
Книги по МРТ КТ на английском языке / Functional Neuroimaging in Child Psychiatry Ernst 1 ed 2000
.pdf
134 M. F. Casanova, D. Buxhoeveden and G. S. Sohal
the macaque monkey and human brain. J. Comp. Neurol., 297, 441±70.
Kreig, W. J. S. (1963). Connections of the Cerebral Cortex. Evanston II: Brain Brooks.
Landlesser, L. (1976). The development of neural circuits in the limb moving segments of the spinal cord. In Neural Control of Locomotion, ed. L. Landlesser. New York: Plenum Press.
Langworthy, O. R. (1933). Behavior patterns and myelination.
Contrib. Embryol., 24, 1±57.
Laval, S. H., Dann, J. C., Butler, R. J. et al. (1998). Evidence for linkage to psychosis and cerebral asymmetry (relative hand skill) on the X chromosome. Am. J. Med. Genet., 81, 420±7.
Le Douarin, N. M. (1982). The Neural Crest. London: Cambridge
University Press.
Leise, E. M. (1990). Modular constructs of nervous systems: a basic principle of design for vertebrates and invertebrates. Brain Res. Rev., 15, 1±23.
LeMay, M. and Geschwind, N. (1975). Hemispheric diVerences in the brains of great apes. Brain Behav. Evol., 11, 48±52.
LeMay, M. (1976). Morphological asymmetry in modern man, fossil man, and non-human primates. Ann. NY Acad. Sci., 280, 349±66.
Lemire, R., Loeser, J., Leech, R. and Alvord, E. (1975). Normal and Abnormal Development of the Human Nervous System. New York: Harper Row.
Li, L. M., Cendes, F., Bastos, A. C., Andermann, F., Dubeau, F. and Arnold, D. L. (1998). Neuronal metabolic dysfunction in patients with cortical developmental malformations: a proton magnetic resonance spectroscopy imaging study. Neurology, 50, 755±9.
Louilot, A. and Choulli, M. K. (1997). Asymmetrical increases in dopamine turn-over in the nucleus accumbens and lack of changes in locomotor responses following unilateral dopaminergic depletions in the entorhinal cortex. Brain Res., 778, 150±7.
Lucas Keene, M. F. and Hewer, E. E. (1931). Some observations on myelination in the human central nervous system. J. Anat., 66, 1±13.
Lund, R. D. and Mustari, M. J. (1977). Development of the geniculorcortical pathway in rats. J. Comp. Neurol., 173, 289±306.
Macneilage, P. F., Studdert-Kennedy, M. G. and Lindblom, B. (1987). Primate handedness reconsidered. Behav. Brain Sci., 10, 247.
Mayeri, E. Koester, J. Kupperman, I., Liebeswar, G. and Kandel, E. R. (1974). Neural control of circulation in aplysia. J. Neurophys.,
37, 458±75.
Mountcastle, V. B. (1957). Modality and topographic properties of single neurons of cat's somatic sensory cortex. J. Neurophysiol.,
20, 408±34.
Mountcastle,V. B. (1978). An organizing principle for cerebral function: the unit module and the distributed system. In G. M. Edelman and V. B. Mountcastle. The Mindful Brain: Cortical Organization and the Group-selective Theory of Higher Brain Function, eds. G. M. Edelman and V. B. Mountcastle, pp. 7±51. Cambridge, MA: MIT Press.
Mountcastle, V. B. (1997). The columnar organization of the neocortex. Brain, 120, 701±22.
Nashner, L. M. (1981). Analysis of stance posture in humans. In
Handbook of Behavioral Neurobiology, Vol. 5, eds. A. L. Towe and E. S. Luschei, pp. 527±65. New York: Plenum Press.
Nelson, J. S., Parisi, J. E. and Schochet, S. S. Jr (1993). Principles and Practice of Neuropathology, p. 24. St Louis, MO: Mosby.
Noback, C. R. and Demarest, R. J. (1981). The Human Nervous System: Basic Principles of Neurobiology, pp. 142±5. New York: McGraw-Hill.
Nottebohm, F. (1970). Ontogeny of bird song. Science, 167, 950±6. Nottebohm, F. (1971). Vocalizations and breeding behavior of surgically deafened ring doves. (Streptopelia risoria). Anim. Behav.,
19, 313±27.
Nudo, R. J. and Masterson, R. B. (1990). Descending pathways to the spinal cord, IV: some factors related to the amount of cortex devoted to the cortical spinal tract. J. Comp. Neurol., 296, 584±97.
O'Leary, D. D. M. and StanWeld, B. B. (1989). Selective elimation of axons extended by developing cortical neurons is dependent on regional locale: experiments utilizing fetal cortical transplants. J. Neurosci., 9, 2230±46.
O'Leary, D. D., Schlaggar, B. L. and StanWeld, B. B. (1992). The speciWcation of sensory cortex: lessons from cortical transplantation. Exp. Neurol., 115, 121±6.
Oppenheim, R. W. (1991). Cell death during development of the nervous system. Annu. Rev. Neurosci., 14, 453±501.
Pandya, D., Seltzer, B. and Barlos, H. (1988). Input±output organization of the primate cerebral cortex. In Comparative Primate Biology, vol. 4, Neurosciences. New York: Alan R. Liss.
Pearson, K. (1976). The control of walking. Science, 235, 72±86. Perry, E. K., Piggott, M. A., Court, J. A., Johnson, M. and Perry, R. H.
(1993). Transmitters in the developing and senescent human brain. Ann. NY Acad. Sci., 695, 69±72.
Placzek, M., Tessier-Lavigne, M., Yamada, T., Jessell, T. M. and Dodd, J. (1990). Mesodermal control of neural cell identify: Xoor plate induction by the notochord. Science, 250, 985±8.
Pryse-Davies, J. and Beard, R. W. (1973). A necropsy study of brain swelling in the newborn with special reference to cerebellar herniation. J. Pathol., 109, 51±73.
Rakic, P. (1976). Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature, 261, 467±71.
Rakic, P. (1978). Neuronal migration and contact guidance in primate telencephalon. Postgrad. Med. J., 54, 25±40.
Rakic, P. (1988a). The speciWcation of cerebral cortical areas: the radial unit hypothesis. Science, 242, 928±31.
Rakic, P. (1988b). Defects of neuronal migration and the pathogenesis of cortical malformations. Prog. Brain Res., 73, 15±37.
Rakic, P. (1990). Principles of neural cell migration. Experientia, 46, 882±91.
Rakic, P., Suner, I. and Williams, R. W. (1991). A novel cytoarchitectonic area induced experimentally within the primate visual cortex. Proc. Natl. Acad. Sci. USA, 88, 2083±7.
Reiss, A. L., Abrams, M. T., Singer, H. S., Ross, J. L. and Denckla, M. B. (1996). Brain development, gender, and IQ in children. A volumetric imaging study. Brain, 119, 1763±74.
Richman, D. P., Stewart, R. M., Hutchinson, J. W. and Caviness, V. S. Jr (1975). Mechanical model of brain convolutional development. Science, 189, 18±21.
Brain development and evolution |
135 |
|
|
Rosen, G. D. Sherman, G. F. and Galaburda, A. M. (1989). Interhemispheric connections diVer between symmetrical and asymmetrical brain regions. Neuroscience, 33, 525±33.
Rumbaugh, D. M. (1977). Language Learning by a chimpanzee: The LANA project. New York: Academic Press.
Sanford, C., Gvin, K. and Ward, J. P. (1984). Posture and laterality in the bushbaby (Galago senegalensis). Brain Behav. Evol., 25, 217±24.
Scammon, R. E. (1932). The central nervous system. In White House Conference on Child Health and Protection. Growth and Development of the Child, section 1 (Medical Service), Part 2:
Anatomy and Physiology. Report of the Committee on Growth and Development, pp. 176±90. London: Appleton.
Schoenwolf, G. C. and Smith, J. L. (1990). Mechanisms of neuralation: traditional viewpoint and recent advances. Development,
109, 243±70.
Scheibel, A. (1984). A dendritic correlate of human speech. In
Cerebral Dominance: The Biological Foundations. eds. N. Geschwind and A. Galaburda. Cambridge, MA: Harvard University Press.
Seldon, H. L. (1981a). Structure of human auditory cortex. I. Cytoarchitectonics and dendritic distributions. Brain Res., 229, 277±94.
Seldon, H. L. (1981b). Structure of human auditory cortex. II. Axon distributions and morphological correlates of speech perception. Brain Res., 229, 295±310.
Sharma, K., Korade, Z. and Frank, E. (1995). Late-migrating neuroepithelial cells from the spinal cord diVerentiate into sensory ganglion cells and melanocytes. Neuron, 14, 143±52.
Shik, M. L., Severen, F. V. and Orlovskii, G. N. (1966). Control of walking and running by means of electrical stimulation of the mid-brain. BioWzyka, 1, 659±66.
Smart, I. H. M. and McSherry, G. M. (1986a). Gyrus formation in the cerebral cortex of the ferret. II. Description of the internal histological changes. J. Anat., 147, 27±43.
Smart, I. H. M. and McSherry, G. M. (1986b). Gyrus formation in the cerebral cortex of the ferret. I. Description of the external changes. J. Anat., 146, 141±52.
Smith, J. F. (1974). Pediatric Neuropathology, pp. 4±8, 10±14. New York: McGraw-Hill.
Sohal, G. S. (1992). The role of the target size in neuronal survival.
J. Neurobiol., 23, 1124±30.
Sohal, G. S., Bockman, D. E., Ali, M. M. and Tsai, N. T. (1996). DiI labeling and homeobox gene islet-1 expression reveal the contribution of ventral neural tube cells to the formation of the avian trigeminal ganglion. Int. J. Dev. Neurosci., 14, 419±27.
Sohal, G. S., Ali, A. A. and Ali, M. M. (1998a). Ventral neural tube cells diVerentiate into craniofacial skeletal muscles. Biochem. Biophys. Res. Commun., 252: 675±8.
Sohal, G. S., Ali, M. M., Ali, A. A. and Bockman, D. E. (1999). Ventral neural tube cells diVerentiate into hepatocytes in the chick embryo. Cell. Mol. Life Sci., 55: 128±30.
Sohal, G. S., Ali, M. M. Galileo, D. S. and Ali, A. A. (1998c). Emigration of neuroepithelial cells from the hindbrain neural tube in the chick embryo. Int. J. Dev. Neurosci., 16, 477±81.
Strumwasser, F. (1975). Neuronal principles organizing periodic behaviors. In Circadian Oscillations and Organization of the Nervous System, ed. C. S. Pittendrigh. Cambridge, MA: MIT Press.
Sullivan, R. M. and Gratton, A. (1998). Relationships between stress-induced increases in medial prefrontal cortical dopamine and plasma corticosterone levels in rats: role of cerebral laterality. Neuroscience, 83(1), 81±91.
Sur, M. Pallas, S. and Roe, A. (1990). Cross-modal plasticity in cortical development: diVerentiation and speciWcation of sensory cortex. Trends Neurosi., 13, 227±33.
Szekely, G. Y., Czeh, G. and Voros, G. Y. (1969). The activity pattern of limb muscles in freely moving normal and deaVerented newts. Exp. Brain Res., 9, 53±62.
Szentagothai, J. (1968). The modular architectonic principle of neural centers. Rev. Physiol. Biochem. Pharmacol., 98, 11±61.
Szentagothai, J. (1978). The neuron network of the cerebral cortex: a functional interpretation. The Ferrier Lecture 1977. Proc. R. Soc. Lond. Ser. B, 201, 219±48.
Tan, U. (1995). Growth hormone limits brain/body development before birth in relation to sex, grasp-reXex asymmetry and familial sinistrality of human neonates. Int. J. Neurosci., 82, 105±11.
Taub, E. (1976). Motor behavior following deaVerentation in the developing and motorically mature monkey. In Neural Control of Locomotion, eds. R. Herman and S. Griller. New York: Plenum Press.
Taub, E., Goldberg, I. and Taub, P. (1975). DeaVerentation in monkeys: pointing at a target without visual feedback. Exp. Neurol., 46, 178±86.
Teyler, T. J. and Fountain, S. B. (1987). Neuronal plasticity in the mammalian brain: relevance to behavioral learning and memory. Child Dev., 58, 698±712.
Varlinskaya, E. I., Petrov, E. S., Robinson, S. R. and Smotherman, W. P. (1995). The asymmetrical development of the dopamine system in the fetal rat as indicated by lateralized administration of SKF-38393 and SCH-23390. Pharmacol. Biochem. Behav., 50, 359±67.
van der Knaap, M. S. and Valk, J. (1990). MR imaging of the various stages of normal myelination during the Wrst year of life.
Neuroradiology, 31, 459±70.
Weiskrantz, L. (1977). On the role of cerebral commisures in animals. In Structure and Function of Cerebral Commisures, eds. I. Russel, M. van Hof and G. Berlucchi, pp. 475±478.
Weller, R. E. and Kaas, J. H. (1981). Retinotopic patterns of connections of area 17 with visual areas V-II and MT in macaque monkeys. J. Comp. Neurol., 220, 253±79.
Willis, J. A. (1974). The role of the electrogenic sodium pump in the modulation of pacemaker discharge of Aplysia neuron. J. Cell Physiol., 84, 463±72.
Wilmer, H. A. (1940). Changes in structural components of the human body from six lunar months to maturity. Proc. Soc. Exp. Biol., 43, 545±7.
Worthen, N. J., Gilbertson, V. and Lau, C. (1986). Cortical sulcal development seen on sonography: relationship to gestational parameters. J. Ultrasound Med., 5, 153±6.

136 M. F. Casanova, D. Buxhoeveden and G. S. Sohal
Yakovlev, P. I. and Lecours, A. R. (1967). The myelogenic cycles of regional maturation of the brain. In Regional Development of Brain in Early Life, ed. A. Minowski, Davis, CA: Blackwells.
Yamada, T., Placzek, M., Tanaka, H., Dodd, J. and Jessell, T. M. (1991). Control of cell pattern in the developing nervous system: polarizing activity of the Xoor plate and notochord. Cell, 64, 635±47.
Yates, M., Morris, C., Cheng, A. V., Ferrier, N. (1991). Laterality and 5HT2 receptors in human brain. Psychiatr. Res., 36, 169±74.
Zeisel, S. H. (1997). Choline: essential for brain development and function. Adv. Pediatr., 44, 263±95.

8
Cognitive development from a neuropsychologic perspective
Daisy M. Pascualvaca and Gloria Morote
Introduction
The Weld of neuropsychology has made great advances in understanding how the adult brain is organized and how it functions. Relatively little is known, however, about the neural systems mediating normal cognitive development. At present, much of our understanding of brain±behavior relationships in children has been based primarily on adult models. Although valuable, these models have serious limitations when applied to children. Concepts such as critical periods, for example, refer exclusively to the developing organism. The immature brain diVers from the mature brain in its neural organization. For example, while studies of laterality provide considerable data to suggest that hemisphere specialization begins early in life, the demarcation between cognitive functions that are diVerentially mediated by the two hemispheres is less clear cut in children than in adults (Luria, 1973). Concepts such as neural plasticity have been mostly applied to the developing brain, as the possibilities for neural re-organization and recovery of function following injury are remarkable in young organisms. Furthermore, the structure and function of the developing brain is profoundly responsive to and aVected by experience. Adversity, whether through deprivation or through abusive environments, can have a deleterious and long-lasting impact on the brain's structural and functional organization (van der Kolk and Greenberg, 1987; Ito et al., 1993).
Recognition of the diVerences between the mature and the immature brain have led to the design of measures that are sensitive to developmental factors. Of all the techniques available neuroimaging provides a unique approach to the study of brain±behavior relationships in children. By allowing the investigator to observe the brain in vivo, neuroimaging now makes it possible to study the mechanisms that govern the development of cognition.
Studies using these powerful tools will challenge some, if not many, of our traditional concepts and oVer invaluable contributions to the advancement of developmental neuroscience.
In this chapter, we will review the developmental trajectories of speciWc cognitive and neuropsychologic functions. Data from the cognitive, neuropsychologic and developmental literatures will be discussed. Although we will cover major developmental milestones and transitions, it is important to keep in mind that development is not a steady or linear process but rather is characterized by leaps, plateau, and even temporary regressions. As such, measurement of brain±behavior relationships in children requires an understanding of the developmental trajectories of various cognitive processes. In studying children, knowledge of these trajectories is critical to distinguish deviance from delay, because the performance of a normal 6-year-old may be indistinguishable from that of an adolescent with a developmental disability (see Fig. 8.1). Having an understanding of normal cognitive development is also critical to the selection of appropriate assessment instruments. This word of caution is important in developmental neuropsychology, where there has been a reliance on measures designed for adults whose brain±behavior relationships are more clearly understood than are those in children. Measures designed to capture deWcits in adults with brain lesions may not be sensitive to the subtleties of deviations in normal development. Whenever possible, we will relate developmental trajectories to documented changes in neural tissue. It is important to note that despite the existence of a signiWcant body of knowledge regarding both neurologic and cognitive development, mapping the correspondence between processes involved in brain maturation, such as myelination, and cognitive development remains a vast Weld of exploration. We will present Piaget's model, which has guided
137

138 D. M. Pascualvaca and G. Morote
(a) |
(b) |
(c ) |
Fig. 8.1. Performance of a normal 6-year-old child (a), a 16-year-old-child with a nonverbal learning disability (b), and a normal 16-year-
old child (c) on the Rey±Osterrieth Complex Figure Test.
much of the developmental research. The neuropsychologic Wndings will be related to Piagetian concepts, which, to this day, continue to inXuence our conceptualization of normal cognitive development.
Piaget's theory
Piaget (1952, 1954, 1976) proposed a comprehensive and ambitious theory of intellectual development. His observations regarding children's abilities to think, reason, and perceive their world have had a tremendous impact on our conceptualization of cognitive maturation. Piaget asserted throughout his work that cognitive changes are the result of qualitative changes in the way children understand their world. Children learn new concepts as they interact with their environment. At the same time, they adjust these concepts to incorporate changes in the environment. Cognitive development takes place as the child Wts new information into his/her notion about the surroundings (ªassimilationº) and revises old concepts to Wt the new information (ªaccommodationº). Piaget viewed intellectual development as reXecting a dynamic equilibrium between these two processes. Through assimilation and accommodation, the child forms ªa schema,º an organizing principle that is used to interpret the world.
Piaget conceptualized cognitive development as consisting of a sequence of stages, although it is important to emphasize that he envisaged a continuity of development over its entire course. Each stage derives logically and inevitably from the preceding one, with old schema incorporating new ones. He broadly summarized the stages of
cognitive development as the stage of sensorimotor intelligence (0±2 years), preoperational thought (2±7 years), concrete operations (7±11 years), and formal operations (11±15 years). The main characteristics of these stages are shown in Table 8.1.
BrieXy, during the Wrst 24 months of life, the infant learns about the environment by manipulating objects (e.g., through reaching, grasping, and pushing objects). Thinking independent of overt behavior is not yet developed. The child gradually begins to understand simple cause-and-eVect relationships and, by the end of the sensorimotor stage, shows clear goal-directed behaviors. Over the next 5 years, during the preoperational stage, the child becomes increasingly able to conceptualize events and situations independent of actions. He/she continues to be egocentric, however, and his/her thoughts are still largely under the control of immediate experiences. By 7 years of age (in the concrete operations stage), the child begins to understand the viewpoint of others and makes logical decisions. He/she can also solve a variety of problems, although it is not until the next stage (the formal operations stage) that the child can apply logic to understand problems that are abstract. In the formal operations stage, there is a complete freeing of thought from direct experience and the adolescent shows hypothetical-deductive, as well as scientiWc-inductive, reasoning.
Piaget's model has generated much research and some of his Wndings have been challenged. Recent studies with infants and young children have consistently shown that mastery of many of the constructs he proposed are manifested much earlier. For example, Piaget proposed that object permanence (the recognition that objects exist even

Cognitive development from a neuropsychologic perspective |
139 |
|
|
|
|
Table 8.1. Piaget's stages of cognitive development
Stage |
Age (years) |
Characteristics |
|
|
|
Sensorimotor |
0±2 |
The child gains knowledge about objects by manipulating them |
|
|
ReXexive behaviors gradually evolve into intentional acts |
|
|
There is a rudimentary understanding of simple cause-and-eVect relationships |
|
|
Object permanence develops |
Preoperational |
2±7 |
Development of internal representations of objects and events (symbols) |
|
|
Thought is egocentric in that the child fails to take others' point of view |
|
|
There is a tendency to Wx attention on a limited aspect of a stimulus (centration) |
|
|
Thought is irreversible (unable to follow line of reasoning back) |
Concrete operations |
7±11 |
Emergence of logical operations (thought is no longer dominated by perceptions) |
|
|
Development of the ability to arrange objects according to a characteristic (seriation) |
|
|
Understanding of common characteristics among objects (classiWcation) |
|
|
The child masters conservation (of number, area, mass, and volume) |
|
|
Reversibility of thought (ability to appreciate the invariant properties of objects) |
|
|
De-centering is attained (ability to consider all the salient features of a stimulus) |
|
|
The child can think logically but cannot apply logic to abstract problems |
Formal operations |
11±151 |
Emergence of hypothetical-deductive reasoning |
|
|
Onset of scientiWc-inductive reasoning |
|
|
The child can understand abstractions from existing knowledge |
|
|
Evidence of logical, abstract, and systematic thinking |
|
|
|
|
|
|
when not in view) is not mastered until approximately 9 months of age. Using a number of occlusion paradigms (e.g., a toy car rolling along a track that is partly hidden by a screen), Baillargeon (1995) has shown that infants as young as 2.5 months understand that the occluded object continues to exist. The diVerence in these timelines is likely to arise through the use of more reWned measures that enable investigators to isolate speciWc cognitive processes involved in a particular task. For instance, Baillargeon (1995) suggested that the infant's failure to search for an object may not represent a lack of object permanence but may instead signal a diYculty in the ability to plan and search for objects. Despite such revisions, Piaget's model continues to provide a useful organizing framework within the developmental literature.
Development of speciWc cognitive functions
Attention
Piaget's description of the sensorimotor and preoperational stages provides some insight into the development of attention. Children in the preoperational stage (ages 2 to 7 years) are drawn by visual appearances and tend to focus on a single attribute, or the most compelling feature, of objects and situations. For example, when identifying geo-
metrical shapes, young children often confuse triangles, rectangles, and squares because they attend to only one feature of the shape (e.g., whether it has angles or not) (Wadsworth, 1984). By contrast, a child in the concrete operations stage (between the ages of 7 and 11) is able to attend to many more features when examining a shape (e.g., number of sides, lengths of sides) and, therefore, can identify it easily.
Neo-Piagetian theorists have found support for this developmental progression using problem-solving tasks. For example, Siegler (1981) found that 5-year-old children attend to and process only one salient dimension of the material. Nine-year-olds, in turn, will add a second, lessobvious dimension. By late childhood or early adolescence, children will take into account multiple features simultaneously. Similar developmental changes have been reported by investigators using a number of measures of attention such as vigilance tasks, speeded classiWcation tasks, incidental learning tasks, selective listening tasks, and visual search tasks (for a review see Davies et al., 1984). These studies demonstrate that the ability to detect target information in the presence of distracting stimuli and to resist interference improves with age.
The improvement in processing eYciency and resistance to interference are, in part, responsible for an increase in short-term processing capacity observed during childhood (Cowan, 1997; Pressley and Schneider,

140 D. M. Pascualvaca and G. Morote
Table 8.2. Neuropsychologic models of attention and their proposed brain correlates
Model |
Elements or processes |
Brain regions |
|
|
|
Mirsky (1987), Mirsky |
Focus±execute (scan information and execute |
Inferior parietal, superior temporal, and striatal |
et al. (1991) |
response) |
regions |
|
Sustain attention |
Mesopontine reticular formation, midline and |
|
|
reticular thalamic nuclei |
|
Encode (retain and manipulate information) |
Hippocampus and amygdala |
|
Shift |
Dorsolateral prefrontal cortex |
Posner and Petersen (1990) |
Orienting to sensory stimuli |
Posterior attention system |
|
Disengaging attention |
Parietal lobe |
|
Moving attentional focus |
Superior colliculus |
|
Engaging attention |
Lateral pulvinar nucleus |
|
Detecting target events |
Anterior attention system (cingulate gyrus and |
|
|
supplementary motor cortex) |
|
Maintaining an alert state |
Noradrenaline (norepinephrine) innervation |
|
|
system particularly in the right hemisphere |
Pribram and McGuinness |
Arousal (orienting response) |
Spinal cord through brainstem reticular formation |
(1975) |
Activation (readiness to respond) |
Basal ganglia |
|
EVort (coordination of attention systems) |
Hippocampus |
|
|
|
|
|
|
1997). Short-term capacity refers to the amount of information that can be held in working memory at a given time and is frequently assessed by presenting subjects with sequences of simple stimuli of increasing length and asking them to repeat them immediately. Regardless of the speciWc type of material presented (e.g., digits or letters), there are clear developmental increases in the number of items children can recall with increasing age, and adult levels of performance typically are reached by 12 years of age (Dempster, 1981).
It is clear that the child's abilities to focus and modulate attentional focus are present as early as infancy (see review by Johnson, 1996) and become more eYcient with age. However, little is known about how the diVerent aspects of attention develop in normal children. Neuropsychologic models of attention propose that attention comprises diVerent skills that are mediated by distinct brain regions (see Table 8.2 for a comparison of the main models of attention). Although the speciWc elements outlined in the models diVer, most models include basic processes such as vigilance or arousal, selective attention or focusing, and the shifting of attention.
In developmental neuropsychology, these models have been applied almost exclusively to the study of children with conditions such as attention-deWcit hyperactivity disorder (e.g., Barkley et al., 1992), autism (e.g., Courchesne et al., 1995; Pascualvaca et al., 1999), traumatic brain injury (e.g., Ewing-Cobbs et al., 1998), and spina biWda (Loss et al., 1998). These studies have noted attention problems in all
the patient groups, although there appear to be some diVerences in the speciWc components that are impaired. For example, individuals with autism seem to have particular diYculties with shifting their focus of attention (Courchesne et al., 1995), whereas children who have sustained a traumatic brain injury show problems primarily in their ability to focus (Ewing-Cobbs et al., 1998).
Only a couple of studies have used these models to investigate the developmental trajectories of attention capacities in normal children. One of these studies was conducted by Rebok and colleagues (1997). Using Mirsky's model (Mirsky, 1987; Mirsky et al., 1991) as a conceptual framework, these investigators charted the developmental process of attentional capacities in an epidemiologic sample of children between 8 and 13 years of age. The results of this study indicate that the most rapid gains in attentional performance are seen between the ages of 8 and 10 years. For example, the percentage of omission errors on the Continuous Performance Test (CPT), a measure of sustained attention, declined by about half in this age range. Performance on the CPT leveled oV between the ages of 10 and 13, except for the more diYcult versions of this task (e.g., auditory CPT) on which performance continued to improve until adolescence. These developmental diVerences on the CPT are consistent with those reported by Greenberg and Waldman (1993) using a diVerent version of this task. The developmental progression in sustained attention is consistent with maturation of the neural circuits that purportedly mediate this element of attention.
Cognitive development from a neuropsychologic perspective |
141 |
|
|
|
|
The ability to sustain attention, according to Mirsky, is associated with cellular activity in regions of the reticular activating system and other structures of the brainstem (Mirsky et al., 1991). Although these structures mature shortly after birth, the ascending projections from them to the cerebral cortex, which are also implicated in the regulation of sustained attention (Deutsch et al., 1987; Cohen et al., 1988), continue to develop well into adolescence (Rabinowicz, 1976; Hudspeth and Pribram, 1992).
Rebok and colleagues (1997) also found a marked improvement between ages 8 and 10 in the abilities to focus and to shift attentional focus. Changes in attentional performance seem to be less rapid after age 10, although there is some indication that attention continues to improve into adolescence. For example, reaction time scores in attention tasks continue to decrease across this time period (McKay et al., 1994). The continued improvement observed in the ability to focus, sustain, and shift attention suggests that the brain areas mediating these processes are not functionally mature in middle childhood.
There is also evidence to indicate that boys and girls show diVerent developmental trajectories, with young girls being more eYcient at focusing and sustaining attention. For example, 8-year-old girls make fewer commission errors on the CPT than do boys and take less time; they also make fewer errors on visual search tasks (Pascualvaca et al., 1997). The diVerences in favor of girls observed at 8 years of age decrease by 10 years and are imperceptible by adolescence (Rebok et al., 1997).
It is important to emphasize that adequate performance on traditional tests of attention depends on the integrity of many functional systems. Most neuropsychologic tests make extensive demands on the subjects and require the integrated activity of many brain regions (Cooley and Morris, 1990). The limitation of traditional tests can be overcome through the use of precise cognitive measures and brain imaging techniques. Brain imaging studies have elucidated the eVects of speciWc variable factors, such as variations in the nature of the stimuli, mode of presentation, visual Weld of presentation, and type of response, on attentional performance (e.g., Posner et al., 1988; Pardo et al., 1991; Posner, 1993). These techniques will undoubtedly advance our understanding of the speciWc brain circuits involved in attention as well as of the development of these systems in normal children.
Executive functions
Attention and executive functions both refer to the ability to respond selectively to information. Consequently, the behavioral boundaries between these capacities are the
subject of debate. Executive functions, however, encompass much more than attention. They incorporate many higher cognitive skills that are necessary to achieve a future goal (Luria, 1973). These include the ability to conceptualize situations Xexibly, plan a course of action or goal, initiate the appropriate steps to accomplish this goal, and inhibit irrelevant or competing responses.
The behavioral descriptions of executive functions have been correlated with prefrontal cortical regions in nonhuman primates (e.g., Goldman-Rakic, 1987, 1988). The prefrontal cortex has intricate connections with the limbic system, caudate nucleus, superior colliculus, posterior association cortex, and motor regions within the frontal cortex. The richness of these connections implies a system that is capable of regulating complex behavior (Luria, 1973). At the present time, it is unclear whether speciWc executive functions are mediated by distinct regions within the prefrontal cortex or whether these functions are subserved by a common prefrontal region and distinguished by its unique sets of connections to other areas. Fuster (1985), for example, proposed that the abilities to resist interference, maintain set, and plan a response are localized in diVerent parts of the prefrontal cortex. In contrast, Goldman-Rakic (1987) hypothesized that each of these component skills is mediated by diVerent pathways that comprise the circuitry of the prefrontal cortex. For example, she proposed that the prefrontal±limbic connections may subserve the working memory component of executive functions, whereas the connections to the striatum, thalamus, and premotor cortex may mediate the selection and execution of speciWc responses.
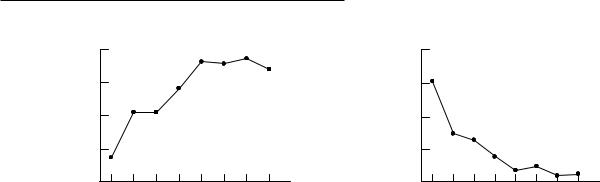
The prefrontal cortex has traditionally been conceptualized as the slowest developing brain region based on the time course of its myelination (Rabinowicz, 1976; Hudspeth and Pribram, 1992). Given that this cortical area is late to mature, many investigators have suggested that executive functions are not fully operational until later in life, perhaps as late as adolescence. This assumption has been supported by the results of studies using clinical measures of executive functions, such as the Wisconsin Card Sorting Test (WCST). For example, Chelune and Baer (1986) administered the WCST to a group of children aged 6±12 years and found that there was a striking improvement in performance between 6 and 10 years of age (Fig. 8.2). Performance continued to improve, at a less accelerated rate, between 10 and 12 years of age, and it reached adult levels at about 12 years. These results have been interpreted as an indication that executive functions mature during early adolescence.
However, rudimentary types of executive function are observed in early childhood and even in infancy when
142 D. M. Pascualvaca and G. Morote
((a) |
|
6 |
|
|
|
|
|
|
|
(b) |
(b) |
|
50 |
|
|
|
|
|
|
|
|
Number of categories |
5 |
|
|
|
|
|
|
|
|
|
Perseverative errors |
40 |
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
30 |
|
|
|
|
|
|
|
||
|
3 |
|
|
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
2 |
7 |
8 |
9 |
10 |
11 |
12 |
Adults |
|
|
|
10 |
7 |
8 |
9 |
10 |
11 |
12 |
Adults |
|
|
6 |
|
|
|
6 |
||||||||||||||
|
|
|
|
|
Age (years) |
|
|
|
|
|
|
|
|
Age (years) |
|
|
||||
Fig. 8.2. Number of categories (a) and perseverative errors (b) on the Wisconsin Card Sorting Test by age (Chelune and Baer, 1986).
developmentally appropriate measures are used. One of these measures is the classic Piagetian object-permanence paradigm. This measure resembles the delayed-response inhibition task, which is sensitive to dorsolateral prefrontal functions in monkeys (Diamond and Goldman-Rakic, 1989). In the object-permanence task, a toy is hidden in one of two identical wells in front of the infant and, after a brief delay, the infant is allowed to reach for the toy. Once the infant reaches for the toy correctly, the toy is hidden in the other well in full view of the infant. Using this paradigm, Diamond (e.g., Diamond and Goldman-Rakic, 1989; Diamond, 1990) found that infants between 7 and 11 months made the classic ªA not Bº error; that is, they reached for the toy in the place where they had found it previously, rather than in the most recent hiding place. By 12 months of age, however, infants completed the task correctly without diYculty. Since this task requires the maintenance of a set over a brief delay (e.g., remembering where the toy was hidden) as well as the execution of an appropriate motor response while inhibiting an inappropriate response (i.e., reaching to the well that contained the toy in the previous trial), it is clear that rudimentary executive skills are present early in infancy.
Infants can not only maintain set and inhibit an inappropriate response but they can also withhold actions to obtain a reward. This ability, observed in children as young as 18 months of age, continues to improve during infancy and is associated with language competence (Vaughn et al., 1984). The association between language and inhibitory control is consistent with Luria's hypothesis (1959) that verbal mediation plays a role in the development of response inhibition.
Taken together, the consensus of recent Wndings indicates that the mastery of behaviors typically conceptualized as executive functions occurs at diVerent ages, depending on the speciWc domains assessed. For example, using a number of executive tasks,Welsh et al. (1991) found
three stages of maturation: at ages 6, 10, and 12 years. Six- year-old children demonstrated simple planning and organized visual search, which included the ability to resist distraction and inhibit irrelevant responding. More developed impulse control and set maintenance and an increased ability to ignore distracting information were evident at 10 years of age. By 12 years of age, complex planning skills, verbal Xuency, and motor sequencing reached adult levels. The speciWc functions mastered at each of these age levels and examples of tasks used to assess these functions are shown in Table 8.3. Using very diVerent measures, a similar multistage progression was found by Passler et al. (1985) and by Becker, et al. (1987). These investigators outlined comparable stages of maturation, with the greatest period of development observed between 6 and 8 years of age.
It is important to note that, even though most studies have indicated complete mastery on tasks of executive functions by age 12, these functions are likely to continue maturing until late adolescence or adulthood. In fact, children of 12 years of age are not as Xexible and eYcient in their planning skills as are adults (Pea, 1982; Welsh et al., 1991). Adolescents are also likely to exhibit only incomplete mastery of tasks that require complex planning, organization, and inhibition of competing responses.
There are no consistent sex diVerences in the development of executive functions. Most studies have found either no diVerences between boys and girls (Welsh et al., 1991) or inconsistent diVerences only at the younger age groups (Passler et al., 1985). Girls are better at inhibiting motor movements (Macoby and Jacklin, 1974) and irrelevant responses (Pascualvaca et al., 1997) than boys, but it is not clear how these sex diVerences impact on their ability to plan and achieve a goal.
The developmental trajectory of executive functions parallels normal cognitive development. As discussed by Welsh and Pennington (1988), Piaget's theory of cognitive

Cognitive development from a neuropsychologic perspective |
143 |
|
|
|
|
Table 8.3. Developmental progression of behaviors associated with frontal lobe functioning
Age |
|
|
(years) |
Processes |
Name of instruments |
|
|
|
6 |
Resistance to distraction |
Visual Search Task |
|
Rudimentary response |
Tower of Hanoi (3 rings) |
|
inhibition |
|
|
Simple strategic and |
Speeded responding |
|
planning |
|
10 |
Inhibition of irrelevant |
Matching Familiar Figures Test |
|
responding |
|
|
Hypothesis generation |
Wisconsin Card Sorting Test |
|
Organized search |
|
|
Maintenance of set |
|
12 |
Verbal Xuency eYciency |
Verbal Fluency Test |
|
Goal setting |
Tower of Hanoi (4 rings) |
|
(intermediate and Wnal) |
|
|
Complex planning skills |
Motor Planning |
|
Motor sequencing |
|
|
|
|
|
|
|
Source: From Welsh et al., 1991.
development resembles current concepts of executive functions and supports the premise that these functions are present during infancy. For instance, the concept of causality, which is mastered during the Wrst year of life, involves rudimentary set maintenance, planning, and Xexibility. Subsequent stages in Piaget's theory also provide examples of how executive functions change during cognitive development. Increased language competence during the preoperational stage (ages 2 to 7) allows greater verbal control, which, in turn, leads to improvement in the attainment of a goal. Subsequently, during the operational and formal operational stages, the child experiences a gradual distancing from the environmental stimuli and begins to reason logically about familiar situations (in the concrete operations stage, between the ages of 7 and 11), as well as about hypothetical events (during the formal operations stage, between 11 and 15 years). This gradual improvement in the ability to reason logically and understand hypothetical situations resembles the developmental progression of executive functions.
Memory
Contemporary views of memory posit that memory is not a unitary trait but rather comprises diVerent skills (Table 8.4) that are mediated by distinct brain pathways (for a
review, see Schacter and Tulvin, 1994). At a general level, memory processes can be divided into short-term and long-term components. Short-term or working memory refers to the ability to represent information internally for brief periods of time (up to 20s). Only a limited amount of information can be consciously processed while in shortterm storage. In contrast, long-term memory contains virtually everything that the person has learned, including records of personal events, facts about the world, and information about how to do things.
The neural systems underlying shortand long-term memory processes also diVer. Short-term capacity is mediated primarily by areas of the prefrontal cortex (Funahashi et al., 1993; Casey et al., 1995; Courtney et al., 1998; Ungerleider et al., 1998), whereas consolidation processes resulting in long-term memory is associated with the medial temporal region and neighboring structures (Bachevalier and Mishkin, 1984; Alvarez et al., 1994). Support for a distinction in the neural substrates subserving shortand longterm memory has been derived from the adult and developmental literature. However, there is evidence to suggest that the neurologic systems that support shortand long-term memory may change during the course of development. For example, compared with adults, normal children have shown a more diVuse pattern of activation on fMRI scans in the prefrontal cortex during working memory tasks (Casey et al., 1995). This Wnding may reXect an immature brain organization in children, particularly in areas that develop late. There is also some indication that the medial temporal region, which matures earlier than the prefrontal cortex, may mediate some aspects of short-term memory early in development (Hershey et al., 1998).
Short-term memory capacities can be further subdivided into verbal, visual (e.g., for objects, patterns, designs), and spatial systems (e.g., relationships among objects in space). Evidence for a dissociation of these systems has been found in cognitive (Baddeley, 1992), lesion (Levine et al., 1985), animal (Goldman-Rakic, 1987), positron emission tomographic (PET) (Jonides, et al., 1993; Courtney et al., 1996), and functional magnetic resonance imaging (fMRI) studies (D'Esposito et al., 1995). The evidence suggests that these memory systems are mediated by neighboring but distinct areas of the prefrontal cortex (e.g., Courtney et al., 1996). It is unclear, however, what speciWc role the prefrontal cortex plays in the working memory process.
Short-term memory improves dramatically during childhood and early adolescence, regardless of the speciWc type of material that children are asked to remember (e.g., verbal, visual, or spatial). For example, the number of unrelated words that children can recall doubles between 4 and 12 years, by which adult levels of performance are gener-
