
Книги по МРТ КТ на английском языке / Functional Neuroimaging in Child Psychiatry Ernst 1 ed 2000
.pdf
154 D. M. Pascualvaca and G. Morote
Rapin, I. and Allen, D. A. (1988). Syndromes in developmental dysphasia and adult aphasia. In Language, Communication, and the Brain, ed. F. Plum. New York: Raven Press.
Rasmussen, T. and Milner, B. (1977). The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann. N.Y. Acad. Sci., 299, 355±69.
Rebok, G. W., Smith, C. B., Pascualvaca, D. M., Mirsky, A. F., Anthony, B. J. and Kellam, S. G. (1997). Developmental changes in attentional performance in urban children from eight to thirteen years. Child Neuropsychol., 3, 28±46.
Reilly, J. S., Bates, E. A. and Marchman, V. A. (1998). Narrative discourse in children with early focal brain injury. Brain Lang., 61, 335±75.
Rovee-Collier, C. and Gerhardstein, P. (1997). The development of infant memory. In The Development of Memory in Childhood, ed. N. Cowan, pp. 5±39. Hove, UK: Psychology Press.
Schacter, D. L. and Tulvin, E. (eds.) (1994). Memory Systems. Cambridge, MA: MIT Press.
Schneider, W. (1997). Memory Development between Two and Twenty. New Jersey: Lawrence Erlbaum.
Shaywitz, B. A., Shaywitz, S. E., Pugh, K. R. et al. (1995). Sex diVerences in the functional organization of the brain for language. Nature, 373, 607±9.
Siegler, R. S. (1981). Developmental sequences within and between concepts. Monogr. Soc. Res. Child Dev., 46, (serial No. 189).
Ungerleider, L. G. and Haxby, J. V. (1994). What and where in the human brain. Curr. Opin. Neurobiol., 4, 157±65.
Ungerleider, L. G. and Mishkin, M. (1982). Two cortical visual systems. In Analysis of Visual Behavior, eds. D. J. Ingle, M. A. Goodale and R. J. W. MansWeld, pp. 549±86. Cambridge, MA: MIT Press.
Ungerleider, L. G., Courtney, S. M. and Haxby, J. V. (1998). A neural system for human working memory. Proc. Natl. Acad. Sci. USA,
95, 883±90.
van der Kolk, B. A. and Greenberg, M. S. (1987). The psychobiology of the trauma response: hyperarousal, constriction, and addiction to the traumatic reexposure. In Psychological Trauma, ed. B. van der Kolk, pp. 63±87. Washington, DC: American Psychiatric Press.
Vargha-Khadem, F., Gadian, D. G., Watkins, K. E., Connelly, A., van Paesschen, W. and Mishkin, M. (1997). DiVerential eVects of early hippocampal pathology on episodic and semantic memory. Science, 277, 376±80.
Vaughn, B. E., Kopp, C. B. and Krakow, J. B. (1984). The emergence and constellation of self-control from eighteen to thirty months of age: normative trends and individual diVerences. Child Dev.,
55, 990±1004.
Wadsworth, B. J. (1984). Piaget's Theory of Cognitive and AVective Development, 3rd edn. New York: Longman.
Warrington, E. K. and Shallice, T. (1984). Category speciWc semantic impairments. Brain, 107, 829±53.
Welsh, M. C. and Pennington, B. F. (1988). Assessing frontal lobe function in children: views from developmental psychology.
Dev. Psychol., 4, 199±230.
Welsh, M. C., Pennington, B. F. and Groisser, D. B. (1991). A norma- tive-developmental study of executive function: a window of prefrontal function in children. Dev. Neuropsychol., 7, 131±49.
Western Psychological Services (1996). Rey Auditory Verbal Learning Test. Los Angeles, CA: Western Psychological Services.
Woods, B. T. and Teuber, H. L. (1973). Early onset of complementary specialization of cerebral hemispheres in man. Trans. Am. Neurolog. Assoc., 98, 113±17.
Introduction
Functional magnetic resonance imaging (fMRI) allows neuroscientists to examine the function of the human brain, especially the developing human brain, in a relatively noninvasive manner. Previous approaches were relatively indirect techniques such as scalp-recorded electroencephalography (EEG) and event-related brain potentials or brain imaging techniques that required exposure to ionizing radiation, for example positron emission tomography (PET), single photon emission computed tomography (SPECT), and X-ray computed tomography (CT). While these latter techniques may be used with pediatric patient populations when clinically warranted, the ethics of exposing children to unnecessary radiation for the advancement of science are still under debate (Casey and Cohen, 1996; Morton, 1996; Zametkin et al., 1996). With fMRI, developmental research is possible. This technique is described in detail in Chapter 3. In the current chapter age-appropriate behavioral paradigms and task designs for use with fMRI in the context of developmental studies are described.
The signiWcance of studying functional brain changes in a developmental context becomes more apparent when we consider aspects of brain development in general (see Chapter 7). The brain continues to develop after birth and well into childhood. Huttenlocher (1990, 1997) has demonstrated that pruning and reorganization of some cortical regions are relatively protracted. While synaptic density reaches adult levels by approximately 5 months in the visual cortex, prefrontal cortex still shows a 10% greater synaptic density at 7 years than in adulthood. Similarly, PET studies of glucose metabolism suggest that maturation of local metabolic rates in prefrontal cortex closely parallel the time course of this overproduction and subsequent pruning of synapses (Chugani et al., 1987). A logical
9
Cognitive and behavioral probes of developmental landmarks for use in functional neuroimaging
B. J. Casey, Kathleen M. Thomas, Tomihisa F. Welsh, Rona Livnat and Clayton H. Eccard
conclusion from these Wndings is that cognitive processes such as memory and language, which rely heavily on the prefrontal, temporal, and association cortices, may function in a physiologically diVerent manner in children than in adults. Whether this diVerence is qualitative or quantitative is yet to be determined.
To date, little is known regarding the neural bases of cognition in normally developing children, and the neural correlates of developmental disorders are even less well understood. Certain developmental disorders (e.g., atten- tion-deWcit hyperactivity disorder and obsessive-compul- sive disorder) have shown abnormal brain metabolism in adult brain imaging studies (Swedo et al., 1989; Zametkin et al. 1990). Despite the signiWcance of these Wndings, the study of childhood-onset disorders in adulthood, long after the appearance of the clinical symptoms, provides relatively limited information about the progression of the disorder with development. Noninvasive fMRI techniques provide a means of addressing the developmental physiologic course of a disorder in vivo. As a Wrst step, the patterns detected by this technology for normal development must be established.
Paradigms for use in fMRI studies of children
There are few published studies using fMRI in children, although the number of studies reported at scientiWc meetings is increasing substantially. An important consideration in taking on this challenge is the development of appropriate cognitive and behavioral probes for children. Table 9.1 summarizes reported studies to date by type of behavioral paradigm; eight of these studies have been published to date, but many have been summarized previously (Thomas and Casey, 1999). Four general areas of research have been addressed. First, several neuroimaging studies
155

156B. J. Casey et al.
Table 9.1. Paradigms used to date in pediatric fMRI studies
|
Study group |
|
|
|
|
|
|
|
|
|
|
|
|
Paradigms |
Subjects |
Age |
Number |
Citation |
||
|
|
|
|
|
|
|
Sensorimotor tasks |
|
|
|
|
|
|
Passive visual stimulation |
Sedated patients |
6 weeks to 36 months |
7 |
|
|
Born et al. (1996)a |
|
Unsedated normal |
Adults |
3 |
|
|
|
|
Sedated and unsedated patients |
28 weeks' gestation to |
30 |
|
|
Born et al. (1997) |
|
|
36 months of age |
|
|
|
|
|
Congenital structural deformities |
3 years |
1 |
|
|
Hunter et al. (1998) |
|
of visual cortex |
Older children |
2 |
|
|
|
|
Sedated |
|
|
|
|
|
|
Unsedated |
|
|
|
|
|
|
Sedated patients |
4 days to 8 years |
7 |
|
|
Joeri et al. (1996a) |
|
Unsedated healthy |
Adults |
10 |
|
|
|
Tactile stimulation |
Neurosurgery patients |
22 months to 18 years |
25 |
|
|
Kiriakopoulos et al. (1996) |
|
Neurosurgery patients, including |
0±22 years |
17 |
(14) |
|
Graveline et al. (1998) |
|
children after a hemispherectomy |
|
|
|
|
|
|
involving the primary sensorimotor |
|
|
|
|
|
|
cortex |
|
|
|
|
|
Unilateral and bilateral |
Patients including child with |
8±14 years |
7 |
|
|
Popp et al. (1996) |
hand and Wnger movements |
parietal tumor |
|
|
|
|
|
|
Neurosurgery patients |
22 months to 18 years |
25 |
|
|
Kiriakopoulos et al. (1996) |
Motor sequence task |
Patients with affective disorders |
9±11 years |
6 |
|
|
Casey et al. (1997c) |
|
Normal volunteers |
Adult |
6 |
|
|
|
Sensorimotor |
ADD boys |
14 years (mean) |
7 |
|
|
Rubia et al. (1998) |
synchronization task |
Healthy boys |
14 years (mean) |
9 |
|
|
|
Language tasks |
|
|
|
|
|
|
Verbal Xuency and language |
Child with epilepsy and learning |
9 years |
1 |
|
|
Benson et al. (1996)a |
task |
disability |
|
|
|
|
|
Spelling and rhyming tasks |
Children and adults |
|
± |
|
|
Dapretto et al. (1996) |
|
with and without dyslexia |
|
|
|
|
|
Word generation |
Complex partial epilepsy |
9±17 years |
7 |
|
|
Hertz-Pannier et al. (1995) |
|
Complex partial epilepsy |
8±18 years |
11 |
|
|
Hertz-Pannier et al. (1997)a |
Phoneme deletion and |
Dyslexic subjects and age-matched |
13±19 years |
20 |
|
|
Frost et al. (1997)a |
discrimination |
controls |
|
|
|
|
|
Passive listening task |
Sedated patient |
15 months |
1 |
|
|
Hirsch et al. (1997) |
|
Sedated patients |
15 months |
1 |
|
|
Hirsch et al. (1998) |
|
|
31 months |
1 |
|
|
|
|
|
39 months |
1 |
|
|
|
Word generation and object |
Normal volunteers |
11±17 years |
15 |
|
|
Bonello et al. (1998) |
naming |
Presurgical patients |
6±16 years |
20 |
|
|
Logan (1998) |
Sentence comprehension |
Children with left hemisphere |
11±12 years |
2 |
|
|
Booth et al. (1999) |
and verb generation |
lesions |
|
|
|
|
|
|
Healthy |
Adults |
4 |
|
|
|
Living/nonliving word |
Normal volunteers |
8±12 years |
6 |
|
|
Vaidya et al. (1998) |
decisions |
|
|
|
|
|
|
Speech task, silent word |
Neurosurgery patients |
22 months to 18 years |
25 |
|
|
Kiriakopoulos et al. (1996) |
generation, pair-judgement |
Patients with complex partial |
14 years (mean) |
11 |
|
|
Hertz-Pannier et al. (1997)a |
and naming tasks |
epilepsy, preand postsurgery |
|
|
|
|
|

Cognitive and behavioral probes of developmental landmarks |
157 |
|
|
|
|
Table 9.1 (cont.)
|
Study group |
|
|
|
|
|
|
|
|
|
|
Paradigms |
Subjects |
Age |
Number |
Citation |
|
|
|
|
|
|
|
|
Healthy and epileptic children and |
12±56 years |
18 |
|
Kato et al. (1998) |
|
adults including presurgical |
|
|
|
|
|
epileptic patients |
|
|
|
|
|
With and without reading disabilities |
Adolescents |
64 |
|
Vincent et al. (1998) |
|
Dyslexics |
Adult |
31 |
|
Pugh et al. (1998) |
Spatial tasks |
|
|
|
|
|
Mental rotation |
Children with left hemisphere lesions |
11±12 years |
2 |
|
Booth et al. (1999) |
|
Healthy |
Adults |
4 |
|
|
Hierarchical spatial analysis |
Children, including 2 with lesions |
10±15 years |
7 |
|
Moses et al. (1997) |
of shape forms |
|
|
|
|
|
Memory and inhibition tasks |
|
|
|
|
|
Nonspatial working memory |
Normal volunteers |
9±11 years |
6 |
|
Casey et al. (1995)a |
|
|
Adult |
6 |
|
|
Spatial working memory |
Normal volunteers |
8±10 years |
6 |
|
Casey et al. (1997a) |
|
Normal volunteers |
8±10 years |
6 |
|
Orendi et al. (1997) |
|
Normal volunteers |
8±12 years |
7 |
|
Truwit et al. (1996) |
|
Normal volunteers |
8±10 years |
6 |
|
Thomas et al. (1999)a |
Go-no-go task |
Normal volunteers |
7±12 years |
9 |
|
Casey et al. (1997b)a |
|
|
Adults |
9 |
|
|
|
Children with perinatal IVH |
6±9 years |
10 |
|
Casey et al. (1998b) |
|
Children with ADD, on and oV |
|
10 |
|
Vaidya et al. (1998)a |
|
medication |
|
|
|
|
Go-no-go task (sinusoidal |
Normal volunteers |
7±11 years |
9 |
|
Casey et al. (1998a) |
target probability) |
|
Adults |
9 |
|
|
Response selection task |
Normal volunteers |
7±11 years |
10 |
|
Casey et al. (1997c) |
(incompatible SRT task) |
Normal volunteers |
Adults |
10 |
|
|
AVect recognition tasks |
|
|
|
|
|
AVective face task |
Normal volunteers |
12±17 years |
14 |
|
Baird et al. (1999)a |
|
Normal volunteers |
Adults |
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes:
aJournal article, not conference abstract.
ADD, attention deWcit disorder; IVH, intraventricular hemorrhage; SRT, serial reaction time
have examined developing sensorimotor systems in infants and young children (Born et al., 1996, 1997; Joeri et al., 1996a,b; Popp et al., 1996). These studies have typically employed the use of sedation during the passive presentation of visual and auditory stimuli or required simple hand and Wnger movements of alert neurologic patients to map the sensorimotor cortex. Although these studies have been performed with the youngest children studied with fMRI to date, the majority of the studies used sedation and/or patient populations. In the studies using sedation, the interpretations of their data are limited because of the
failure to include unsedated children or sedated adults as comparison groups. With studies using neurologic patients, the interpretations about the normal development of sensorimotor cortex based on mapping studies with neurosurgical patients is unclear. Second, a number of investigators (Hertz-Pannier et al., 1995, 1997; Benson et al., 1996; Dapretto et al., 1996; Kiriakopoulos et al., 1996; Frost et al., 1997; Hirsh et al., 1997; Logan, 1998; Vaidya et al., 1998) have begun to use fMRI to study language in children. These studies have investigated language function primarily in children with epilepsy, dyslexia, or early left

158B. J. Casey et al.
hemisphere lesions, thereby limiting conclusions on the normal development of language. Nonetheless, these studies represent an important Wrst step toward understanding the neural circuitry involved in language development. Third, the reorganization of the brain following early lateralized lesions has been a topic of much interest and the focus of at least two fMRI studies. These imaging studies were attempts to determine whether early left hemisphere lesions result in the reorganization of hemi- sphere-specialized functions (e.g., language) to the contralateral side. Booth et al. (1999) report preliminary 3T fMRI data examining this question with regard to language. For the child in their sample with the largest early lesion, they observed regions of activation in the undamaged hemisphere that were homologous to the regions activated in healthy adults. These results may have implications for the plasticity of function following early brain lesions, speciWcally the relation between lesion size and location and subsequent cortical reorganization. Moses et al. (1997) report similar preliminary Wndings from a study of spatial analytic processing that showed activity in the contralateral hemisphere to that observed in normal developing children and adults. Finally, a number of neuroimaging studies have begun to address the functional development of the prefrontal cortex (Casey et al., 1995, 1997a,b,c, 1998a; Orendi et al., 1997; Truwit et al., 1996; Vaidya et al., 1998). These studies have addressed two cognitive processes that have been attributed to the prefrontal cortex: working memory and response inhibition. These studies will be described in greater detail later in this chapter.
Across the pediatric neuroimaging studies reviewed, two important issues emerged. First, in the process of developing cognitive and behavioral paradigms, it is important that the tasks be appropriate for children. Often tasks used with adults are modiWed or simpliWed for use with children. When taking this approach, investigators should ensure that the task is not too diYcult or complex for children. Even simple tasks that require the subject to press more than one button can be diYcult for young children because they are more likely to rely on visual input together with somatosensory feedback to position their Wngers on the correct button. In the scanner environment, the child is lying down and typically cannot see her/his Wnger movements, which presents a problem for the child. Furthermore, when using a similar task with children and adults, task diYculty should be titrated across the diVerent age ranges. Otherwise, diVerences in patterns of brain activity between groups may simply reXect diVerences in overall eVort on the task rather than maturational changes in the behavior or the brain. Likewise, the lack of activity in certain brain regions of subjects performing poorly does
not necessarily reXect the integrity of those brain regions, but rather a failure of the subject to recruit those areas. This issue is not speciWc to developmental research but applies to clinical research comparing patients with normal volunteers as well. Examples of how to titrate performance to a subject's individual ability include increasing task diYculty by degrading stimulus information, increasing the stimulus presentation rate, or increasing the memory load. Slowing the stimulus presentation rate or self-pacing tasks as a strategy for equating task performance between groups should be used with caution. Distinct patterns of brain activity have been observed as a function of varying stimulus presentation rate or self-pacing a task. For example, diVerent brain regions were shown to be activated during the Stroop task (Stroop, 1935) when the stimulus presentation rate varied in a Wxed-pace design (Bench et al., 1993) and similarly between subjects in a self-paced design (George et al., 1994). However, D'Esposito et al. (1997) argue that Wxed-pace designs are sensitive to diVerences in both the duration and intensity of neural processing, whereas self-paced designs are less sensitive to diVerences in the duration of neural processing and more sensitive to diVerences in the intensity of local neural processing. For these reasons, when studying a patient or age group that is likely to show increased reaction times relative to controls, self-paced designs may be preferred.
Another concern that emerged when reviewing the current pediatric neuroimaging literature was the importance of developing appropriate comparison conditions within the behavioral paradigms. Most functional neuroimaging studies rely on subtractive methodology to identify active brain regions. Statistical subtractions are performed, comparing one condition with another. A wellrecognized concern about subtractive methodology (Donders, 1969; Sergent et al., 1992) is whether it is possible to design conditions or tasks such that all of the ªirrelevantº processes ± those that are supposed to be subtracted out ± are being performed in the same way or (in the case of neuroimaging studies) by the same anatomic structures in the control and experimental conditions. Failures to satisfy this assumption can produce misleading results. One approach to this problem is to analyze multiple subtraction pairings of conditions (e.g., Sergent, et al., 1992). For example, if a brain region shows activation in subtractions with two diVerent control conditions (e.g., A ± B and A ± C), it is more likely to reXect processes related to condition A. This design can be further exploited by using a standard analysis of variance and comparing multiple conditions simultaneously (e.g., Sanders and Orrison, 1993).

Cognitive and behavioral probes of developmental landmarks |
159 |
|
|
|
|
Comparison conditions can be introduced by using extended periods of ªonº versus ªoVº activations, commonly referred to as ªblocked designsº or by using intermixed trial designs referred to as ªevent-related fMRIº. Conventional blocked designs incorporate the use of experimental and control blocks that last several seconds or even minutes and have been used extensively with PET and SPECT studies of sensory and higher cortical function. Such block designs are a necessity when imaging hemodynamic responses that occur over periods of approximately 1min but are not required for fMRI studies where activity can be observed within a few seconds. Event-related fMRI studies examine MR signal changes to brief stimulus events. For example, Savoy et al. (1995) showed that a clearly detectable signal change could be elicited with visual stimulation as brief as 34 ms in duration (see Rosen et al. (1998) for a review). These data suggest that it is possible to interpret transient changes in the MR signal analogous to electrophysiologic evoked potentials. Buckner and colleagues (Buckner et al., 1996; Dale and Buckner, 1997) were among the Wrst to use event-related fMRI studies to demonstrate detectable changes related to single-task events. Since that time, a number of studies (Buckner et al., 1996; Konishi et al., 1997; Cohen et al., 1997; Courtney et al., 1997; Zarahn et al., 1998) have explored the use of event-related fMRI to separate sensorimotor activation temporally from cognitive-related activation (e.g., memory and inhibition). These issues, age-appropriate tasks, and appropriate task designs will be discussed within the context of a number of empirical studies.
Behavioral and cognitive paradigms of prefrontal cortical functioning
Given the prolonged physiologic development and reorganization of the frontal lobes, tasks believed to involve this region are ideal for investigating development. Two cognitive processes that have been attributed to the frontal lobe are working memory and response inhibition (Fuster, 1989). A number of normative pediatric fMRI studies have examined prefrontal cortical activity in children during working memory and response inhibition tasks (Casey et al., 1995, 1997b). In fact, the Wrst published pediatric fMRI study examined prefrontal cortical activation in children performing a working memory task (Casey et al., 1995). One of the central themes of this chapter is the importance of examining the functional development of the frontal lobes and related circuitry. This is important for a number of reasons. First, as stated above, there is considerable development and reorganization of the frontal lobes
throughout childhood and adolescence. Second, the frontal lobes and related circuitry have been implicated in a number of developmental disorders.
One view of prefrontal function consistent with the approach that our laboratory has taken is that the prefrontal cortex supports representations of information (e.g., verbal, spatial, motor, emotional) against interference over time or from competing sources (Goldman-Rakic, 1987; Cohen and Servan-Schreiber, 1992). A number of classic developmental studies have demonstrated that these memory-related processes develop throughout childhood and adolescence (Flavell et al., 1966; Pascual-Leone, 1970; Case, 1972; Keating and Bobbitt, 1978).
In the current chapter we address the important themes of the development of age-appropriate behavioral paradigms and appropriate task designs in the context of four developmental fMRI studies. These empirical studies revolve around two central themes: maintenance of information in prefrontal cortex over time and suppression of competing responses in prefrontal cortex.
Maintenance of information in prefrontal cortex
The Wrst published pediatric fMRI study examined prefrontal activation in children performing a working memory task (Casey et al., 1995). The purpose of this study, at the time, was to determine the feasibility of using fMRI to examine higher level cognitive processing in children. Six children between the ages of 9 and 11 years were scanned with fMRI while performing a working memory task used previously in adults (Cohen et al., 1994). The study included two task conditions: a memory condition and a comparison/control condition. The memory condition required children to observe sequences of letters and to respond whenever the current letter was the same as the letter occurring two trials back (ª2-back memory taskº). In the comparison condition, subjects monitored similar sequences of letters for any occurrence of a single, prespeciWed letter. Both the memory and comparison conditions required subjects to monitor sequences of letters presented visually one at a time, encode each letter, evaluate its identity, and respond to a target by pressing a button. The conditions diVered in that the memory task required the subject to keep in mind both the identity and the order of the two previous letters, continuously updating this mental record over time. These latter cognitive operations are central to the concept of working memory (Baddeley, 1986) and consistent with our view of prefrontal involvement in the representation of information over time and against interference from competing sources.
Echo-planar images were acquired on a 1.5T gradient
160 B. J. Casey et al.
(a) |
|
5 |
|
Memory |
Control |
Memory |
|
|
signal |
Control |
|
||||
|
4 |
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
MR |
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
in |
|
|
|
|
|
|
|
1 |
|
|
|
|
|
|
|
change |
|
|
|
|
|
|
|
0 |
|
|
|
|
|
|
|
±1 |
|
|
|
|
|
|
|
Percentage |
|
|
|
|
|
|
|
±2 |
|
|
|
|
|
|
|
±3 |
|
|
|
|
|
|
|
±4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
±5 |
|
|
|
|
|
|
|
0 |
20 |
40 |
|
60 |
80 |
Scan number
(b) |
5 |
|
Memory |
Control |
Memory |
|
signal |
Control |
|
||||
4 |
|
|
|
|
|
|
3 |
|
|
|
|
|
|
MR |
2 |
|
|
|
|
|
in |
1 |
|
|
|
|
|
change |
|
|
|
|
|
|
0 |
|
|
|
|
|
|
±1 |
|
|
|
|
|
|
Percentage |
|
|
|
|
|
|
±2 |
|
|
|
|
|
|
±3 |
|
|
|
|
|
|
±4 |
|
|
|
|
|
|
|
±5 |
|
|
|
|
|
|
0 |
20 |
40 |
|
60 |
80 |
|
|
|
Scan number |
|
|
|
Fig. 9.1. Change in MR signal intensity as a function of the experimental manipulation (two-back memory task) for (a) the inferior frontal
gyrus; (b) the middle frontal gyrus.
echo scanner using 5inch(15 cm) surface coils while the children performed the memory and comparison conditions. Eight 5mm coronal slices covering the frontal poles were acquired. Images were registered to a reference image to correct for movement using a modiWed version of Woods et al. (1992) three-dimensional automated image registration (AIR) algorithm. Movement did not correlate with the experimental manipulation but rather appeared to increase as a function of time on task and was minimal. The average movement across the entire study was less than 0.5mm with 0.34mm of movement in the x direction, and 0.47mm in the y direction. Areas of signiWcant activation were identiWed by performing pixel-wise t-tests comparing the memory and comparison conditions using a split-halves method, as follows. The Wrst and second half of the data were analyzed separately and areas with signiWcant values (e.g., 3.56, p#0.001, one-tailed) in both comparisons were accepted as reliable regions of activity (the split-halves statistic approximates the p value of
#0.0012 5 0.000001).
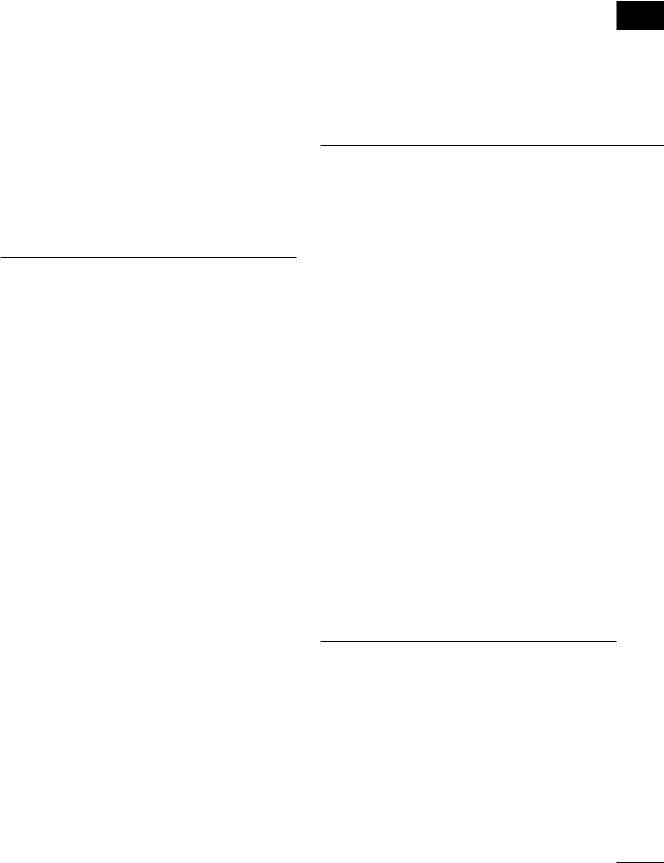
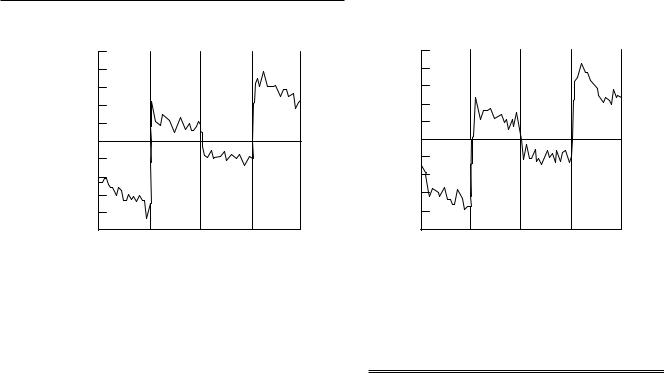
The results demonstrated reliable activity in the middle and inferior frontal gyri in Wve of the six children (Fig. 9.1). The graph depicts the change in MR signal intensity as a function of scans across time. The increases in activity nicely map onto the experimental manipulation. These results replicate an earlier fMRI study with adults showing inferior and middle frontal gyri activity using the same paradigm (Cohen et al., 1994) and a more recent event-related fMRI study of working memory (Cohen et al., 1997) showing dorsolateral activation during active maintenance of stimulus information. Table 9.2 illustrates the distribution of activity across frontal gyri for children and adults taken from the Casey et al. (1995) and Cohen et al. (1994) studies, respectively. Taken together, these two
Table 9.2. Distribution of prefrontal cortical activity in two fMRI studies deWned by the number of active particles as a function of gyri
|
|
Number of active particles |
|
|
|
|
|
Frontal gyri |
Adults |
Children |
|
|
|
|
|
Superior frontal |
4 |
3 |
|
Middle frontal |
32 |
27 |
|
Inferior frontal |
48 |
46 |
|
Anterior cingulate |
4 |
9 |
|
Orbitofrontal |
9 |
10 |
|
|
|
|
|
|
|
|
|
Sources: Adult data from the study of Cohen et al. (1994) and child data from that of Casey et al. (1995).
initial studies suggest a similar distribution of prefrontal cortical activity in children and adults during performance of a working memory task. However, the percentage change in signal observed for the children was on average two to three times that observed for the adults in the Cohen et al. (1994) study. Based on the behavioral data, the children had more diYculty with the task. On average, children performed at 70 to 75% accuracy in the Casey et al. (1995) study while adults performed at or above 90% accuracy in the Cohen et al. (1994) experiment. This study demonstrates the importance of collecting behavioral responses in the scanner but also raises concerns with regard to the interpretation of our Wndings given the behavioral diVerences. Are the diVerences maturational or strategic in nature or both?
A study that may help address this question is one by Braver et al. (1997) that examined prefrontal cortical activity as a function of increasing memory load in adults. They
Cognitive and behavioral probes of developmental landmarks |
161 |
|
|
|
|
Fig. 9.2. Depiction of the n-back task. For the 0-back control condition, the target is the letter X. For the three memory load conditions, the target is any repeat of a letter presented one, two, or three trials back.
performed the same type of memory task but varied the memory load from 0 to 3, as demonstrated in Fig. 9.2. The subject monitored a sequential display of single letters and responded only when the current letter was the same as the letter n trials before it (e.g., if n5 2, then A±F±A or G±B±G, but not A±F±G±A or A±A). Subjects were practiced until they reached 90% accuracy on the highest memory load of three trials. The results revealed monotonic increases in percentage change in prefrontal cortex (Fig. 9.3). We mention this study because it is an elegant example of a task that may be especially well suited for developmental populations. This paradigm allows for the manipulation of memory load (number of trials back the subject must remember) by age and/or ability.
While our Wrst study demonstrated the use of fMRI in pediatric populations, there was no direct comparison between children and adults since the child and adult studies were performed at diVerent sites and with diVerent scanning parameters. A similar working memory task was used to perform a more direct comparison of brain activation for children and adults. This study was an extension of a multisite collaboration with the primary goals of demonstrating the reproducibility of fMRI results across several sites in the USA and determining feasibility of using fMRI with pediatric populations.
Each participating group examined brain activity in both adults and children during performance of a spatial working memory task designed as an analog of the verbal working memory task. Instead of letters, colored dots were presented in one of four adjacent locations. The task was to press one of four buttons that corresponded to the location of the colored dot. In the memory condition, the task was to press the button that corresponded to the location of the
Fig. 9.3. Illustration of the monotonic increase in the MR signal intensity as a function of increasing memory load of zero, one, two, and three trials back for the dorsolateral prefrontal cortex.
dot n-trials back. While adults can perform the task at two and three trials back, typically children between the ages of 6 and 8 years can only perform the task at 1-back while older children (9±12 years) can do the task at 2-back. The number of trials back the subject had to remember was determined by individual ability. In the comparison or motor condition, the task was to press the button that corresponded to the location of the dot in the current trial (no memory load). Therefore, these conditions are identical except in the instructions to the subject and the memory demands.
As reported in Casey et al. (1998c), the results for adults were reproducible and reliable across all participating

162B. J. Casey et al.
sites. The results from six children and eight adults at the Pittsburgh site demonstrated reliable activity in the right dorsolateral prefrontal cortex, right superior parietal cortex, and bilaterally in inferior parietal cortex during the memory condition relative to the motor condition (Fig. 9.4, p. 242). In part, these results suggest that spatial working memory tasks activate very similar cortical regions for school-age children and adults. However, despite an attempt to equate performance between age groups by varying memory load as a function of age, children performed less well than adults on both the memory and motor tasks. Adults performed near ceiling (99%) on the motor and memory tasks compared with the children, whose performance was signiWcantly poorer (93% and 69%, respectively). Regional diVerences in activation (e.g., insular cortex and cingulate gyrus) between groups may reXect these performance diVerences.
In sum, task designs that inherently allow the manipulation of memory load or task diYculty yield promise for examining developmental progressions in behavior. However, the increment in memory load or diYculty may need to be gradual and Wne-tuned to the individual subject in order to observe these progressions. For example, the use of degraded stimuli or varying stimulus presentation rates may allow for Wner manipulations than increasing memory load by a factor of 1, 2 or 3. These manipulations in conjunction with event-related fMRI will provide a more reWned understanding of the maturation of sensorimotor brain regions compared with higher cortical regions (e.g., prefrontal cortex).
Suppression of competing information in prefrontal cortex
Given the prevalence of inhibitory problems in children and related symptomatology in various developmental disorders (e.g., attention-deWcit hyperactivity disorder, obsessive-compulsive disorder, Tourette's syndrome), the neural substrate of inhibitory control has received much attention. Consistent with the view put forth by GoldmanRakic and others (Goldman-Rakic, 1987; Cohen and Servan-Schrieber, 1992), inhibitory control reXects the ability to support representations of information against interference from competing sources. Accordingly, when goals or task demands are well represented, then competing alternatives are suppressed. What prefrontal systems are involved in representing information against competing sources? Two examples of studies addressing this question are described below.
One classic paradigm for examining inhibition is the go- no-go task. At least one fMRI study using a go-no-go para-
Fig. 9.5. Volume of activation of dorsolateral prefrontal cortex for
children and adults during performance of a go-no-go task.
digm with healthy children has been published to date (Casey et al., 1997b). Nine children (7 to 12 years) and nine young adults (21 to 24 years) were scanned while performing a version of the go-no-go paradigm. The task required that the subject respond to all sequentially presented letters except X. Stimulus duration was 500ms and the interstimulus interval was 1500ms. The percentage of targets was maintained at 75% to build up a prepotent tendency to respond. Gradient-echo, echo-planar images (time to repetition (TR) 6000ms, time to echo (TE) 40ms, acquisition matrix 128!64) were acquired in eight 5mm contiguous coronal slice locations during three task conditions: (i) inhibitory trials deWned by the presence of 50% nontargets (i.e., Xs); (ii) control trials consisting of 100% targets (i.e., nonXs); and (iii) control trials consisting of 100% targets but with interstimulus intervals of 3500ms, resulting in an equal number of motor responses as the inhibitory condition. The two comparison conditions thus controlled for stimulus parameters (number of stimuli and interstimulus interval) and response parameters (number of responses and inter-response interval), respectively.
An analysis of variance with a Bonferroni adjustment showed reliable activation in the anterior cingulate, inferior and middle frontal gyri, and orbitofrontal gyri for both the children and adults. In this investigation, the general location of activation in prefrontal cortex did not diVer for children compared with adults, but the overall volume of prefrontal activation, particularly in dorsolateral prefrontal regions, was greater for children than adults (Fig. 9.5). This diVerence in the volume of dorsolateral prefrontal activity resulted from a lack of robust activity in these areas for the adults. Adults showed the most robust activity in more ventral regions of prefrontal cortex. Activity in the orbitofrontal and anterior cingulate cortex in children and adults correlated with behavioral performance on this
Cognitive and behavioral probes of developmental landmarks |
163 |
|
|
|
|
response inhibition task such that greater activity in the orbitofrontal cortex was associated with the better performance, and greater activity in the anterior cingulate cortex with worse performance. It should be noted that those children with the best performance and the most orbitofrontal activity also had the most dorsolateral prefrontal activity. This observation suggests that children may be less selective in the portions of prefrontal cortex recruited in performance of the go-no-go task and/or rely on diVerent strategies to perform the go-no-go task compared with adults. An event-related design may provide a clearer understanding of the neural mechanisms underlying response inhibition and their development. In a recent fMRI study by Konishi et al. (1997) using a go-no-go paradigm with a mixed trial design, right ventral prefrontal activation discriminated no-go from go trials in healthy adults. This Wnding is particularly interesting given that when we grouped our subjects (children and adults) by performance using a median split on the number of false alarms (i.e., responses to nontarget stimuli), we observed signiWcant diVerences between groups only in ventral prefrontal cortex (Casey et al., 1996).
More recently we have begun to examine prefrontal activity related to the representation and suppression of information with emotional content. This work is based largely on Davidson's model of prefrontal asymmetries related to approach and avoidance behaviors (Davidson, 1994). Accordingly, appetitive or approach-related behavior is supported by the left prefrontal system, while negative or avoidance-related behaviors are presumably supported by the right prefrontal system. To examine this view of prefrontal asymmetries, we developed a task that we refer to as the ªemotional n-back taskº. This task is a modiWed version of the working memory task described previously (Cohen et al., 1993; Casey et al., 1995) and includes emotionally evocative positive, negative, and neutral stimuli. The n-back task was superimposed onto backgrounds of emotionally evocative stimuli. Backgrounds of emotional and neutral content employed a subset of digitized slides from the International AVective Picture System (Lang et al., 1988) modiWed for use with children (McManis et al., 1995). This manipulation allowed us to assess the ability of a subject to suppress the representation of an emotional stimulus during maintenance of verbal information. The neutral background condition allows one to determine whether disruption in performance of the memory task is simply owing to distracting information in the background regardless of emotional type or whether it is a consequence of a speciWc type of interfering emotional response to the background. The control memory condition of 0-back (i.e., the detection of
a given letter) when superimposed on the diVerent emotional backgrounds keeps subjects' vision Wxated centrally and permits the examination of diVerences in patterns of brain activation as a function of emotional content irrespective of memory load (Fig. 9.6).
We had several predictions regarding the manipulations of memory and emotional content in this paradigm. First, we expected that the greatest disruption in performance of the memory condition would occur when the letters were superimposed on a withdrawal-related negative background and the least disruption would be observed when letters were superimposed on an approach-related positive background. These hypotheses were based on the assumption that the negative condition requires competing frontal systems (approach and withdrawal) to be activated simultaneously. In other words, representation and maintenance of verbal information (e.g., letters) is thought to be predominantly subserved by the left prefrontal cortex (Smith et al., 1996) or approach frontal system (Davidson, 1994) while representation of negative information is presumably subserved by the right prefrontal cortex or withdrawal/avoidant system. Therefore, when negative-related stimuli (e.g., a pointed gun, a wounded soldier, or a snarling dog) are presented, there is a conXict in the representation of behavioral goals. The negative-related stimuli are represented as an event to avoid whereas the letters are represented as events that are important to maintain (i.e., approach). For this reason, it was assumed that the negative stimulus information would be more disruptive because it required the representation of competing behavioral systems. When positive or approach-related stimuli are presented in conjunction with the task, the representation of behavioral goals are consistent so the subject's performance may even be primed or facilitated since a congruent behavioral system is activated.
Our behavioral data conWrmed our hypothesis of worse memory performance during the presentation of negative information (81%) compared with performance during the presentation of positive (87%) and neutral information (84%), although these values did not reach signiWcance for the small number of subjects (n5 10, p#0.11).
The results from our initial imaging studies using this paradigm were promising with regard to our hypothesis of laterality of activity. For the Wrst three subjects scanned during this task, all showed predominantly right prefrontal activity during the presentation of the negative backgrounds and predominantly left prefrontal activity during the presentation of the positive backgrounds. Figure 9.7 illustrates the pattern of activity for the two adult subjects. Similarly, the third subject, an 8-year-old child, showed a 3:1 ratio in right-to-left prefrontal activity during the presentation of
