
Книги по МРТ КТ на английском языке / PLUM AND POSNER S DIAGNOSIS OF STUPOR AND COM-1
.pdf
166 Plum and Posner’s Diagnosis of Stupor and Coma
where her blood pressure was 180/100 mm Hg. She had sighing respirations, which shortly changed to a Cheyne-Stokes pattern. The pupils were 4 mm in diameter and unreactive to light. The oculocephalic responses were absent, but cold caloric irrigation induced abduction of the eye only on the side being irrigated. She responded to noxious stimuli with extensor posturing and occasionally was wracked by spontaneous waves of extensor rigidity.
CT scan was initially read as normal, and she was brought to the neurology intensive care unit. The CSF pressure on lumbar puncture was 140 mm of water; the fluid was clear, without cells, and contained 35 mg/dL of protein. Two days later, the patient continued in coma with extensor responses to noxious stimulation; the pupils remained fixed in midposition, and there was no ocular response to cold caloric irrigation. Respirations were eupneic. Repeat CT scan showed lucency in the medial pons and midbrain. The next day she died and the brain was examined postmortem. The basilar artery was occluded in its midportion by a recent thrombus 1 cm in length. There was extensive infarction of the rostral portion of the base of the pons, as well as the medial pontine and midbrain tegmentum. The lower portion of the pons and the medulla were intact.
Comment: This woman suffered an acute brainstem infarction with unusually symmetric neurologic signs. She was initially diagnosed with an infarct at the midbrain level based on her clinical picture. Other considerations included a thalamic hemorrhage with sudden acute transtentorial her-
niation producing a picture of acute midbrain transection. However, such rapid progression to a midbrain level almost never occurs in patients with supratentorial intracerebral hemorrhages. The CT scan and the absence of red blood cells on the lumbar puncture ruled out subarachnoid hemorrhage as well. Finally, the neurologic signs of midbrain damage in this patient remained nearly constant from onset, whereas transtentorial herniation would rapidly have produced further rostral-caudal deterioration.
Brainstem Hemorrhage
Relatively discrete brainstem hemorrhage can affect the midbrain,249 the pons,250 or the me-
dulla.251 The causes of brainstem hemorrhage include hypertension, vascular malformations, clotting disorders, or trauma. Hypertensive brainstem hemorrhages tend to lie deep within the brainstem substance, are rather diffuse, frequently rupture into the fourth ventricle, occur in elderly persons, and have a poor prognosis for recovery.252 Brainstem hematomas caused by vascular malformations occur in younger individuals, are usually subependymal in location, tend to be more discrete, do not rupture into the ventricle, and have a good prognosis for recovery. Surgery generally does not have a place in treating brainstem hypertensive hemorrhages, but it is sometimes possible to remove a vascular malformation, particularly a cavernous angioma.
Table 4–17 Clinical Findings in Patients With Spontaneous
Midbrain Hemorrhage
|
Literature Cases |
Mayo Cases |
Combined Series |
Findings |
(N¼ 66) |
(N¼ 7) |
(N¼ 73) |
Cranial nerve III or IV paresis |
58 |
6 |
64 |
Disturbance of consciousness |
33 |
6 |
39 |
Headache |
34 |
4 |
38 |
Corticospinal tract deficits |
32 |
4 |
36 |
Corticobulbar deficits |
22* |
2 |
24 |
Hemisensory deficits |
21 |
3 |
24 |
Gait ataxia |
22 |
2 |
24 |
Visual hallucinations |
3 |
0 |
3 |
Tinnitus or hyperacusis |
3 |
2 |
5 |
|
|
|
|
*One patient had corticobulbar deficit without a corticospinal deficit.
From Link et al.,249 with permission.

Primary midbrain hemorrhages, which may be of either type, are rare. Most patients present acutely with headache, alterations of consciousness, and abnormal eye signs (Table 4–17). The diagnosis is obvious on imaging. Most patients recover completely from bleeds from cavernous angiomas; some remain with mild neurologic deficits.
Hemorrhage into the pons typically arises from the paramedian arterioles, beginning at the base of the tegmentum, and usually dissecting in all directions in a relatively symmetric fashion (Figure 4–9A). Rupture into the fourth ventricle is frequent, but dissection into the medulla is rare. Although most patients lose consciousness immediately, in a few cases (such as Patient 2–1) this is delayed, and in others when the hematoma is small, and particularly when it is confined to the base of the pons, consciousness can be retained. However, such patients often have other focal signs (e.g., a bleed into the base of the pons can present with an acute locked-in state). Such patients, however, often have considerable recovery.254
Coma caused by pontine hemorrhage begins abruptly, usually during the hours when patients are awake and active and often without a prodrome. When the onset is witnessed, only a few patients complain of symptoms such as sudden occipital headache, vomiting, dyscoordination, or slurred speech before losing consciousness.255 Almost every patient with pontine hemorrhage has respiratory abnormalities of the brainstem type: Cheyne-Stokes breathing, apneustic or gasping breathing, and progressive slowing of respiration or apnea250 (Table 4–18).
In patients who present in coma, the pupils are nearly always abnormal and usually pinpoint. The pupils are often thought to be fixed to light on initial examination, but close examination with a magnifying glass usually demonstrates further constriction. The ciliospinal response disappears. If the hemorrhage extends into the midbrain, pupils may become asymmetric or dilate to midposition. About onethird of patients suffer from oculomotor abnormalities such as skewed or lateral ocular deviations or ocular bobbing (or one of its variants), and the oculocephalic responses disappear. Motor signs vary according to the extent of the hemorrhage. Some subjects become diffusely rigid, tremble, and suffer repeated waves
Specific Causes of Structural Coma |
167 |
of decerebrate rigidity. More frequently, however, patients are quadriplegic and flaccid with flexor responses at the hip, knee, and great toe to plantar stimulation, a reflex combination characteristic of acute low brainstem damage when it accompanies acute coma. Nearly all patients with pontine hemorrhage who survive more than
a few hours develop fever with body temperatures of 38.58C to 408C.256,257
The diagnosis of pontine hemorrhage is usually straightforward. Almost no other lesion, except an occasional cerebellar hemorrhage with secondary dissection into the brainstem, produces sudden coma with periodic or ataxic breathing, pinpoint pupils, absence of oculovestibular responses, and quadriplegia. The pinpoint pupils may suggest an opiate overdose, but the other eye signs and the flaccid quadriplegia are not seen in that condition. If there is any question in an ambiguous case, naloxone can be administered to reverse any opiate intoxication.
Table 4–18 Clinical Findings in 80
Patients With Pontine Hemorrhage
Level of Consciousness |
|
|
Alert |
15 |
(0) |
Drowsy |
21 |
(3) |
Stuporous |
4 |
(3) |
Coma |
40 |
(32) |
Respiratory Disturbance |
|
|
Yes |
37 |
(29) |
Brachycardia |
|
|
Yes |
34 |
(23) |
Hyperthermia |
|
|
Yes |
32 |
(30) |
Pupils |
|
|
Normal |
29 |
(1) |
Anisocoria |
29 |
(11) |
Pinpoint |
23 |
(17) |
Mydriasis |
9 |
(9) |
Motor Disturbance |
|
|
Hemiplegia |
34 |
(4) |
Tetraplegia |
22 |
(17) |
Decerebrate posture* |
16 |
(14) |
|
|
|
*Number that died in parentheses. Modified from Murata.250

168 Plum and Posner’s Diagnosis of Stupor and Coma
Patient 4–7
A 54-year-old man with poorly treated hypertension was playing tennis when he suddenly collapsed on the court. The blood pressure was 170/ 90 mm Hg; the pulse was 84 per minute; respirations were Cheyne-Stokes in character and 16 per minute. The pupils were pinpoint but reacted equally to light; eyes were slightly dysconjugate with no spontaneous movement, and vestibuloocular responses were absent. The patient was flaccid with symmetric stretch reflexes of normal amplitude and bilateral flexor withdrawal responses in the lower extremities to plantar stimulation. CT scan showed a hemorrhage into the pontine tegmentum. The next morning he was still in deep coma, but now was diffusely flaccid except for flexor responses to noxious stimuli in the legs. He had slow, shallow, eupneic respiration; small, equally reactive pupils; and eyes in the neutral position. Shortly thereafter, breathing became irregular and he died. A 3-cm primary hemorrhage destroying the central pons and its tegmentum was found at autopsy.
The clinical features in Patient 4–7, including coma in the absence of motor responses, corneal reflexes, and oculocephalic responses, predicted the poor outcome.258 In addition, if CT scanning shows a hematoma greater than 4 mL, hemorrhage in a ventral location,259 evidence of extension into the midbrain and thalamus, or hydrocephalus on admission, the prognosis is poor.258
Primary hemorrhage into the medulla is rare.251 Patients present with ataxia, dysphagia, nystagmus, and tongue paralysis. Respiratory and cardiovascular area may occur, leaving the patient paralyzed and unable to breathe, but not unconscious.
Basilar Migraine
Altered states of consciousness are an uncommon but distinct aspect of what Bickerstaff calledbasilar artery migraine,260 associated with prodromal symptoms that suggest brainstem dysfunction. The alteration in consciousness can take any of four major forms: confusional states, brief syncope, stupor, and unarousable coma. Although not technically a destructive
lesion, and with a pathophysiology that is not understood, basilar migraine clearly causes parenchymal dysfunction of the brainstem that is often mistaken for a brainstem ischemic attack.
Alterations in consciousness often last longer than the usual sensorimotor auras seen with migraine. Encephalopathy and coma in migraine occur in patients with familial hemiplegic migraine associated with mutations in a calcium channel261 and in patients with the disorder known as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)262 (see page 276, Chapter 5). The former often have fixed cerebellar signs and the latter multiple hyperintensities of the white matter on MR scanning. Blood flow studies concurrent with migraine aura have demonstrated both diffuse and focal cerebral vasoconstriction, but this is an insufficient explanation for the striking focal symptoms in basilar migraine; however, some clinical lesions suggestive of infarction can be found in patients with migraine significantly more often than in controls.263
Selby and Lance264 observed that among 500 consecutive patients with migraine, 6.8% had prodromal episodes of confusion, automatic behavior, or transient amnesia, while 4.6% actually fainted. The confusional and stuporous attacks can last from minutes to as long as 24 hours or, rarely, more. They range in content from quiet disorientation through agitated delirium to unresponsiveness in which the patient is barely arousable. Transient vertigo, ataxia, diplopia, hemianopsia, hemisensory changes, or hemiparesis changes may immediately precede the mental changes. During attacks, most observers have found few somatic neurologic abnormalities, although occasional patients are reported as having oculomotor palsies, pupillary dilation, or an extensor plantar response. A few patients, at least briefly, have appeared to be in unarousable coma.
Posterior Reversible
Leukoencephalopathy Syndrome
Once believed to be associated only with malignant hypertension (hypertensive encephalopathy),265 posterior reversible leukoencephalopathy syndrome (PRES) is known to be caused by several illnesses that affect endothe-

|
|
Specific Causes of Structural Coma |
169 |
|
Table 4–19 Common Differential Diagnoses of Posterior Leukoencephalopathy |
|
|||
Syndrome |
|
|
|
|
|
|
|
|
|
|
Posterior |
Central Venous |
Top of Basilar |
|
|
Leukoencephalopathy |
Thrombosis |
Syndrome |
|
|
|
|
|
|
Predisposing |
Eclampsia, renal failure, |
Pregnancy, puerperium, |
Risk factors for |
|
factors |
cytotoxic and |
dehydration |
stroke, cardiac |
|
|
immunosuppressive |
|
disorders |
|
|
agents, hypertension |
|
|
|
Onset and |
Acute, evolves in days |
Acute, evolves in days |
Sudden, evolves |
|
progression |
|
|
in hours |
|
Clinical features |
Seizures precede all other |
Headaches, seizures, |
Cortical blindness, |
|
|
manifestations, visual |
stupor or coma, focal |
hemianopia, |
|
|
aura, cortical blindness, |
neurologic deficits |
confusional state, |
|
|
confusion, headache, |
(monoparesis or |
brainstem signs, |
|
|
rarely focal deficit |
hemiparesis), papilledema, |
cerebral signs, |
|
|
|
evidence of venous |
rarely seizures |
|
|
|
thrombosis elsewhere, |
|
|
|
|
infrequently hypertensive |
|
|
Imaging features |
Predominantly white |
Hemorrhage and ischemic |
Infarcts of bilateral |
|
|
matter edema in |
infarcts, small ventricles, |
paracalcarine |
|
|
bilateral occipital and |
‘‘cord sign’’ caused by |
cortex, thalamus, |
|
|
posterior parietal regions, |
hyperdense thrombosed |
inferior medial |
|
|
usually spares paramedian |
vein, evidence of major |
temporal lobe, |
|
|
brain parenchyma |
venous sinus thrombosis |
and brainstem |
|
|
|
on MRI |
|
|
Prognosis |
Completely resolves after |
|
rapid control of BP and |
|
removal of offending drug |
Intensive management is |
No recovery |
needed; mortality |
or only partial |
high in severe cases |
eventual recovery |
BP, blood pressure; MRI, magnetic resonance imaging.
From Garg,266 with permission.
lial cells, particularly in the posterior cerebral circulation.266 Among the illnesses other than hypertension, pre-eclampsia and immunosuppressive and cytotoxic agents (e.g., cyclosporin, cisplatin) are probably the most common causes. Vasculitis, porphyria, and thrombotic thrombocytopenic purpura are also reported causes, as is occasionally migraine. Posterior leukoencephalopathy is characterized by vasogenic edema of white matter of the posterior circulation, particularly the occipital lobes, but sometimes including the brainstem. Clinically, patients acutely develop headache, confusion, seizures, and cortical blindness; coma is rare. The MRI reveals vasogenic edema primarily affecting the occipital and posterior parietal lobes. Brainstem and cerebellum may also be affected. With appropriate treatment (controlling hypertension or discontinuing drugs), symptoms resolve. In patients with pre-eclampsia
who are pregnant, intravenous infusion of magnesium sulfate followed by delivery of the fetus has a similar effect. If PRES due to preeclampsia occurs in the postpartum period, immediate administration of magnesium sulfate followed by treatment for several weeks with verapamil is often effective, in our experience. The differential diagnosis includes posterior circulation infarction, venous thrombosis, and metabolic coma (Table 4–19 and Patient 5–8).
INFRATENTORIAL INFLAMMATORY DISORDERS
The same infective agents that affect the cerebral hemispheres can also affect the brainstem and cerebellum. Encephalitis, meningitis, and abscess formation may either be part of a more generalized infective process or be
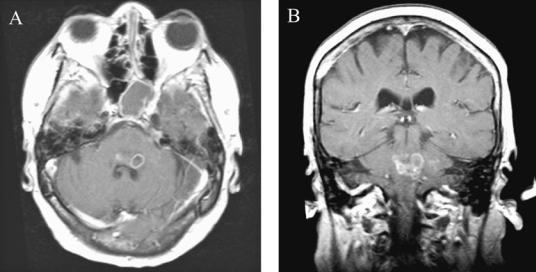
170 Plum and Posner’s Diagnosis of Stupor and Coma
Figure 4–13. A pair of magnetic resonance images demonstrating a multiloculated pontine abscess in a 73-year-old woman (Patient 2–2) who had been taking chronic prednisone for ulcerative colitis. She developed a fever, nausea and vomiting, left facial numbness, left gaze paresis, left lower motor neuron facial weakness, and left-sided ataxia. Lumbar puncture showed 47 white blood cells/mm3, but culture was negative. She was treated for suspected Listeria monocytogenes and recovered slowly, but had residual facial and oropharyngeal weakness requiring chronic tracheostomy.
restricted to the brainstem.267 Organisms that have a particular predilection for the brainstem include L. monocytogenes, which often causes brainstem abscesses 268 (Figure 4–13). Occasionally herpes zoster or simplex infection that begins in one of the sensory cranial nerves may cause a segmental brainstem encephalitis.269 Behc¸et’s disease may also cause brainstem inflammatory lesions.270 These disorders usually cause headache with or without nuchal rigidity, fever, and lethargy, but rarely coma. In a minority of instances, the CT scan may show brainstem swelling. The MR scan is usually more sensitive. CSF usually contains an increased number of cells. In bacterial infections cultures are usually positive; in viral infections PCR may establish the diagnosis. Stereotactic drainage of a brain abscess often identifies the organisms; appropriate antimicrobial therapy is usually successful.271
A brainstem disorder often confused with infection is Bickerstaff’s brainstem encephalitis.272 Patients with this disorder have often had a preceding systemic viral infection, then acutely develop ataxia, ophthalmoplegia, longtract signs, and alterations of consciousness including coma. In some patients, MRI reveals brainstem swelling and increased T2 signal273;
in others, the scan is normal. The CSF protein may be elevated, but there are no cells. The disease is believed to be autoimmune in origin related to postinfectious polyneuropathy (the Guillain-Barre´ syndrome) and the related Miller Fisher syndrome.272 The diagnosis can be established by the identification of antiGQ1b ganglioside antibodies in serum.272 Patients recover spontaneously.
INFRATENTORIAL TUMORS
Tumors within the brainstem cause their symptoms by a combination of compression and destruction. Although relatively common in children, primary tumors of the brainstem (brainstem glioma) are rare in adults. Metastatic tumors are more common, but with both primary and metastatic tumors, slowly or subacutely evolving brainstem signs typically establish the diagnosis long before impairment of consciousness occurs. An exception is the rare instance of an acute hemorrhage into the tumor, causing the abrupt onset of paralysis and sometimes coma, in which case the signs and treatment are similar to other brainstem hemorrhages.
CENTRAL PONTINE
MYELINOLYSIS
This is an uncommon disorder in which the myelin sheaths in the central basal pons are destroyed in a single confluent and symmetric lesion. Similar lesions may be found in the corpus callosum or cerebral hemispheres.274 Lesions vary from a few millimeters across to ones that encompass almost the entire base of the pons, sparing only a rim of peripheral myelin. The typical clinical picture is one of quadriparesis, with varying degrees of supranuclear paresis of lower motor cranial nerves and impairment of oculomotor or pupillary responses. A majority of patients become ‘‘locked in.’’ Approximately one-quarter of patients demonstrate impairment of level of consciousness, reflecting extension of the lesion into the more dorsal and rostral regions of the pons.
It is now recognized that most cases of central pontine myelinolysis are due to overly vigorous correction of hyponatremia, giving rise tothe‘‘osmoticdemyelinationsyndrome.’’Since the adoption of current regimens that recommend that hyponatremia be reversed at a rate no greater than 10 mEq/day, the frequency of this once-feared complication has decreased dramatically. On the other hand, a similar syndrome is seen in patients with liver transplantation, possibly due to the use of cyclosporine.274 As liver transplant has become more common, this population is increasing.
REFERENCES
1.Mut M, Cataltepe O, Bakar B, et al. Eosinophilic granuloma of the skull associated with epidural haematoma: a case report and review of the literature. Childs Nerv Syst 2004; 20, 765–769.
2.Simmons NE, Elias WJ, Henson SL, et al. Small cell lung carcinoma causing epidural hematoma: case report. Surg Neurol 1999; 51, 56–59.
3.Griffiths SJ, Jatavallabhula NS, Mitchell RD. Spontaneous extradural haematoma associated with craniofacial infections: case report and review of the literature. Br J Neurosurg 2002; 16, 188–191.
4.Miller DJ, Steinmetz M, McCutcheon IE. Vertex epidural hematoma: surgical versus conservative management: two case reports and review of the literature. Neurosurgery 1999; 45, 621–624.
5.Mishra A, Mohanty S. Contre-coup extradural haematoma: a short report. Neurol India 2001; 49, 94–95.
Specific Causes of Structural Coma |
171 |
6.Sunderland S, Bradley KC. Disturbances of oculomotor function accompanying extradural haemorrhage. J Neurochem 1953; 16, 35–46.
7.Jamieson KG, Yelland JD. Extradural hematoma. Report of 167 cases. J Neurosurg 1968; 29, 13–23.
8.GallagherJP,BrowderEJ.Extraduralhematoma.Experience with 167 patients. J Neurosurg 1968; 29, 1–12.
9.Browne GJ, Lam LT. Isolated extradural hematoma in children presenting to an emergency department in Australia. Pediatr Emerg Care 2002; 18, 86–90.
10.Dunn LT, Fitzpatrick MO, Beard D, et al. Patients with a head injury who ‘‘talk and die’’ in the 1990s. J Trauma 2003; 54, 497–502.
11.Mower WR, Hoffman JR, Herbert M, et al. Devel-
oping a decision instrument to guide computed tomographic imaging of blunt head injury patients. J Trauma 2005; 59, 954–959.
12.Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA 2005; 294, 1511–1518.
13.Zee CS, Go JL. CT of head trauma. Neuroimaging Clin N Am 1998; 8, 525–539.
14.Shimizu S, Endo M, Kan S, et al. Tight Sylvian cisterns associated with hyperdense areas mimicking subarachnoid hemorrhage on computed tomography— four case reports. Neurol Med Chir (Tokyo) 2001; 41, 536–540.
15.Servadei F, Teasdale G, Merry G. Defining acute mild head injury in adults: a proposal based on prognostic factors, diagnosis, and management. J Neurotrauma 2001; 18, 657–664.
16.Servadei F. Prognostic factors in severely head injured adult patients with epidural haematoma’s. Acta Neurochir (Wien) 1997; 139, 273–278.
17.Servadei F, Piazza G, Seracchioli A, et al. Extradural haematomas: an analysis of the changing characteristics of patients admitted from 1980 to 1986. Diagnostic and therapeutic implications in 158 cases. Brain Inj 1988; 2, 87–100.
18.Sakas DE, Bullock MR, Teasdale GM. One-year outcome following craniotomy for traumatic hematoma in patients with fixed dilated pupils. J Neurosurg 1995; 82, 961–965.
19.Pozzati E, Tognetti F. Spontaneous resolution of acute extradural hematoma—study of twenty-five selected cases. Neurosurg Rev 1989; 12 (Suppl 1), 188–189.
20.Lin J, Hanigan WC, Tarantino M, et al. The use of recombinant activated factor VII to reverse warfarininduced anticoagulation in patients with hemorrhages in the central nervous system: preliminary findings. J Neurosurg 2003; 98, 737–740.
21.Abe M, Udono H, Tabuchi K, et al. Analysis of ischemic brain damage in cases of acute subdural hematomas. Surg Neurol 2003; 59, 464–472.
22.Servadei F. Prognostic factors in severely head injured adult patients with acute subdural haematoma’s. Acta Neurochir (Wien) 1997; 139, 279–285.
23.Adhiyaman V, Asghar M, Ganeshram KN, et al. Chronic subdural haematoma in the elderly. Postgrad Med J 2002; 78, 71–75.
24.Lee KS. Natural history of chronic subdural haematoma. Brain Inj 2004, 18, 351–358.
172 Plum and Posner’s Diagnosis of Stupor and Coma
25.Cameron MM. Chronic subdural haematoma: a review of 114 cases. J Neurol Neurosurg Psychiatry 1978; 41, 834–839.
26.Jones S, Kafetz K. A prospective study of chronic subdural haematomas in elderly patients. Age Ageing 1999; 28, 519–521.
27.Nobbe FA, Krauss JK. Subdural hematoma as a cause of contralateral dystonia. Clin Neurol Neurosurg 1997; 99, 37–39.
28.Moster ML, Johnston DE, Reinmuth OM. Chronic subdural hematoma with transient neurological deficits: a review of 15 cases. Ann Neurol 1983; 14, 539–542.
29.Ingvar DH, Lundberg N. Paroxysmal systems in intracranial hypertension, studied with ventricular fluid pressure recording and electroencephalography. Brain 1961; 84, 446–459.
30.Inao S, Kawai T, Kabeya R, et al. Relation between brain displacement and local cerebral blood flow in patients with chronic subdural haematoma. J Neurol Neurosurg Psychiatry 2001; 71, 741–746.
31.Voelker JL. Nonoperative treatment of chronic subdural hematoma. Neurosurg Clin N Am 2000; 11, 507–513.
32.Olson JJ, Poor MM Jr, Beck DW. Methylprednisolone reduces the bulk flow of water across an in vitro blood-brain barrier. Brain Res 1988; 439, 259–265.
33.Hasbun R, Abrahams J, Jekel J, et al. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345, 1727–1733.
34.Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry 2003; 74, 937–943.
35.Asfora WT, Schwebach L. A modified technique to treat chronic and subacute subdural hematoma: technical note. Surg Neurol 2003; 59, 329–332.
36.Pichert G, Henn V [Conservative therapy of chronic subdural hematomas]. Schweiz Med Wochenschr 1987; 117, 1856–1862.
37.Nathoo N, Nadvi SS, Van Dellen JR. Cranial extradural empyema in the era of computed tomography: a review of 82 cases. Neurosurgery 1999; 44, 748–753.
38.Hlavin ML, Kaminski HJ, Fenstermaker RA, et al. Intracranial suppuration: a modern decade of postoperative subdural empyema and epidural abscess. Neurosurgery 1994; 34, 974–980.
39.Nathoo N, Nadvi SS, Van Dellen JR. Traumatic cranial empyemas: a review of 55 patients. Br J Neurosurg 2000; 14, 326–330.
40.Tamaki T, Eguchi T, Sakamoto M, et al. Use of diffusion-weighted magnetic resonance imaging in empyema after cranioplasty. Br J Neurosurg 2004; 18, 40–44.
41.Tsuchiya K, Osawa A, Katase S, et al. Diffusionweighted MRI of subdural and epidural empyemas. Neuroradiology 2003; 45, 220–223.
42.Heran NS, Steinbok P, Cochrane DD. Conservative neurosurgical management of intracranial epidural abscesses in children. Neurosurgery 2003; 53, 893–897.
43.Kleinschmidt-DeMasters BK. Dural metastases—a retrospective surgical and autopsy series. Arch Pathol Lab Med 2001; 125, 880–887.
44.Johnson MD, Powell SZ, Boyer PJ, et al. Dural lesions mimicking meningiomas. Hum Pathol 2002; 33, 1211–1226.
45.Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet 2004; 363, 1535–1543.
46.Bosnjak R, Derham C, Popovic M, et al. Spontaneous intracranial meningioma bleeding: clinicopathological features and outcome. J Neurosurg 2005; 103, 473–484.
47.Pistolesi S, Fontanini G, Camacci T, et al. Menin- gioma-associated brain oedema: the role of angiogenic factors and pial blood supply. J Neuro-Oncol 2002; 60, 159–164.
48.Engelhard HH. Progress in the diagnosis and treatment of patients with meningiomas. Part I: diagnostic imaging, preoperative embolization. Surg Neurol 2001; 55, 89–101.
49.Wiesmann M, Gliemroth J, Kehler U, et al. Pituitary apoplexy after cardiac surgery presenting as deep coma with dilated pupils. Acta Anaesthesiol Scand 1999; 43, 236–238.
50.Sibal L, Ball SG, Connolly V, et al. Pituitary apoplexy: a review of clinical presentation, management and outcome in 45 cases. Pituitary 2004; 7, 157–163.
51.Elsasser Imboden PN, De TN, Lobrinus A, et al. Apoplexy in pituitary macroadenoma: eight patients presenting in 12 months. Medicine (Baltimore) 2005; 84, 188–196.
52.Jassal DS, McGinn G, Embil JM. Pituitary apoplexy masquerading as meningoencephalitis. Headache 2004; 44, 75–78.
53.Prabhu VC, Brown HG. The pathogenesis of craniopharyngiomas. Childs Nerv Syst 2005; 21, 622– 627.
54.Haupt R, Magnani C, Pavanello M, et al. Epidemiological aspects of craniopharyngioma. J Pediatr Endocrinol Metab 2006; 1, 289–293.
55.Swaroop GR, Whittle IR. Pineal apoplexy: an occurrence with no diagnostic clinicopathological features. Br J Neurosurg 1998; 12, 274–276.
56.Polmear A. Sentinel headaches in aneurysmal subarachnoid haemorrhage: what is the true incidence? A systematic review. Cephalalgia 2003; 23, 935–941.
57.Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med 2000; 342, 29–36.
58.Landtblom AM, Fridriksson S, Boivie J, et al. Sudden onset headache: a prospective study of features, incidence and causes. Cephalalgia 2002; 22, 354–360.
59.Schievink WI, Wijdicks EF, Parisi JE, et al. Sudden death from aneurysmal subarachnoid hemorrhage. Neurology 1995; 45, 871–874.
60.Liebenberg WA, Worth R, Firth GB, et al. Aneurysmal subarachnoid haemorrhage: guidance in making the correct diagnosis. Postgrad Med J 2005; 81, 470–473.
61.Boesiger BM, Shiber JR. Subarachnoid hemorrhage diagnosis by computed tomography and lumbar puncture: are fifth generation CT scanners better at identifying subarachnoid hemorrhage? J Emerg Med 2005; 29, 23–27.
62.Mohamed M, Heasly DC, Yagmurlu B, et al. Fluidattenuated inversion recovery MR imaging and subarachnoid hemorrhage: not a panacea. AJNR Am J Neuroradiol 2004; 25, 545–550.
63.Edlow JA, Wyer PC. Evidence-based emergency medicine/clinical question. How good is a negative cranial computed tomographic scan result in excluding subarachnoid hemorrhage? Ann Emerg Med 2000; 36, 507–516.
64.Petzold A, Keir G, Sharpe TL. Why human color vision cannot reliably detect cerebrospinal fluid xanthochromia. Stroke 2005; 36, 1295–1297.
65.Klimo P Jr, Kestle JR, MacDonald JD, et al. Marked reduction of cerebral vasospasm with lumbar drainage of cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg 2004; 100, 215–224.
66.Klopfenstein JD, Kim LJ, Feiz-Erfan I, et al. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: a prospective randomized trial. J Neurosurg 2004; 100, 225–229.
67.Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol 2004; Suppl 4, iv285-iv291.
68.Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev 1999; 25, 103–119.
69.Yung WA, Horten BC, Shapiro WR. Meningeal gliomatosis: a review of 12 cases. Ann Neurol 1980; 8, 605–608.
70.Cinalli G, Sainte-Rose C, Lellouch-Tubiana A, et al. Hydrocephalus associated with intramedullary lowgrade glioma. J Neurosurg 1995; 83, 480–485.
71.Chen HS, Shen MC, Tien HF, et al. Leptomeningeal seeding with acute hydrocephalus—unusual central nervous system presentation during chemotherapy in Ki-1- positive anaplastic large-cell lymphoma. Acta Haematol 1996; 95, 135–139.
72.Floeter MK, So YT, Ross DA, et al. Miliary metastasis to the brain: clinical and radiologic features. Neurology 1987; 37, 1817–1818.
73.Broderick JP, Cascino TL. Nonconvulsive status epilepticus in a patient with leptomeningeal cancer. Mayo Clin Proc 1987; 62, 835–837.
74.Klein P, Haley EC, Wooten GF, et al. Focal cerebral infarctions associated with perivascular tumor infiltrates in carcinomatous leptomeningeal metastases. Arch Neurol 1989; 46, 1149–1152.
75.Herman C, Kupsky WJ, Rogers L, et al. Leptomeningeal dissemination of malignant glioma simulating cerebral vasculitis—case report with angiographic and pathological studies. Stroke 1995; 26, 2366– 2370.
76.Weller M, Stevens A, Sommer N, et al. Tumor cell dissemination triggers an intrathecal immune response in neoplastic meningitis. Cancer 1992; 69, 1475–1480.
77.Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer 1998; 82, 733–739.
78.van Zanten AP, Twijnstra A, Ongerboer DE, et al. Cerebrospinal fluid tumour markers in patients treated for meningeal malignancy. J Neurol Neurosurg Psychiatry 1991; 54, 119–123.
79.Wasserstrom WR, et al. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 1982; 49, 759–772.
80.Siegal T, Lossos A, Pfeffer MR. Leptomeningeal metastases: analysis of 31 patients with sustained offtherapy response following combined-modality therapy. Neurology 1994; 44, 1463–1469.
Specific Causes of Structural Coma |
173 |
81.DeAngelis LM, Boutros D. Leptomeningeal metastasis. Cancer Invest 2005; 23, 145–154.
82.Scheld WM, Koedel U, Nathan B, et al. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. J Infect Dis 2002; 186, S225–S233.
83.van de BD, De Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004; 351, 1849–1859.
84.Mylonakis E, Hohmann EL, Caderwood SB. Central nervous system infection with Listeria monocytogenes—33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine 1998; 77, 313–336.
85.Gerner-Smidt P, Ethelberg S, Schiellerup P, et al. Invasive listeriosis in Denmark 1994–2003: a review of 299 cases with special emphasis on risk factors for mortality. Clin Microbiol Infect 2005; 11, 618–624.
86.Drevets DA, Leenen PJ, Greenfield RA. Invasion of the central nervous system by intracellular bacteria. Clin Microbiol Rev 2004; 17, 323–347.
87.Hussein AS, Shafran SD. Acute bacterial meningitis in adults. A 12-year review. Medicine (Baltimore) 2000; 79, 360–368.
88.Podlecka A, Dziewulska D, Rafalowska J. Vascular changes in tuberculous meningoencephalitis. Folia Neuropathol 1998; 36, 235–237.
89.Attia J, Hatala R, Cook DJ, et al. The rational clinical examination. Does this adult patient have acute meningitis? JAMA 1999; 282, 175–181.
90.Risch L, Lisec I, Jutzi M, et al. Rapid, accurate and non-invasive detection of cerebrospinal fluid leakage using combined determination of beta-trace protein in secretion and serum. Clin Chim Acta 2005; 351, 169–176.
91.Romer FK. Difficulties in the diagnosis of bacterial meningitis. Evaluation of antibiotic pretreatment and causes of admission to hospital. Lancet 1977; 2, 345–347.
92.Romer FK. Bacterial meningitis: a 15-year review of bacterial meningitis from departments of internal medicine. Dan Med Bull 1977; 24, 35–40.
93.Clark T, Duffell E, Stuart JM, et al. Lumbar puncture in the management of adults with suspected bacterial meningitis-a survey of practice. J Infect 2005; 52, 316–319.
94.Begg N, Cartwright KA, Cohen J, et al. Consensus statement on diagnosis, investigation, treatment and prevention of acute bacterial meningitis in immunocompetent adults. British Infection Society Working Party. J Infect 1999; 39, 1–15.
95.Roos KL, Tunkel AR, Scheld WM. Acute bacterial meningitis. In: Scheld WM, Whitley RJ, Marra CM, eds. Infections of the Central Nervous System, 3rd ed. Philadelphia: Lippincott Williams & Wilkins, pp 347–422, 2004.
96.Chaudhuri A. Adjunctive dexamethasone treatment in acute bacterial meningitis. Lancet Neurol 2004; 3, 54–62.
97.Kastrup O, Wanke I, Maschke M. Neuroimaging of infections. NeuroRx 2005; 2, 324–332.
98.Zimmerman RA, Wong AM, Girard N. Imaging of intracranial infections. In: Scheld WM, Whitley RJ, Marra CM, eds. Infections of the Central Nervous System, 3rd ed. Philadelphia: Lippincott Williams & Wilkins, pp 31–55, 2004.
174 Plum and Posner’s Diagnosis of Stupor and Coma
99.Massaro AR, Sacco RL, Mohr JP, et al. Clinical discriminators of lobar and deep hemorrhages: the Stroke Data Bank. Neurology 1991; 41, 1881–1885.
100.Chung CS, Caplan LR, Yamamoto Y, et al. Striatocapsular haemorrhage. Brain 2000; 123, 1850–1862.
101.Kumral E, Kocaer T, Ertubey NO, et al. Thalamic hemorrhage. A prospective study of 100 patients. Stroke 1995; 26, 964–970.
102.Choi KD, Jung DS, Kim JS. Specificity of ‘‘peering at the tip of the nose’’ for a diagnosis of thalamic hemorrhage. Arch Neurol 2004; 61, 417–422.
103.Darby DG, Donnan GA, Saling MA, et al. Primary intraventricular hemorrhage: clinical and neuropsychological findings in a prospective stroke series. Neurology 1988; 38, 68–75.
104.Engelhard HH, Andrews CO, Slavin KV, et al. Current management of intraventricular hemorrhage. Surg Neurol 2003; 60, 15–21.
105.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365, 387–397.
106.Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005; 352, 777–785.
107.Fisher CM. Some neuro-ophthalmological observations. J Neurol Neurosurg Psychiatry 1967; 30, 383– 392.
108.Pessin MS, Adelman LS, Prager RJ, et al. ‘‘Wrongway eyes’’ in supratentorial hemorrhage. Ann Neurol 1981; 9, 79–81.
109.Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology 1965; 15, 774–784.
110.Greenberg SM, Gurol ME, Rosand J, et al. Amyloid angiopathy-related vascular cognitive impairment. Stroke 2004; 35, 2616–2619.
111.Yamada M. Cerebral amyloid angiopathy: an overview. Neuropathology 2000; 20, 8–22.
112.Miller JH, Wardlaw JM, Lammie GA. Intracerebral haemorrhage and cerebral amyloid angiopathy: CT features with pathological correlation. Clin Radiol 1999; 54, 422–429.
113.Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology 2006; 66, 165–171.
114.Barami K, Ko K. Ruptured mycotic aneurysm presenting as an intraparenchymal hemorrhage and nonadjacent acute subdural hematoma: case report and review of the literature. Surg Neurol 1994; 41, 290– 293.
115.Chun JY, Smith W, Halbach VV, et al. Current multimodality management of infectious intracranial aneurysms. Neurosurgery 2001; 48, 1203–1213.
116.Mathiesen T, Edner G, Kihlstrom L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg 2003; 99, 31–37.
117.Porter RW, Detwiler PW, Spetzler RF, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg 1999; 90, 50–58.
118.Kim MS, Pyo SY, Jeong YG, et al. Gamma knife surgery for intracranial cavernous hemangioma. J Neurosurg 2005; 102 (Suppl), 102–106.
119.Liscak R, Vladyka V, Simonova G, et al. Gamma knife surgery of brain cavernous hemangiomas. J Neurosurg 2005; 102 (Suppl), 207–213.
120.Choi JH, Mohr JP. Brain arteriovenous malformations in adults. Lancet Neurol 2005; 4, 299–308.
121.Posner JB. Neurologic Complications of Cancer. Philadelphia: F.A. Davis, 1995.
122.DeAngelis LM, Gutin PH, Leibel SA, et al. Intracranial Tumors: Diagnosis and Treatment. London: Martin Dunitz Ltd., 2002.
123.Behin A, Hoang-Xuan K, Carpentier AF, et al. Primary brain tumours in adults. Lancet 2003; 361, 323–331.
124.Engelhard HH. Current diagnosis and treatment of oligodendroglioma. Neurosurg Focus 2002; 12, E2.
125.Panageas KS, Elkin EB, DeAngelis LM, et al. Trends in survival from primary central nervous system lymphoma, 1975–1999: a population-based analysis. Cancer 2005; 104, 2466–2472.
126.Patchell RA, Tibbs PA, Walsh JW. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990; 322, 494–500.
127.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors—report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 54, 1886–1893.
128.Fuentes R, Bonfill X, Exposito J. Surgery versus radiosurgery for patients with a solitary brain metastasis from non-small cell lung cancer. Cochrane Database Syst Rev 2006; (1), CD004840.
129.Roche M, Humphreys H, Smyth E, et al. A twelveyear review of central nervous system bacterial abscesses; presentation and aetiology. Clin Microbiol Infect 2003; 9, 803–809.
130.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol 2005; 4, 653–661.
131.Tuzun Y, Kadioglu HH, Izci Y, et al. The clinical, radiological and surgical aspects of cerebral hydatid cysts in children. Pediatr Neurosurg 2004; 40, 155–160.
132.Collazos J. Opportunistic infections of the CNS in patients with AIDS: diagnosis and management. CNS Drugs 2003; 17, 869–887.
133.Kastenbauer S, Pfister H-W, Wispelwey B, et al. Brain abscess. In: Scheld WM, Whitley RJ, Marra CM, eds. Infections of the Central Nervous System, 3rd ed. Philadelphia: Lippincott Williams & Wilkins, pp 479–507, 2004.
134.Bozbuga M, Izgi N, Polat G, et al. Posterior fossa epidural hematomas: observations on a series of 73 cases. Neurosurg Rev 1999; 22, 34–40.
135.Khwaja HA, Hormbrey PJ. Posterior cranial fossa venous extradural haematoma: an uncommon form of intracranial injury. Emerg Med J 2001; 18, 496–497.
136.Berker M, Cataltepe O, Ozcan OE. Traumatic epidural haematoma of the posterior fossa in childhood: 16 new cases and a review of the literature. Br J Neurosurg 2003; 17, 226–229.
137.Bor-Seng-Shu E, Aguiar PH, de Almeida Leme RJ, et al. Epidural hematomas of the posterior cranial fossa. Neurosurg Focus 2004; 16, ECP1.
138.Parkinson D, Hunt B, Shields C. Double lucid interval in patients with extradural hematoma of the posterior fossa. J Neurosurg 1971; 34, 534–536.
139.Karasawa H, Furuya H, Naito H, et al. Acute hydrocephalus in posterior fossa injury. J Neurosurg 1997; 86, 629–632.
140.Pozzati E, Tognetti F, Cavallo M, et al. Extradural hematomas of the posterior cranial fossa. Observations on a series of 32 consecutive cases treated after the introduction of computed tomography scanning. Surg Neurol 1989; 32, 300–303.
141.Wong CW. The CT criteria for conservative treatment—but under close clinical observation— of posterior fossa epidural haematomas. Acta Neurochir (Wien) 1994; 126, 124–127.
142.Roda JM, Gimenez D, Perez-Higueras A, et al. Posterior fossa epidural hematomas: a review and synthesis. Surg Neurol 1983; 19, 419–424.
143.Nathoo N, Nadvi SS, Van Dellen JR. Infratentorial empyema: analysis of 22 cases. Neurosurgery 1997; 41, 1263–1268.
144.Roberti F, Sekhar LN, Kalavakonda C, et al. Posterior fossa meningiomas: surgical experience in 161 cases. Surg Neurol 2001; 56, 8–20.
145.Cantore G, Ciappetta P, Delfini R, et al. Meningiomas of the posterior cranial fossa without dural attachment. Surg Neurol 1986; 25, 127–130.
146.Psiachou-Leonard E, Paterakis G, Stefanaki K, et al. Cerebellar granulocytic sarcoma in an infant with CD56þ acute monoblastic leukemia. Leuk Res 2001; 25, 1019–1021.
147.d’Avella D, Servadei F, Scerrati M, et al. Traumatic acute subdural haematomas of the posterior fossa: clinicoradiological analysis of 24 patients. Acta Neurochir (Wien) 2003; 145, 1037–1044.
148.Stendel R, Schulte T, Pietila TA, et al. Spontaneous bilateral chronic subdural haematoma of the posterior fossa. Case report and review of the literature. Acta Neurochir (Wien) 2002; 144, 497–500.
149.Sahjpaul RL, Lee DH. Infratentorial subdural empyema, pituitary abscess, and septic cavernous sinus thrombophlebitis secondary to paranasal sinusitis: case report. Neurosurgery 1999; 44, 864–866.
150.Sadato N, Numaguchi Y, Rigamonti D, et al. Bleeding patterns in ruptured posterior fossa aneurysms: a CT study. J Comput Assist Tomogr 1991; 15, 612–617.
151.Logue V. Posterior fossa aneurysms. Clin Neurosurg 1964; 11, 183–219.
152.Duvoisin RC, Yahr MD. Posterior fossa aneurysms. Neurology 1965; 15, 231–241.
153.Jamieson KG. Aneurysms of the vertebrobasilar system. Further experience with nine cases. J Neurosurg 1968; 28, 544–555.
154.Flaherty ML, Haverbusch M, Kissela B, et al. Perimesencephalic subarachnoid hemorrhage: incidence, risk factors, and outcome. J Stroke Cerebrovasc Dis 2005; 14, 267–271.
155.Van der Schaap I, Velthius BK, Gouw A, Rinkel GJ. Venous drainage in perimesencephalic hemorrhage. Stroke 2004; 35, 1614–1618.
156.Kirollos RW, Tyagi AK, Ross SA, et al. Management of spontaneous cerebellar hematomas: a prospective treatment protocol. Neurosurgery 2001; 49, 1378–1386.
157.Mezzadri JJ, Otero JM, Ottino CA. Management of 50 spontaneous cerebellar haemorrhages. Importance of obstructive hydrocephalus. Acta Neurochir (Wien) 1993; 122, 39–44.
Specific Causes of Structural Coma |
175 |
158.Da Pian R, Bazzan A, Pasqualin A. Surgical versus medical treatment of spontaneous posterior fossa haematomas: a cooperative study on 205 cases. Neurol Res 1984; 6, 145–151.
159.Itoh Y, Yamada M, Hayakawa M, et al. Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J Neurol Sci 1993; 116, 135–141.
160.Siu TL, Chandran KN, Siu T. Cerebellar haemorrhage following supratentorial craniotomy. J Clin Neurosci 2003; 10, 378–384.
161.Brennan RW, Bergland RM. Acute cerebellar hemorrhage. Analysis of clinical findings and outcome in 12 cases. Neurology 1977; 27, 527–532.
162.Fisher CM, Picard EH, Polak A, et al. Acute hypertensive cerebellar hemorrhage: diagnosis and surgical treatment. J Nerv Ment Dis 1965; 140, 38–57.
163.Messert B, Leppik IE, Sato S. Diplopia and involuntary eye closure in spontaneous cerebellar hemorrhage. Stroke 1976; 7, 305–307.
164.St Louis EK, Wijdicks EF, Li H. Predicting neurologic deterioration in patients with cerebellar hematomas. Neurology 1998; 51, 1364–1369.
165.Coplin WM, Kim DK, Kliot M, et al. Mutism in an adult following hypertensive cerebellar hemorrhage: nosological discussion and illustrative case. Brain Lang 1997; 59, 473–493.
166.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain 1998; 121, 561– 579.
167.Aarsen FK, Van Dongen HR, Paquier PF, et al. Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology 2004; 62, 1311–1316.
168.Tohgi H, Takahashi S, Chiba K, et al. Cerebellar infarction. Clinical and neuroimaging analysis in 293 patients. The Tohoku Cerebellar Infarction Study Group. Stroke 1993; 24, 1697–1701.
169.Barinagarrementeria F, Amaya LE, Cantu C. Causes and mechanisms of cerebellar infarction in young patients. Stroke 1997; 28, 2400–2404.
170.Geller T, Loftis L, Brink DS. Cerebellar infarction in adolescent males associated with acute marijuana use. Pediatrics 2004; 113, 365–370.
171.Hornig CR, Rust DS, Busse O, et al. Spaceoccupying cerebellar infarction. Clinical course and prognosis. Stroke 1994; 25, 372–374.
172.Jauss M, Krieger D, Hornig C, et al. Surgical and medical management of patients with massive cerebellar infarctions: results of the German-Austrian Cerebellar Infarction Study. J Neurol 1999; 246, 257– 264.
173.Nadvi SS, Parboosing R, Van Dellen JR. Cerebellar abscess: the significance of cerebrospinal fluid diversion. Neurosurgery 1997; 41, 61–66.
174.Sennaroglu L, Sozeri B. Otogenic brain abscess: review of 41 cases. Otolaryngol Head Neck Surg 2000; 123, 751–755.
175.Shaw MD, Russell JA. Cerebellar abscess. A review of 47 cases. J Neurol Neurosurg Psychiatry 1975; 38, 429–435.
176.Agrawal D, Suri A, Mahapatra AK. Primary excision of pediatric posterior fossa abscesses—towards zero mortality? A series of nine cases and review. Pediatr Neurosurg 2003; 38, 63–67.
