postural changes. J Neurosurg 44, 687–697, 1976.
18.Ingvar DH, Lundberg N. Paroxysmal systems in intracranial hypertension, studied with ventricular fluid pressure recording and electroencephalography. Brain 84, 446–459, 1961.
19.Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Neurol Scand Supp 149, 1–193, 1960.
20.Ethelberg S, Jensen VA. Obscurations and further time-related paroxysmal disorders in intracranial tumors: syndrome of initial herniation of parts of the brain through the tentorial incisure. J Neurol Psychiatry 68, 130–149, 1952.
21.Lemaire JJ. [Slow pressure waves during intracranial hypertension]. Ann Fr Anesth Reanim 16 (4), 394– 398, 1997.
22.Sullivan HC. Fatal tonsillar herniation in pseudotumor cerebri. Neurology 41, 1142–1144, 1991.
23.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature 438 (7070), 954–959, 2005.
24.Fishman RA. Cerebrospinal Fluid in Diseases of the Nervous System, 2nd ed. 1992.
25.Fishman RA. Brain edema. N Engl J Med 293 (14), 706–711, 1975.
26.Mokri B. The Monro-Kellie hypothesis—applications in CSF volume depletion. Neurology 56, 1746–1748, 2001.
27.Cushing H. Some experimental and clinical observations concerning states of increased intracranial tension. Am J Med Sci 124, 375–400, 1902.
28.MacEwen W. Pyrogenic Infective Diseases of the Brain and Spinal Cord. Glasgow: James Maclehose, 1893.
29.Meyer A. Herniation of the brain. Arch Neurol Psychiatry 4, 387–400, 1920.
30.Kernohan JW. Incisura of the crus due to contralateral brain tumor. Arch Neurol Psychiatry 21, 274–287, 1929.
31.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med 314, 953–958, 1986.
32.Fisher CM. Brain herniation: a revision of classical concepts. Can J Neurol Sci 22 (2), 83–91, 1995.
33.Gadda D, Carmignani L, Vannucchi L, et al. Traumatic lesions of corpus callosum: early multidetector CT findings. Neuroradiology 46 (10), 812–816, 2004.
34.Adler DE, Milhorat TH. The tentorial notch: anatomical variation, morphometric analysis, and classification in 100 human autopsy cases. J Neurosurg 96, 1103–1112, 2002.
35.Bogousslavsky J, Regli F, Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology 38 (6), 837–848, 1988.
36.Neau JP, Bogousslavsky J. The syndrome of posterior choroidal artery territory infarction. Ann Neurol 39 (6), 779–788, 1996.
37.Kerr FW, Hallowell OW. Localization of the pupillomotor and accommodation fibers in the oculomotor nerve: experimental observations on paralytic mydriasis. J Neurol Neurosurg Psychiatry 27, 473– 481, 1964.
38.Adams JH, Graham DI, Murray LS, et al. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 12 (6), 557–563, 1982.
39.Adams JH, Graham DI, Gennarelli TA, et al. Diffuse axonal injury in non-missile head injury. J Neurol Neurosurg Psychiatry 54 (6), 481–483, 1991.
40.Burgerman RS, Wolf AL, Kelman SE, et al. Traumatic trochlear nerve palsy diagnosed by magnetic resonance imaging: case report and review of the literature. Neurosurgery 25 (6), 978–981, 1989.
41.Giuseffi V, Wall M, Siegel PZ, et al. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case-control study. Neurology 41, 239–244, 1991.
42.Nishio I, Williams BA, Williams JP. Diplopia: a complication of dural puncture. Anesthesiology 100, 158–164, 2004.
43.Simonetti F, Pergami P, Ceroni M, et al. About the original description of cerebellar tonsil herniation by Pierre Marie. J Neurol Neurosurg Psychiatry 63 (3), 412, 1997.
44.Rothfus WE, Goldberg AL, Tabas JH, et al. Callosomarginal infarction secondary to transfalcial herniation. AJNR Am J Neuroradiol 8, 1073–1076, 1987.
45.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med 314, 953–958, 1986.
46.Weiner LP, Porro RS. Total third nerve paralysis: a case with hemorrhage in the oculomotor nerve in subdural hematoma. Neurology 15, 87–90, 1965.
47.Binder DK, Lyon R, Manley GT. Transcranial motor evoked potential recording in a case of Kernohan’s notch syndrome: case report. Neurosurgery 54, 999– 1002, 2004.
48.Derakhsan I. Kernohan notch. J Neurosurg 100, 741–742, 2004.
49.Marshman LA, Polkey CE, Penney CC. Unilateral fixed dilation of the pupil as a false-localizing sign with intracranial hemorrhage: case report and literature review. Neurosurgery 49, 1251–1255, 2001.
50.Sato M, Tanaka S, Kohama A, et al. Occipital lobe infarction caused by tentorial herniation. Neurosurgery 18 (3), 300–305, 1986.
51.Keane JR. Blindness following tentorial herniation. Ann Neurol 8, 186–190, 1980.
52.Barton JJ. Disorders of face perception and recognition. Neurol Clin 21(2), 521–548, 2003.
Structural Causes of Stupor and Coma |
117 |
53.Parizel PM, Makkat S, Jorens PG, et al. Brainstem hemorrhage in descending transtentorial herniation (Duret hemorrhage). Intensive Care Med 28 (1), 85– 88, 2002.
54.Klintworth GK. The pathogenesis of secondary brainstem hemorrhages as studied in an experimental model. Am J Pathol 47 (4), 525–536, 1965.
55.Friede RL, Roessmann U. The pathogenesis of secondary midbrain hemorrhages. Neurology 16 (12), 1210–1216, 1966.
56.Duffy GP. Lumbar puncture in the presence of raised intracranial pressure. BMJ 1, 407–409, 1969.
57.Osborn AG, Heaston DK, Wing SD. Diagnosis of ascending transtentorial herniation by cranial computed tomography. AJR Am J Roentgenol 130 (4), 755–760, 1978.
58.Reeves AG, Posner JB. The ciliospinal response in man. Neurology 19 (12), 1145–1182, 1969.
59.Reich JB, Sierra J, Camp W, et al. Magnetic resonance imaging measurements and clinical changes accompanying transtentorial and foramen magnum brain herniation. Ann Neurol 33, 159–170, 1993.
60.Wijdicks EFM, Miller GM. MR imaging of progressive downward herniation of the diencephalon. Neurology 48, 1456–1459, 1997.
61.Zervas NT, Hedley-Whyte J. Successful treatment of cerebral herniation in five patients. N Engl J Med 286 (20), 1075–1077, 1972.
62.Brendler SJ, Selverstone B. Recovery from decerebration. Brain 93 (2), 381–392, 1970.
63.Schild SE, Scheithauer BW, Schomberg PJ, et al. Pineal parenchymal tumors. Clinical, pathologic, and therapeutic aspects. Cancer 72, 870–880, 1993.
64. Kretschmar CS. Germ cell tumors |
of |
the brain |
in children: a review of current literature and new |
||
advances in therapy. Cancer Invest |
15, |
187–198, |
1997. |
|
|
65.Kaufmann GE, Clark K. Continuous simultaneous monitoring of intraventricular and cervical subarachnoid cerebrospinal fluid pressure to indicate development of cerebral or tonsillar herniation. J Neurosurg 33 (2), 145–150, 1970.
66.Lubic LG, Marotta JT. Brain tumor and lumbar puncture. AMA Arch Neurol Psychiatry 72 (5), 568– 572, 1954.
67.Korein J, Cravioto H, Leicach M. Reevaluation of lumbar puncture; a study of 129 patients with papilledema or intracranial hypertension. Neurology 9 (4), 290–297, 1959.
68.Grant FC. Cerebellar symptoms produced by supratentorial tumors: a further report. Arch Neurol Psychiat 20, 292–308, 1928.
69.Battaglia-Mayer A, Caminiti R. Optic ataxia as a result of the breakdown of the global tuning fields of parietal neurones 2. Brain 125, 225–237, 2002.
70.Weller M. Anterior opercular cortex lesions cause dissociated lower cranial nerve palsies and anarthria but no aphasia: Foix-Chavany-Marie syndrome and ‘‘automatic voluntary dissociation’’ revisited. J Neurol 240 (4), 199–208, 1993.
71.Adams JH. Hypoxic brain damage. Br J Anaesth 47 (2), 121–129, 1975.
72.Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to
118 Plum and Posner’s Diagnosis of Stupor and Coma
field CA1 of the hippocampus. J Neurosci 6 (10), 2950–2967, 1986.
73.Snider BJ, Gottron FJ, Choi DW. Apoptosis and necrosis in cerebrovascular disease. Ann N Y Acad Sci 893, 243–253, 1999.
74.Peter L, Nighoghossian N, Jouvet A, et al. [Delayed post-anoxic leukoencephalopathy]. Rev Neurol (Paris) 160 (11), 1085–1088, 2004.
75.Lerman-Sagie T, Leshinsky-Silver E, Watemberg N, et al. White matter involvement in mitochondrial diseases. Mol Genet Metab 84 (2), 127–136, 2005.
76.Caplan LR. ‘‘Top of the basilar’’ syndrome. Neurology 30, 72–79, 1980.
77.Montagna P, Gambetti P, Cortelli P, et al. Familial and sporadic fatal insomnia. Lancet Neurol 2(3), 167– 176, 2003.
78.Akman-Demir G, Bahar S, Coban O, et al. Cranial MRI in Behcet’s disease: 134 examinations of 98 patients. Neuroradiology 45 (12), 851–859, 2003.
79.Rosenfeld MR, Eichen JG, Wade DF, et al. Molecular and clinical diversity in paraneoplastic immunity to Ma proteins. Ann Neurol 50, 339–348, 2001.
80.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized
absence of hypocretin peptides in human narcoleptic brains. Nat Med 6, 991–997, 2000.
81.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474, 2000.
82.Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 53 (2), 154–166, 2003.
83.Scammell TE, Nishino S, Mignot E, et al. Narcolepsy and low CSF orexin (hypocretin) concentration after a diencephalic stroke. Neurology 56 (12), 1751–1753, 2001.
84.Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain 126, 1524–1536, 2003.
85.Levy DE, Sidtis JJ, Rottenberg DA, et al. Differences in cerebral blood flow and glucose utilization in vegetative versus locked-in patients. Ann Neurol 22 (6), 673–682, 1987.
86.Armstrong RW, Fung PC. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin Infect Dis 16, 689–702, 1993.

Chapter 4
Specific Causes of Structural Coma
INTRODUCTION
SUPRATENTORIAL COMPRESSIVE LESIONS
EPIDURAL, DURAL, AND SUBDURAL
MASSES
Epidural Hematoma
Subdural Hematoma
Epidural Abscess/Empyema
Dural and Subdural Tumors
SUBARACHNOID LESIONS
Subarachnoid Hemorrhage
Subarachnoid Tumors
Subarachnoid Infection
INTRACEREBRAL MASSES
Intracerebral Hemorrhage
Intracerebral Tumors
Brain Abscess and Granuloma
INFRATENTORIAL COMPRESSIVE LESIONS
EPIDURAL AND DURAL MASSES
Epidural Hematoma
Epidural Abscess
Dural and Epidural Tumors
SUBDURAL POSTERIOR FOSSA
COMPRESSIVE LESIONS
Subdural Empyema
Subdural Tumors
SUBARACHNOID POSTERIOR FOSSA LESIONS
INTRAPARENCHYMAL POSTERIOR
FOSSA MASS LESIONS
Cerebellar Hemorrhage
Cerebellar Infarction
Cerebellar Abscess
Cerebellar Tumor
Pontine Hemorrhage
SUPRATENTORIAL DESTRUCTIVE LESIONS CAUSING COMA
VASCULAR CAUSES OF SUPRATENTORIAL
DESTRUCTIVE LESIONS
Carotid Ischemic Lesions
Distal Basilar Occlusion
Venous Sinus Thrombosis
Vasculitis
INFECTIONS AND INFLAMMATORY CAUSES OF SUPRATENTORIAL DESTRUCTIVE LESIONS
Viral Encephalitis
Acute Disseminated Encephalomyelitis
CONCUSSION AND OTHER TRAUMATIC
BRAIN INJURIES
Mechanism of Brain Injury During Closed
Head Trauma
Mechanism of Loss of Consciousness
in Concussion
Delayed Encephalopathy After
Head Injury
INFRATENTORIAL DESTRUCTIVE LESIONS
119
120 Plum and Posner’s Diagnosis of Stupor and Coma
BRAINSTEM VASCULAR DESTRUCTIVE
DISORDERS
Brainstem Hemorrhage
Basilar Migraine
Posterior Reversible Leukoencephalopathy
Syndrome
INTRODUCTION
The previous chapter divided structural lesions causing coma into compressive and destructive lesions. It further indicated that lesions could be supratentorial, compressing or destroying the diencephalon and upper midbrain, or infratentorial, directly affecting the pons and cerebellum. A physician attempting to determine the cause of coma resulting from a structural lesion must establish first the site of the lesion, determining whether the lesion is supratentorial or infratentorial, and second whether the lesion is causing its symptoms by compression or destruction or both. Those considerations were the focus of Chapter 3. This chapter discusses, in turn, the specific causes of supratentorial and infratentorial compressive and destructive lesions that cause coma.
Although these designations are useful for rapid bedside diagnosis, it is of course possible for a lesion such as an intracerebral hemorrhage both to destroy and to compress normal tissues. Extracerebral mass lesions can also cause sufficient compression to lead to infarction (i.e., tissue destruction). Thus, in some instances, the division is arbitrary. However, the types of conditions that cause the compression versus destruction of neural tissue tend to be distinct, and often they have distinct clinical presentations as well. The guide provided in this chapter, while not exhaustive, is meant to cover the most commonly encountered causes and ones where understanding their pathophysiology can influence diagnosis and treatment (Table 4–1).
When any structural process impairs consciousness, the physician must find a way to halt the progression promptly or the patient will run the risk of irreversible brain damage or death. Beyond that generality, different structural lesions have distinct clinical properties that govern the rate of progression, hint at the diagnosis, and may dictate the treatment.
INFRATENTORIAL INFLAMMATORY DISORDERS
INFRATENTORIAL TUMORS
CENTRAL PONTINE MYELINOLYSIS
Structural causes of unconsciousness often cause focal signs that help localize the lesion, particularly when the lesion develops acutely. However, if the lesion has developed slowly, over a period of many weeks or even months, it may attain a remarkably large size without causing focal neurologic signs. In those cases, the first evidence of a space-occupying lesion may be signs of increased intracranial pressure (e.g., headache, nausea) or even herniation itself (see Patient 3–2).
SUPRATENTORIAL
COMPRESSIVE LESIONS
The supratentorial compartments are dominated by the cerebral hemispheres. However, many of the most dangerous and difficult lesions to diagnose involve the overlying meninges. Within the hemisphere, a compressive lesion may originate in the gray matter or the white matter of the hemisphere, and it may directly compress the diencephalon from above or laterally (central herniation) or compress the midbrain by herniation of the temporal lobe through the tentorial notch (uncal herniation). In addition, there are a number of compressive lesions that affect mainly the diencephalon.
EPIDURAL, DURAL, AND
SUBDURAL MASSES
Tumors, infections, and hematomas can occupy the epidural, dural, and subdural spaces to eventually cause herniation. Most epidural tumors result from extensions of skull lesions that grow into the epidural space. Their growth is relatively slow; they mostly occur in patients with known cancer and are usually discovered long before they affect consciousness. Dural tumors, by contrast, are usually primary tumors of the meninges, or occasionally metastases.

Specific Causes of Structural Coma |
121 |
Table 4–1 Examples of Structural Causes of Coma
Compressive Lesions |
Destructive Lesions |
|
|
Cerebral hemispheres |
Cerebral hemispheres |
Epidural and subdural hematomas, tumors, |
Hypoxia-ischemia |
and abscesses |
Hypoglycemia |
Subarachnoid hemorrhages, |
Vasculitis |
infections (meningitis), and tumors |
Encephalitis |
(leptomeningeal neoplasms)* |
Leukoencephalopathy |
Intracerebral hemorrhages, infarcts, tumors, |
Prion diseases |
and abscesses |
Progressive multifocal |
|
leukoencephalopathy |
Diencephalon |
Diencephalon |
Basal ganglia hemorrhages, tumors, |
Thalamic infarct |
infarcts, and abscesses* |
Encephalitis |
Pituitary tumor |
Fatal familial insomnia |
Pineal tumor |
Paraneoplastic syndrome |
|
Tumor |
Brainstem |
Brainstem |
Cerebellar tumor |
Infarct |
Cerebellar hemorrhage |
Hemorrhage |
Cerebellar abscess |
Infection |
|
|
*Both compressive and destructive.
Epidural or subdural hematomas, on the other hand, may develop acutely or subacutely and can be a diagnostic problem.
Epidural Hematoma
Because the external leaf of the dura mater forms the periosteum of the inner table of the skull, the space between the dura and the skull is a potential space that accumulates blood only when there has been an injury to the skull itself. Epidural hematomas typically result from head trauma with a skull fracture that crosses a groove in the bone containing a meningeal vessel (see Figure 4–1). The ruptured vessel may be either arterial or venous; venous bleeding usually develops slowly and often is self-limiting, having a course more similar to subdural hematomas, which are discussed below. On rare occasions, epidural hematomas may result from bleeding into skull lesions such as eosinophilic granuloma,1 metastatic skull or dural tumors,2 or craniofacial infections such as sinusitis.3
Arterial bleeding is usually under high pressure with the result that the vessel may not seal and blood continues to accumulate. Thus, in-
stead of causing symptoms that develop slowly or wax and wane over days or weeks, a patient with an epidural hematoma may pass from having only a headache to impairment of consciousness and signs of herniation within a few hours after the initial trauma.
Although epidural hematomas can occur frontally, occipitally, at the vertex,4 or even on the side opposite the side of trauma (contrecoup),5 the most common site is in the lateral temporal area as a result of laceration of the middle meningeal artery. Trauma sufficient to cause such a fracture may also fracture the skull base. For this reason, it is necessary for the examiner to be alert to signs of basal skull fracture on examination, such as blood behind the tympanic membrane or ecchymosis of the skin behind the ear (Battle’s sign) or around the eyes (raccoon eyes). The epidural hemorrhage pushes the brain medially, and in so doing stretches and tears pain-sensitive meninges and blood vessels at the base of the middle fossa, causing headache. However, the headache is often attributed to the original head injury, and unless the lesion causes sufficiently increased intracranial pressure (ICP) to produce nausea and vomiting, the condition may
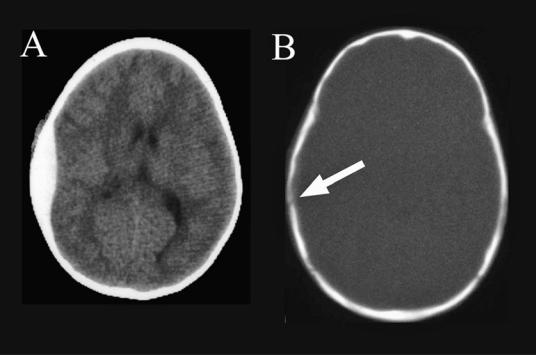
122 Plum and Posner’s Diagnosis of Stupor and Coma
Figure 4–1. A pair of computed tomography scans showing an epidural hematoma. The image in (A) shows the lensshaped (biconvex), bright mass along the inner surface of the skull. In (B), the skull is imaged with bone windows, showing a fracture at the white arrow, crossing the middle meningeal groove.
not be recognized. Subsequently, the hematoma compresses the adjacent temporal lobe and causes uncal herniation with gradual impairment of consciousness. Early dilation of the ipsilateral pupil is often seen followed by complete ophthalmoparesis and then impairment of the opposite third nerve as the herniation progresses.6 Motor signs often occur late in such cases.
In many patients the degree of head trauma is less than one might expect to cause a fracture. In Jamieson and Yelland’s series, for example, of 167 patients with epidural hematoma, nearly one-half had no initial loss of consciousness,7 and in Gallagher and Browder’s equally large series, two-thirds of such patients had an initial injury too mild to command hospital attention.8 This is particularly true in children, one-half of whom have suffered a fall of less than one-half meter, and many of whom complained of nonspecific symptoms.9 Only 15% to 20% of patients had the ‘‘classic’’ history of traumatic loss of consciousness, followed by a lucid interval and then a relapse into coma (patients who ‘‘talk and die’’).10 Thus,
even though most epidural hematomas are identified by computed tomography (CT) scans performed acutely in emergency departments on trauma patients by using current evidencebased decision paradigms,11,12 the examiner must remain alert to the possibility of an epidural hematoma that develops or rapidly enlarges after an apparently negative CT. It is therefore important to review the CT scan of trauma patients with attention directed to whether there is a skull fracture that crosses the middle meningeal groove. The hematoma appears as a hyperdense, lens-shaped mass between the skull and the brain (i.e., the hematoma is convex on both surfaces; subdural hematomas, by comparison, are concave on the surface facing the brain; see Figure 4–1). A vertex hematoma may be missed on a routine axial CT scan,13 but a coronal reconstruction should identify the lesion.4 A magnetic resonance imaging (MRI) scan is not required for evaluation of an epidural hematoma, but may be necessary to evaluate contusions and edema in the underlying brain. In addition, mass lesions outside the brain may cause hyperdensity
of subarachnoid cisterns that may be mistaken for subarachnoid hemorrhage on CT, but is probably an artifact of partial volume averaging.14 In these instances, MRI helps rule out subarachnoid hemorrhage.
In those circumstances where CT scan is not readily available, a plain skull film can often identify the fracture. Certainly, all patients with head trauma should be cautioned that it is important to remain under the supervision of a family member or friend for at least 24 hours; the patient must be returned to the hospital immediately if a lapse of consciousness occurs. Careful follow-up is required even in patients in whom the original CT was negative, as occasionally the development of the hematoma is delayed.15
In comatose patients with epidural hematomas, the treatment is surgical evacuation. The surgery is an emergency, as the duration from time of injury to treatment is an important determinant of the prognosis.16 Other factors in determining outcome are age, depth of coma, degree of midline shift, and size of the hematoma.17 Most patients operated on promptly recover, even those whose pupils are dilated and fixed before surgery.18 Rarely, acute epidural hematomas resolve spontaneously, probably a result of tamponade of the bleeding vessel by underlying edematous brain.19
Subdural Hematoma
The unique anatomy of the subdural space also can produce much slower, chronic subdural hematomas in patients in whom the history of head trauma is remote or trivial. The potential space between the inner leaf of the dura mater and the arachnoid membrane (subdural space) is traversed by numerous small draining veins that bring venous blood from the brain to the dural sinus system that runs between the two leaves of the dura. These veins can be damaged with minimal head trauma, particularly in elderly individuals with cerebral atrophy in whom the veins are subject to considerable movement of the hemisphere that may occur with acceleration-deceleration injury. When focal signs are absent, these cases can be quite difficult to diagnose. A useful rule when faced with a comatose patient is that ‘‘it could always be a subdural,’’ and hence imaging is needed even in cases where focal signs are absent.
Specific Causes of Structural Coma |
123 |
Subdural bleeding is usually under low pressure, and it typically tamponades early unless there is a defect in coagulation. Acute subdural bleeding is particularly dangerous in patients who take anticoagulants for vascular thrombotic disease. Continued venous leakage over several hours can cause a mass large enough to produce herniation. Warfarin inhibits the synthesis of vitamin K-dependent clotting factors II, VII, IX, and X and the anticoagulant proteins C and S. The conventional treatment includes administering fresh frozen plasma and vitamin K. However, these measures take hours to days to become effective and are too slow to stop subdural bleeding. Hence, in the case of a subdural (or epidural) bleed in a patient on warfarin, it is important to administer pooled cryoprecipitate of factors II, VII, IX, and X immediately. Recombinant factor VII has also been used,20 but data are lacking as to its effectiveness.
Acute subdural hematomas, which are usually the result of a severe head injury, are often associated with underlying cerebral contusions. Rarely, acute subdural hematomas may occur without substantial trauma, particularly in patients on anticoagulants. Rupture of an aneurysm into the subdural space, sparing the subarachnoid space, can also cause an acute subdural hematoma. The mass accumulates rapidly, causing underlying brain edema and herniation. Ischemic brain edema results when herniation compresses the anterior or posterior cerebral arteries and causes ischemic brain damage.21 Patients with acute subdural hematomas usually present with coma, and such cases are surgical emergencies. Early evacuation of the mass probably improves outcome, but because of underlying brain damage, mortality remains significant. Prognostic factors include age, time from injury to treatment, presence of pupillary abnormalities, immediate and persisting coma as opposed to the presence of a lucid interval, and volume of the mass.22
Chronic subdural hematomas usually occur in elderly patients or those on anticoagulants. Chronic alcoholism, hemodialysis, and intracranial hypotension are also risk factors. A history of trauma can be elicited in only about onehalf of patients, and then the trauma is usually minor. The pathogenesis of chronic subdural hematomas is controversial. One hypothesis is that minor trauma to an atrophic brain causes a small amount of bleeding. A membrane forms

124 Plum and Posner’s Diagnosis of Stupor and Coma
around the blood. Vessels of the membrane are quite friable and this, plus an increase of fi- brinolytic products in the fluid, leads to repetitive bleeding, causing an enlarging hematoma.23 Another hypothesis is that minor trauma leads to the accumulation of either serum or cerebrospinal fluid (CSF) in the subdural space. This subdural hygroma also causes membrane formation that leads to repetitive bleeding and an eventual mass lesion.24 If the hemorrhage is small and no additional bleeding occurs, the hematoma may resorb spontaneously. However, if the hematoma is larger or it is enlarged gradually by recurrent bleeds, it may swell as the breakdown of the blood into small molecules causes the hematoma to take on additional water, thus further compressing the adjacent brain.24 In addition, the membrane surrounding the hematoma contains luxuriant neovascularization that lacks a blood-brain barrier and may cause additional edema in the underlying brain. Chronic subdural hematomas are usually unilateral, overlying the lateral cerebral cortex, but may be subtemporal. They are bilateral in about 20% of patients, and occasionally are interhemispheric (i.e., within the falx cerebri), sometimes causing bilateral leg weakness by compression of the medial frontal lobes.
Table 4–2 lists the clinical features of the typical patient with a chronic subdural hematoma who presents with a fluctuating level of consciousness.
A majority of patients, but no more than
70%, complain of headache. A fluctuating level of consciousness is common.23,25,26 There may
be tenderness to percussion of the skull at the site of the hematoma. About 15% to 30% of patients present with parenchymal signs such as seizures, hemiparesis, or visual field defects. Unusual focal signs such as parkinsonism, dystonia,27 or chorea occasionally confuse the clinical picture. Focal signs such as hemiparesis or aphasia may fluctuate, giving an appearance similar to transient ischemic attacks.28 Occasionally patients may have unilateral asterixis. Because subdural hematoma can appear identical to a metabolic encephalopathy (Chapter 5), imaging is required in any patient without an obvious cause of the impairment of consciousness.
The symptoms of subdural hematoma have a remarkable tendency to fluctuate from day to day or even from hour to hour, which may
Table 4–2 Diagnostic Features
of 73 Patients With Fluctuating Level of Consciousness Due to Subdural Hematoma
Unilateral hematoma |
62 |
Bilateral hematomas |
11 |
Mortality |
14 |
|
(3 unoperated) |
Number of patients |
27 |
in stupor or coma |
|
Principal clinical diagnosis before |
|
hematoma discovered |
|
Intracranial mass lesion |
|
or subdural hematoma |
24 |
Cerebral vascular disease, but |
|
subdural hematoma possible |
17 |
Cerebral infarction |
|
or arteriosclerosis |
12 |
Cerebral atrophy |
5 |
Encephalitis |
8 |
Meningitis |
3 |
Metabolic encephalopathy |
|
secondary to systemic illness |
3 |
Psychosis |
1 |
|
|
suggest the diagnosis. The pathophysiology of fluctuations is not clear. Some may reflect increases in ICP associated with plateau waves,29 and careful clinical observations suggest that the level of consciousness reflects the patient moving in and out of diencephalic or uncal herniation. Given the breakdown in the bloodbrain barrier along the margin of the hematoma, this fluctuation may be due to fluid shifts into and out of the brain, a situation from which the brain is normally protected. When the brain is critically balanced on the edge of herniation, such fluid shifts may rapidly make the difference between full consciousness and an obtunded state. Cerebral blood flow in the hemisphere underlying a subdural hematoma is reduced, perhaps accounting for some of the unusual clinical symptoms.30
In favor of the vasogenic edema hypothesis is the observation that oral administration of corticosteroids rapidly and effectively reverses the symptoms in subdural hematoma.31 Corticosteroids reduce the leakage of fluid from capillaries,32 and they are quite effective in minimizing the cerebral edema associated with subdural hematomas.
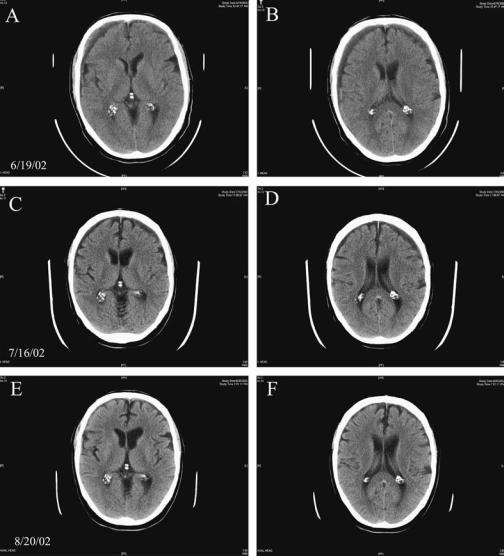
Specific Causes of Structural Coma |
125 |
Figure 4–2. A series of magnetic resonance imaging scans through the brain of Patient 4–1 demonstrating bilateral subdural hematomas and their evolution over time. In the initial scan from 6/19/02 (A, B), there is an isodense subdural hematoma of 11.5 mm thickness on the right (left side of image) and 8 mm thickness on the left. The patient was treated conservatively with oral prednisone, and by the time of the second scan 1 month later (C, D), the subdural hematomas were smaller and hypodense and the underlying brain was less edematous. By the end of the second month (E, F), the subdural hematomas had been almost completely resorbed.
Subdural hematoma can usually be diagnosed by CT scanning. Depending on the age of the bleeding, the contents of the mass between the dura and the brain may be either hyperdense or isodense (Figure 4–2). Acute subdural hematomas are hyperdense, with the rare exception of those occurring inextremely anemic patients and
those in whom CSF has entered the subdural space, diluting the blood. Although the hematoma may become isodense with brain after 2 to 3 weeks, it may still contain areas of hyperdense fresh blood, assisting with the diagnosis. However, if the entire mass is isodense and contrast is not given, the subdural hematoma may be
