
Книги по МРТ КТ на английском языке / Functional Neuroimaging in Child Psychiatry Ernst 1 ed 2000
.pdf
Table 12.1 (cont.)
|
|
Age (mean" |
Imaging modality |
|
|
|
|
|
SD or range, |
spatial resolution; |
|
|
|
Study |
Subjects (No.) |
years) |
acquisition parameters |
Probes |
Control task |
Results |
|
|
|
|
|
|
|
Breiter et al. |
Normal males (18) |
22±33; mean |
Functional MRI 1.5 T; |
Two experiments: |
Ekman Neutral |
The amygdala was preferentially activated in |
(1996) |
|
26.5 |
asymmetric spin-echo |
Ekman fearful faces |
Faces |
response to fearful versus neutral faces and |
|
|
|
T *-weighted, TR |
and Ekman happy |
|
the amygdala also responded preferentially to |
|
|
|
2 |
|
|
|
|
|
|
3000/2000ms, TE 50 |
faces |
|
happy versus neutral faces |
|
|
|
ms, slice thickness |
|
|
|
|
|
|
3.125mm |
|
|
|
Reiman |
Normal females (12) |
23.3"3.2 |
PET H 15O; 10mm |
Emotion-generating |
Neutral Wlm clips |
Filmand recall-generated emotion increased |
|
|
|
2 |
|
|
|
et al. (1997) |
|
|
|
Wlm clips of |
and neutral |
activity in the medial prefrontal cortex and |
|
|
|
|
happiness, sadness, |
autobiographical |
thalamus |
|
|
|
|
and disgust; recall of |
scripts |
Film-generated emotion was associated with |
|
|
|
|
autobiographical |
|
increases in activity bilaterally in the |
|
|
|
|
scripts |
|
occipitotemporoparietal cortex, lateral |
|
|
|
|
|
|
cerebellum, hypothalamus, and in a region |
|
|
|
|
|
|
that included the anterior temporal cortex, |
|
|
|
|
|
|
amygdala, and hippocampal formation |
|
|
|
|
|
|
Recall-generated sadness was associated with |
|
|
|
|
|
|
increases in activity in the vicinity of the |
|
|
|
|
|
|
anterior insular cortex |
Lane et al. |
Normal females (12) |
23.3"3.2 |
PET H 15O; 10mm |
Film clips of |
Three neutral Wlm |
Happiness, sadness, and disgust increased |
|
|
|
2 |
|
|
|
(1997) |
|
|
|
happiness, sadness, |
clips and three |
activity in the thalamus and medial prefrontal |
|
|
|
|
and disgust and recall |
neutral |
cortex; these three emotions were also |
|
|
|
|
of autobiographical |
autobiographical |
associated with activation of anterior and |
|
|
|
|
scripts of the same |
scripts |
posterior temporal structures, primarily when |
|
|
|
|
three emotions |
|
induced by Wlm |
|
|
|
|
|
|
Recalled sadness was associated with increased |
|
|
|
|
|
|
activation in the anterior insula |
|
|
|
|
|
|
Happiness was distinguished from sadness by |
|
|
|
|
|
|
greater activity in the vicinity of ventral |
|
|
|
|
|
|
mesial frontal cortex |
Schneider |
Normal males (7) |
29.7"4.3 |
Functional MRI 1.5 T; |
Mood induction |
Resting baseline, |
A signiWcant increase in signal intensity was |
et al. (1997) Normal females (5) |
|
T *-weighted FLASH, |
using PENN facial |
eyes open |
found during sad as well as happy mood |
|
|
|
|
2 |
|
|
|
|
|
|
TR 240ms, TE 60ms, |
photographs: |
|
induction in the left amygdala |
|
|
|
slice thickness 4mm |
happy, sad |
|
|
Elliott et al. |
Depressed adults (6) |
Mean 34.7 |
PET H 15O |
Easy and hard Tower |
Visual |
Depressed subjects, compared with their |
|
|
|
2 |
|
|
|
(1997) |
Normal controls (6) |
|
|
of London tasks |
perceptuomotor task |
controls, failed to show signiWcant activation |
|
|
|
|
|
|
in the cingulate and striatum and also failed |
|
|
|
|
|
|
to show the normal augmentation of |
|
|
|
|
|
|
activation in the caudate nucleus, anterior |
|
|
|
|
|
|
cingulate, and right prefrontal cortex that was |
|
|
|
|
|
|
associated with increasing task diYculty |
Beauregard |
Depressed males (3) |
Mean 42 |
Functional MRI: 1.5 T; |
Color Wlm clip to elicit |
A neutral Wlm clip of |
Transient sadness elicited signiWcant activation |
et al. (1998) |
Depressed females (4) |
Mean 42 |
echo-planar, TE |
transient sadness |
house renovation |
in both groups of subjects in the medial and |
|
Normal males (3) |
Mean 45 |
54ms, slice thickness |
|
|
inferior prefrontal cortices, the middle |
|
Normal females (4) |
Mean 45 |
5mm |
|
|
temporal cortex, the cerebellum, and the right |
|
|
|
|
|
|
caudate. While viewing the sad Wlm clip, the |
|
|
|
|
|
|
depressed subjects showed signiWcantly |
|
|
|
|
|
|
greater activations in the left medial |
|
|
|
|
|
|
prefrontal cortex and in the right cingulate |
|
|
|
|
|
|
than did the control group |
Baird et al. |
Normal males (5) |
Mean 13.9 |
Functional MRI: 1.5 T |
Facial discrimination |
Three unique |
A signiWcant increase in signal intensity was |
(1999) |
Normal females (7) |
|
echo planar, TE 40ms, |
and labeling of fearful |
nonsense gray-scale |
found in both amygdalae in response to |
|
|
|
slice thickness 3mm |
expressions |
images |
recognition of fearful facial expressions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes: CBF, cerebral blood Xow; rCBF, regional cerebral blood Xow; TE, time to echo; TR time to repetition.

216R. A. Kowatch, P. A. Davanzo and G. J. Emslie
mamillary body rCBF, whereas unsolvable anagrams increased mamillary body and amygdalar CBF and decreased hippocampal rCBF.
To explore the neural bases of externally versus internally generated human emotion, Reiman et al. (1997) measured rCBF using PET in 12 healthy normal females while they watched short Wlm clips with either emotionally laden or neutral content. Tasks alternated between emotion-generating and control Wlm and recall tasks. Both Wlmand recall-generated emotion signiWcantly increased activity in the medial prefrontal cortex and thalamus. Filmgenerated emotion increased rCBF bilaterally in the occipitotemporoparietal cortex, lateral cerebellum, and hypothalamus. Recall-generated sadness was associated with increases in the anterior insular cortex.
Activation studies of adult mood disorders
Using a Tower of London task to study frontal lobe function, Elliott et al. (1997) used PET to measure rCBF in Wve patients with unipolar depression (mean Hamilton score 23.8) and controls matched for age, gender, and educational levels. Unlike controls, depressed subjects failed to show signiWcant activation in the cingulate and striatum and failed to show the normal augmentation of activation in the caudate nucleus, anterior cingulate, and right prefrontal cortex that was associated with increasing task diYculty, suggesting impaired function of these regions in depression.
Blunted activation of the left cingulate in depression has also been demonstrated using a Stroop task with 15O PET. George et al. (1997) studied 11 mood-disordered adults (nine men, two women; six unipolar, three biopolar II, and two bipolar I) and an equal number of ageand sexmatched controls. A control task involved color naming, a standard Stroop task required subjects to name the colors of ink in which incongruent color names appeared (e.g., ªredº printed in blue ink), and a sad Stroop task required the naming of the colors of ink in which emotional words (e.g., grief, misery, sad, bleak) appeared. Depressed subjects were able to activate the left dorsolateral prefrontal cortex, a region commonly hypoactive at rest in depression and hypoactive during the control task in this study. In contrast, activation seen during the interference task (i.e., naming of incongruent colors) was reduced in the left midcingulate, as well as in left insula and right superior temporal gyrus. No signiWcant group diVerences were seen during the sad Stroop test.
Using fMRI, Beauregard et al. (1998) studied seven adults (four women and three men) with major depression (mean age 42 years) and ageand gender-matched controls.
Subjects were imaged while watching a color Wlm clip designed to elicit sadness and while watching a neutral Wlm clip. Transient sadness elicited signiWcant activation in both groups in the medial and inferior prefrontal cortices, the middle temporal cortex, the cerebellum, and the right caudate, suggesting the participation of circuits involving these regions in this emotion. However, relative to controls, depressed subjects showed signiWcantly greater activation while viewing the sad Wlm clip in the left medial prefrontal cortex and right cingulate.
Neuroimaging of pediatric mood disorders
Studies of children and adolescents with mood disorders are just emerging. There have been a few structural studies of children and adolescents with mood disorders, the interpretation of which must be integrated with the changes that are known to occur with brain maturation (Chugani et al., 1987; Chugani and Phelps, 1991; Chiron et al., 1992). This limits direct comparisons with studies of adults. Structural imaging studies of children and adolescents with mood disorders using MRI have begun to implicate the frontal and temporal lobes and basal ganglia.
Hendren et al. (1991) reported clinically abnormal MRI scans in two of three children hospitalized for MDD. The Wrst was a 13-year-old male whose MRI showed abnormally asymmetrical lateral ventricles (right larger than the left), suggesting right-sided ventricular enlargement and decreases in surrounding brain tissue. The second subject was a 12-year-old male whose MRI showed a small area of abnormal signal intensity in the area of the left anterior basal ganglia or medial temporal lobe.
In a volumetric MRI study, Steingard et al. (1996) compared 65 children and adolescents (mean age 13 years) who were hospitalized for either MDD or dysthymia with 18 hospitalized psychiatric controls (mean age 10.7 years) without a depressive disorder: 11 with conduct disorder/ oppositional deWant disorder, two with ADHD, three with post-traumatic stress disorder, and two with an adjustment disorder. Volumetric analyses were used to measure frontal lobe volumes, lateral ventricular volumes, and total cerebral volumes for all subjects. To correct for diVerences in absolute cerebral volume associated with diVerent body and head size, the ratios of frontal lobe and lateral ventricular volumes to total cerebral volume were used to compare diVerences between the two groups; a multivariate analysis was used to control for the eVects of age, gender, and diagnosis. The depressed group had reduced frontal lobe volumes (signiWcantly smaller frontal lobe to total cerebral volume ratio) and increased ventricular volumes (signiWcantly larger ventricular to total cerebral

Pediatric mood disorders and neuroimaging |
217 |
|
|
volume ratio), but normal total cerebral volumes relative to their nondepressed psychiatric controls. The reduction in frontal lobe volume resembles that reported in depressed adults with MDD, suggesting some continuity of deviations in brain anatomy.
In the Wrst anatomic MRI study of pediatric bipolar patients, Botteron et al. (1995) compared T1- and T2- weighted MRI scans from eight manic children and adolescents and Wve age-, but not gender-matched normal subjects. They reported increased rates of subcortical white matter signal hyperintensities, abnormal temporal horn asymmetries, and ventricular abnormalities in four of the bipolar patients, and only one of the normal controls by clinical interpretation. Areas of hyperintensity on MRI usually are caused by increased water content and have been associated with ischemia, inXammation, and demyelination. While the signiWcance of these hyperintensities in children and adolescents with bipolar disorders is uncertain at this time, these Wndings are consistent with those of MRI studies of adults with bipolar I disorders, which frequently Wnd white matter signal hyperintensities in the periventricular space (Altshuler et al., 1995).
Using 1H MRS, Steingard et al. (1998) studied 14 adolescents with MDD (mean age 15.6 years) and 26 normal controls (mean age 14.3 years). Depressed adolescents showed an increase in the ratio of choline to creatine in the orbitofrontal cortex relative to controls, but normal N-acetylas- partate to creatine ratios. These Wndings are similar to Wndings in adult MDD (Charles et al., 1994) and suggest alterations in cholinergic neurotransmission in the orbitofrontal cortex.
In a recent SPECT study with 99mTc-labeled hexamethylpropyleneamine oxime (HMPAO), Kowatch et al. (1999) compared the rCBF in a resting condition of a group of seven adolescents (mean age 15.5 years; range 13±18 years) with symptomatic MDD (DSM-III-R criteria) to those of seven ageand gender-matched normal controls. Previously, the authors have obtained SPECT brain scans on depressed children and adolescents who were inpatients, and visual analysis of these studies suggested abnormalities (relative bilateral hypoperfusion) of the frontal cortex and mesial temporal lobes (Kowatch et al., 1993). This controlled study used a voxel-based T-image analysis to compare the rCBF patterns of a group of adolescents with MDD with those of control adolescents. After normalizing regional to whole brain counts, higher relative rCBF was found in the right mesial temporal cortex, the right superior±anterior temporal lobe, and the left inferolateral temporal lobe in the depressed group relative to controls. Decreased relative rCBF in the depressed group
compared with the control group was localized to the left parietal lobe, the anterior thalamus, and the right caudate. The most spatially extensive area of abnormally low rCBF was in the left superior parietal cortex. Some of these diVerences are shown in Figs. 12.2 and 12.3.
These preliminary Wndings suggest that portions of the right and left temporal lobes, the left parietal lobe, the anterior thalamus, and the right caudate may be involved in adolescent MDD. Parietal lobe rCBF abnormalities in MDD adults have been reported in a number of SPECT studies (Sackeim et al., 1990; Austin et al., 1992; Philpot et al., 1993; Lesser et al., 1994). Mesulam (1985) noted that, in monkeys, the superior parietal lobe receives projections from the primary somatosensory cortex and is designated as the unimodal somatosensory association area. Although lesions of the left parietal cortex area in humans are associated with deWcits in motor planning (de Renzi et al., 1983), the role of the left superior parietal cortex in MDD has yet to be elucidated. The authors also found high levels of relative rCBF in the right mesial temporal cortex, the right superior anterior temporal lobe, and the left inferolateral temporal lobe. The temporal lobes are central to the limbic±thalamic±cortical circuit believed to contribute to the regulation of mood (Ketter et al., 1996; Mayberg 1997). In addition, the majority of adult SPECT studies have reported decreases in temporal lobe rCBF (Soares and Mann, 1997b). Although the above studies implicate the temporal lobes in the pathophysiology of MDD in both adults and adolescents, the direction of the abnormality (low in adult MDD and high in adolescent MDD) is inconsistent. These Wndings raise the possibility of developmental diVerences in the neurobiology of adolescent versus adult MDD. Finally, adolescents with MDD show rCBF abnormalities implicating the limbic±thalamic±cortical circuit and portions of the basal ganglia, consistent with rCBF Wndings in adults with MDD.
Conclusions and future directions
Functional imaging studies have the potential to provide valuable information about the neurobiologic basis of child and adolescent mood disorders and their development into adult mood disorders. SigniWcant progress in this type of research is expected from improvements in functional imaging technologies, the development of normative data bases, and the use of multimodal imaging methods. The goals of brain imaging research will be to delineate the neural circuits underlying mood disorders, the neurobiologic diVerences among speciWc mood disorders and etiological subgroups, and the biology of
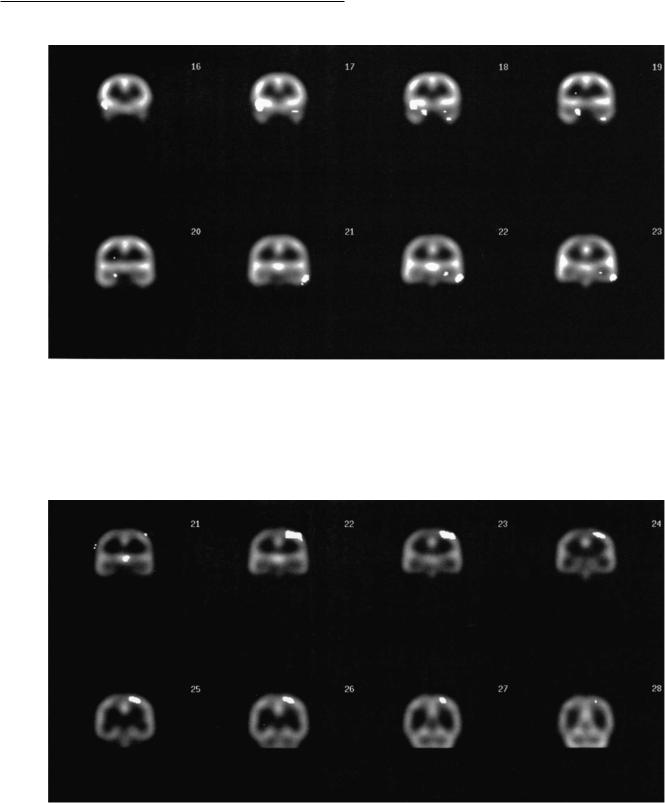
218 R. A. Kowatch, P. A. Davanzo and G. J. Emslie
Fig. 12.2. SPECT T images of coronal brain slices illustrating relative increases in rCBF in seven adolescents with major depression, relative to normal controls (p#0.01). Slices 16±20 illustrate increases in the right mesial and lateral temporal lobe and slices 21±23 illustrate the increases in the left lateral temporal lobe. These T images have been overlaid on the normal adolescent model brain in which the individual brain slices have been averaged together in a 3:1 ratio.
Fig. 12.3. SPECT T images of coronal brain slices illustrating relative decreases in rCBF in seven adolescents with major depression, relative to normal controls (p#0.01). Slice 21 illustrates the thalamic decreases and slices 22±27 illustrate the left parietal decreases.

transient mood states and ongoing trait vulnerabilities, and, ultimately, to contribute to the development of focused and rational treatments, both pharmacologic and behavioral.
To achieve these aims, speciWc behavioral subtyping (i.e., the study of homogeneous diagnostic groups) is needed. Longitudinal designs of speciWc diagnostic subgroups will be particularly helpful in elucidating stateversus trait-related brain abnormalities, correlates of depressive and manic phases of bipolar illness, and the impact of developmental inXuences on brain physiology. Age and gender, as well as other maturational variables such as sexual maturity (Tanner stage), will need to be considered when designing and interpreting functional neuroimaging studies in children and adolescents with mood disorders. Studies of integrated neural activity will predominantly utilize fMRI because of its lack of ionizing radiation and its excellent temporal resolution. Behavioral paradigms that target speciWc aVective and mood-related cognitive processes and that take into consideration age and gender eVects should prove useful. Such paradigms can be developed to examine the neural correlates of behavioral aspects of mood disorders (e.g. eVects on the perception of facial aVect), as well as to evaluate the responsiveness of certain neural structures, such as the amygdala or other limbic structures. The ability to link abnormalities in integrated neural activity with abnormalities of brain neurochemistry may fuel the development of rational therapies for addressing pathophysiology.
Functional imaging studies should be complemented by controlled, quantitative, and, preferably, longitudinal structural imaging studies. Quantitative image analysis techniques, as well as improved characterization of qualitative abnormalities such as white matter hyperintensities (their extent, number, and localization), are needed for use in well-controlled studies of speciWc subgroups.Where volumetric diVerences are identiWed, appropriate adjustments are required in functional image data analysis to avoid partial volume artifacts introduced by such anatomic diVerences.
Acknowledgements
Support from the National Institute of Mental Health (NIMH), Bethesda, MD (grant K07-MH01057 to R.A.K.) is gratefully acknowledged. We appreciate the administrative support of Kenneth Z. Altshuler M.D., Stanton Sharp Distinguished Chair, Professor and Chairman, Department of Psychiatry.
Pediatric mood disorders and neuroimaging |
219 |
|
|
iReferencesi
Adolphs, R., Tranel, D., Damasio, H. and Damasio, A. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372, 669±72.
Adolphs, R., Tranel, D., Damasio, H. and Damasio, A. R. (1995). Fear and the human amygdala. J. Neurosci., 15, 5879±91.
Agren, H. (1980). Symptom patterns in unipolar and biopolar depression correlating with monoamine metabolites in crebrospinal Xuid, I. General patterns. Psychiatry Res., 3, 211±23.
Altshuler, L. L., Curran, J. G., Hauser, P., Mintz, J., DenicoV, K. and Post, R. (1995). T2 hyperintensities in bipolar disorder: magnetic resonance imaging comparison and literature meta-analysis.
Am. J. Psychiatry, 152, 1139±44.
American Psychological Association (1989). Diagnostic and Statistical Manual of Mental Disorders, 3rd edn. Washington, DC: American Psychiatric Association.
Austin, M. P., Dougall, N., Ross, M. et al. (1992). Single proton emission tomography with 99mTc-exametazime in major depression and the pattern of brain activity underlying the psychotic/neurotic continuum. J. AVect. Disord., 26, 31±43.
Baird, A., Gruber, S., Fein, D. et al. (1999). Functional magnetic resonance imaging of facial aVect recognition in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry, 38, 195±9.
Beauregard, M., Leroux, J. M., Bergman, S. et al. (1998). The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. Neuroreport, 9, 3253±8.
Beck, A. T. (1971). Cognition, aVect, and psychopathology. Arch. Gen. Psychiatry, 24, 495±500.
Bench, C. J., Frackowiak, R. S. and Dolan, R. J. (1995). Changes in regional cerebral blood Xow on recovery from depression.
Psychol. Med., 25, 247±61.
Birmaher, B., Ryan, N. D., Williamson, D. E., Brent, D. A. Kaufman, J., Dahl, R. E., Perel, J. and Nelson, B. (1996). Childhood and adolescent depression: a review of the past 10 years. Part I. J. Am. Acad. Child Adolesc. Psychiatry, 35, 1427±39.
Botteron, K. N., Vannier, M. W., Geller, B., Todd, R. D. and Lee, B. C. P. (1995). Preliminary study of magnetic resonance imaging characteristics in 8- to 16-year olds with mania. J. Am. Acad. Child Adolesc. Psychiatry, 34, 742±9.
Bowring, M. A. and Kovacs, M. (1992). DiYculties in diagnosing manic disorders among children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry, 31, 611±14.
Bradley, W. G., Waluch, V., Brant-Zawadzki, M., Yardley, R. A. and WycoV, R. R. (1984). Patchy, perivascular white matter lesions in the elderly: a common observation during NMR imaging.
Noninvasive Med. Imaging, 1, 35±41.
Breiter, H. C., EtcoV, N. L., Whalen, P. J. et al. (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron, 17, 875±87.
Bunney,W. E., Jr and Davis, J. M. (1965). Norepinephrine in depressive reactions: a review. Arch. Gen. Psychiatry, 13, 483±94.
Burke, K. C., Burke, J. D., Rae, D. S. and Regier, D. A. (1991). Comparing age at onset of major depression and other psychiatric

220 R. A. Kowatch, P. A. Davanzo and G. J. Emslie
disorders by birth cohorts in Wve US community populations.
Arch. Gen. Psychiatry, 48, 789±95.
Charles,H. C., Lazeyras, F., Krishnan, K. R., Boyko, O. B., Payne, M. and Moore, D. (1994). Brain choline in depression: in vivo detection of potential pharmacodynamic eVects of antidepressant therapy using hydrogen localized spectroscopy. Prog. Neuropsychopharmacol. Biol. Psychiatry, 18, 1121±7.
Chiron, C., Raynaud, C., Maziere, B. et al. (1992). Changes in regional cerebral blood Xow during brain maturation in children and adolescents. J. Nucl. Med., 33, 696±703.
Chugani, H. T. and Phelps, M. E. (1991). Imaging human brain development with positron emission tomography. J. Nucl. Med.,
32, 23±6.
Chugani, H. T., Phelps, M. E. and Mazziotta, J. C. (1987). Positron emission tomography study of human brain functional development. Ann. Neurol., 22, 487±97.
Cicchetti, D., Rogosch, F. A., Toth, S. L. and Spagnola, M. (1997). AVect, cognition, and the emergence of self-knowledge in the toddler oVspring of depressed mothers. J. Exp. Child Psychol., 67, 338±62.
Cooley, E. L. and Nowicki, S. Jr (1989). Discrimination of facial expressions of emotion by depressed subjects. Genet. Soc. Gen. Psychol. Monogr., 115, 449±65.
Coyle, J. T. (1987). Biochemical development of the brain: neurotransmitters and child psychiatry. In Psychiatric Pharmacosciences of Children and Adolescents, ed. C. Popper, pp. 3±26. Washington, DC: American Psychiatric Press.
Dahl, R., Puig-Antich, J., Ryan, N. et al. (1989). Cortisol secretion in adolescents with major depressive disorder. Acta Scand., 80, 18±26.
Deicken, R. F., Fein, G. and Weiner, M. W. (1995a). Abnormal frontal lobe phosphorous metabolism in bipolar disorder. Am. J. Psychiatry, 152, 915±18.
Deicken, R. F., Weiner, M. W. and Fein, G. (1995b). Decreased temporal lobe phosphomonoesters in bipolar disorder. J. AVect. Disord., 33, 195±9.
de Renzi, E., Faglioni, P., Lodesani, M. and Vecchi, A. (1983). Performance of left brain-damaged patients on imitation of single movements and motor sequences. Frontal and parietalinjured patients compared. Cortex, 19, 333±43.
De Villiers, A. S., Russell, V. A., Carstens, M. E. et al. (1989). Noradrenergic function and hypothalamic±pituitary±adrenal axis activity in adolescents with major depressive disorder.
Psychiatry Res., 27, 101±9.
Doherty, M. B., Madansky, D., Kraft, J. et al. (1986). Cortisol dynamics and test performance of the dexamethasone suppression test in 97 psychiatrically hospitalized children aged 3±16 years. J. Am. Acad. Child Psychiatry, 25, 400±8.
Drevets, W. C. (1998). Functional neuroimaging studies of depression: the anatomy of melancholia. Annu. Rev. Med., 49, 341±61.
Drevets, W. C. and Raichle, M. E. (1992). Neuroanatomical circuits in depression: implications for treatment mechanisms.
Psychopharmacol. Bull., 28, 261±74.
Drevets, W. C., Videen, T. O., Price, J. L., Peskorn, S. H., Carmichael, S. T. and Raichle, M. E. (1992). A functional anatomical study of unipolar depression. J. Neurosci., 12, 3628±41.
Drevets, W. C., Price, J. L., Simpson, J. R., Jr et al. (1997). Subgenual prefrontal cortex abnormalities in mood disorders. Nature, 386, 824±7.
Ekman, P. and Oster, H. (1979). Facial expressions of emotion.
Annu. Rev. Psychol., 30, 527±54.
Elliott, R., Baker, S. C., Rogers, R. D. et al. (1997). Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol. Med.,
27, 931±42.
Emslie, G. J., Weinberg, W. A., Rush, A. J., Weissenburger, J. and Parkin-Feigenbaum, L. (1987). Depression and dexamethasone suppression testing in children and adolescents. J. Child Neurol.,
2, 31±7.
Emslie, G. J., Weinberg, W. A., Rush, A. J., Adams, R. M. and Rintelmann, J. W. (1990). Depressive symptoms by self report in adolescence: phase I of the development of a questionnaire for depression by self-report. J. Child Neurol., 3, 114±21.
Emslie, G. J., Rush, A. J., Weinberg, W. A., Gullion, C. M., Rintelmann, J. W. and Hughes, C. W. (1997). Recurrence of major depressive disorder in hospitalized children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry, 36, 785±92.
Erwin, R. J., Gur, R. C., Gur, R. E., Skolnick, B., Mawhinney-Hee, M., Smailis, J. (1992). Facial emotion discrimination: I. Task construction and behavioral Wndings in normal subjects. Psychiatry Res., 42, 231±40.
Extein, I., Rosenberg, G., Pottash, A., Gold, M. (1982). The dexamethasone suppression test in depressed adolescents. Am. J. Psychiatry, 139, 1617±19.
Faedda, G. L., Baldessarini, R. J., Suppes, T., Tondo, L., Becker, I. and Lipschitz, D. S. (1995). Pediatric-onset bipolar disorder: a neglected clinical and public health problem. Harvard Rev. Psychiatry, 3, 171±95.
Feinberg, T. E., Rifkin, A., SchaVer, C. and Walker, E. (1986). Facial discrimination and emotional recognition in schizophrenia and aVective disorders. Arch. Gen. Psychiatry, 43, 276±79.
Fleming, J. E. and OVord, D. R. (1990). Epidemiology of childhood depressive disorders: a critical review. J. Am. Acad. Child Adolesc. Psychiatry, 29, 571±80.
Gaebel, W. and Wolwer, W. (1992). Facial expression and emotional face recognition in schizophrenia and depression. Eur. Arch. Psychiatr. Clin. Neurosci., 242, 46±52.
Garber, J., Kriss, M. R., Koch, M. and Lindholm, L. (1988). Recurrent depression in adolescents: a follow-up study. J. Am. Acad. Child. Adolesc. Psychiatry, 27, 49±54.
Geller, B., Fox, L. W. and Clark, K. A. (1994). Rate and predictors of prepubertal bipolarity during follow-up of 6- to 12-year-old depressed children. J. Am. Acad. Child Adolesc. Psychiatry, 33, 461±8.
Geller, B., Sun, K., Zimerman, B., Luby, J. and Frazier, J. (1995). Complex and rapid-cycling in bipolar children and adolescents: a preliminary study. J. AVect. Disord., 34, 259±68.
George, M. S., Ketter, T. A., Parekh, P. I., Horwitz, B., Herscovitch, P. and Prost, R. M. (1995). Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatry, 152, 341±51.
George, M. S., Ketter, T. A., Parekh, P. I. et al. (1997). Blunted left cingulate activation in mood disorder subjects during a response
Pediatric mood disorders and neuroimaging |
221 |
|
|
interference task (the Stroop). J. Neuropsychiatry Clin. Neurosci.,
9, 55±63.
Glennon, R. A. (1987). Central serotonin receptors as targets for drug research. J. Med. Chem., 30, 1±12.
Gold, P. W., Goodwin, F. K. and Chrousos, G. P. (1988). Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. N. Engl. J. Med., 319, 348±53.
Golden, R. M. and Potter, W. Z. (1986). Neurochemical and neuroendocrine dysregulation in aVective disorders. Psychiatr. Clin. North Am., 9, 313±27.
Gray, J. A., Davis, N., Feldon, J., Nicholas, J., Rawlins, P. and Owen, S. R. (1981). Animal models of anxiety. Prog. Neuropsychopharmacol., 5, 143±57.
Gur, R. C., Erwin, R. J. and Gur, R. E. (1992). Neurobehavioral probes for physiologic neuroimaging studies. Arch. Gen. Psychiatry, 49, 409±14.
Gur, R. C., Ragland, J. D., Resnick, S. M. and Skolnick, B. E. (1994). Lateralized increases in cerebral blood Xow during performance of verbal and spatial tasks: relationship with performance level.
Brain and Cognit., 24, 244±58.
Harrington, R., Fudge, H., Rutter, M. and Hill, J. (1990). Adult outcomes of childhood and adolescent depression. Arch. Gen. Psychiatry, 47, 465±73.
Hendren, R. L., Hodde-Vargas, J. E., Vargas, L. A., Orrison, W. W. and Dell, L. (1991). Magnetic resonance imaging of severely disturbed children ± a preliminary study. J. Am. Acad. Child Adolesc. Psychiatry, 30, 466±70.
Hofer, P. A. (1973). Urbach±Wiethe disease (lipoglycoproteinosis; lipoid proteinosis; hyalinosis cutis et mucosae). A review. Acta Derm.Venereol., 53(Suppl.), 1±52.
Hofer, P. A., Larsson, P. A., Goller, H., Laurell, H. and Lorentzon, R. (1974). A clinical and histopathological study of twenty-seven cases of Urbach±Wiethe disease. Dermatologic, gastroenterologic, neurophysiologic, ophthalmologic and roentgendiagnostic aspects, as well as the results of some clinico-chemical and histochemical examinations. Acta Pathol. Microbiol. Scand., Pathol., (Suppl.) 245, 1±87.
Kandal, D. B. and Davies, M. (1986). Adult sequela of adolescent depressive symptoms, Arch. Gen. Psychiatry, 43, 255±62.
Kashani, J. H., Beck, N. C., Hoeper, E. et al. (1987a). Psychiatric disorders in a community sample of adolescents. Am. J. Psychiatry,
144, 584±9.
Kashani, J. H., Carlson, G. A., Beck, N. C. et al. (1987b). Depression, depressive symptoms, and depressed mood among a community sample of adolescents. Am. J. Psychiatry, 144, 931±4.
Kato, T., Takahashi, S., Shiori, T. and Inubushi, T. (1992). Brain phosphorous metabolism in depressive disorders detected by phos- phorus-31 magnetic resonance spectroscopy. J. AVect. Disord.,
26, 223±30.
Kato, T., Takahashi, S., Shiori, T. and Inubushi, T. (1993). Alterations in brain phosphorous metabolism in bipolar disorder detected in vivo 31P and 7Li magnetic resonance spectroscopy. J. AVect. Disord., 27, 53±9.
Kato, T., Inubushi, T. and Kato, N. (1998). Magnetic resonance spectroscopy in aVective disorders. J. Neuropsychiatr. Clin. Neurosci., 10, 133±47.
Kessler, R. C., McGonagle, K. A., Nelson, C. B., Hughes, M., Swartz, M. and Blazer, D. G. (1994). Sex and depression in the national comorbidity survey. II. Cohort eVects. J. AVect. Disord.,
30, 15±26.
Ketter, T. George, M., Kimbrell, T., Benson, B. and Post, R. (1996). Functional brain imaging, limbic function, and aVective disorders. Neuroscientist, 2, 55±65.
Kovacs, M., Feinberg, T. L. and Crouse-Novak, M. A. (1984). Depressive disorders in childhood. II. A longitudinal study of the risk for a subsequent major depression. Arch. Gen. Psychiatry, 41, 643±9.
Kowatch, R. A., Devous, M. D. S., Grannemann, B. G., Emslie, G. D., Trivedi, M. H. and Weinberg, W. A. (1993). A preliminary report of regional cerebral blood Xow in depressed adolescents. In
Proceedings of a Meeting of the American Academy of Child and Adolescent Psychiatry, San Antonio, Texas, p. 34.
Kowatch, R. A., Devous, M. D. S., Harvey, D. C. et al. (1999). A SPECT HMPAO study of regional cerebral blood Xow in depressed adolescents and normal controls. Prog. Neuropsychopharm. Biol. Psychiatry, 23, 643±56.
Kutcher, S., Malkin, D., Silverberg, J. et al. (1991). Nocturnal cortisol, thyroid stimulating hormone, and growth hormone secretory proWles in depressed adolescents. J. Am Acad. Child Adolesc. Psychiatry, 30, 407±14.
LaBarbera, J. D., Izard, C. E., Vietze, P. and Parisi, S. A. (1976). Fourand six-month-old infants' visual responses to joy, anger, and neutral expressions. Child Dev., 47, 535±8.
Lane, R. D., Reiman, E. M., Ahern, G. L., Schwartz, G. E. and Davidson, R. J. (1997). Neuroanatomical correlates of happiness, sadness, and disgust. Am. J. Psychiatry, 154, 926±33.
Lesser, I. M., Mena, I., Boone, K. B., Miller, B. L., Mehringer, C. M. and Wohl, M. (1994). Reduction of cerebral blood Xow in older depressed patients. Arch. Gen. Psychiatry, 51, 677±86.
Lewinsohn, P. M., Duncan, E. M., Stanton, A. K. and Hantzine, M. (1986). Age at onset for Wrst unipolar depression. J. Abnorm. Psychol., 95, 387±3.
Lewinsohn, P. M. Hops, H., Roberts, R. E., Seeley, J. R. and Andrews, J. A. (1993). Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. J. Abnorm. Psychol., 102, 133±44.
Lewinsohn, P. M., Clarke, G. N., Seeley, J. R. and Rhodes, P. (1994). Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J. Am. Acad. Child Adolesc. Psychiatry, 33, 809±18.
Lewinsohn, P. M., Klein, D. N. and Seeley, J. R. (1995). Bipolar disorder in a community sample of older adolescents: presence, phenomenology, comorbidity, and course. J. Am. Acad. Child Adolesc. Psychiatry, 34, 454±63.
Maas, J. W. Fawcett, J. and Dekirmenjian, H. (1968). 3-Methoxy-4- hydroxyphenylglycol (MHPG) excretion in depressed states: a pilot study. Arch Gen. Psychiatry, 19, 129±34.
Mandal, M. K and Bhattacharya, B. B. (1985). Recognition of facial aVect in depression. Percept. Motor Skills, 61, 13±14.
Mayberg, H. S. (1997). Limbic-cortical dysregulation: a proposed model of depression. J.Neuropsychiatr. Clin. Neurosci., 9, 471±81.
Mayberg, H. S., Lewis, P. J., Regenold, W. and Wagner, H. N. Jr (1994).

222 R. A. Kowatch, P. A. Davanzo and G. J. Emslie
Paralimbic hypertension in unipolar depression. J. Nucl. Med., 35, 929±34.
McCauley, E., Myers, K., Mitchell, J., Calderon, R., Schloredt, K. and Treder, R. (1993). Depression in young people: initial presentation and clinical course. J. Am. Acad. Child Adolesc. Psychiatry, 32, 714±22.
McCracken, J. T., Rubin, R. T. and Poland, R. E. (1988). Neuroendocrine aspects of primary endogenous depression. VI: Receiver operating characteristic analysis of the cortisol suppression index versus the dexamethasone suppression test in patients and matched controls. Psychiatry Res., 26, 69±78.
McGlashen, T. H. (1988). Adolescent versus adult onset of mania.
Am. J. Psychiatry, 145, 221±3.
Mendelson, W. B. Sitaram, N., Wyatt, R. J., Gillin, J. C. and Jacobs, L. S. (1978). Methoscopolamine inhibition of sleep related growth hormone secretion. Evidence for a cholinergic secretory mechanism. J. Clin. Invest., 61, 1683±90.
Mesulam, M. (1985). Principles of Behavioral Neurology.
Philadelphia, PA: Davis.
Mesulam, M. M. (1990). Large-scale neurocognitive networks and distributed processing for attention, language, and memory.
Ann. Neurol., 28, 597±613.
Moore, C. M., Christensen, J. D., Lafer, B., Fava, M. and Renshaw, P. F. (1997). Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am. J. Psychiatry, 154, 116±18.
Naylor, M. W., Greden, J. F. and Alessi, N. E. (1990). Plasma dexamethasone levels in children given the dexamethasone suppression test. Biol. Psychiatry, 27, 592±600.
Pardo, J. V., Pardo, P. J. and Raichle, M. E. (1993). Neural correlates of self-induced dysphoria. Am. J. Psychiatry, 150, 713±19.
Persad, S. M. and Polivy, J. (1993). DiVerences between depressed and nondepressed individuals in the recognition of and response to facial emotional cues. J. Abnorm. Psychol., 102, 358±68.
Philpot, M. P., Banerjee, S., Needham-Bennett, H., Costa, D. C. and Ell, P. J. (1993). 99mTc-HMPAO single photon emission tomography in late life depression: a pilot study of regional cerebral blood Xow at rest and during a verbal Xuency task. J. AVect. Disord., 28, 233±40.
Poznanski, E. O., Carroll, V. J., Benegas, M. E., Cook, S. C. and Grossman, J. A. (1982). The dexamethasone suppression test in prepubertal depressed children. Am. J. Psychiatry, 139, 321±4.
Prange, A. J. Jr, Wilson, I. C., Lynn, C. W., Alltop, L. B. and Stikeleather, R. A. (1974). l-Tryptophan in mania: contribution to a permissive hypothesis of aVective disorders. Arch. Gen. Psychiatry, 30, 56±62.
Puig-Antich, J., Dahl, R., Ryan, N. et al. (1989). Cortisol secretion in prepubertal children with major depressive disorder. Arch. Gen. Psychiatry, 46, 801±9.
Rao, U., Ryan, N. D., Birmaher, B. et al. (1995). Unipolar depression in adolescents: clinical outcome in adulthood. J. Am. Acad. Child Adolesc. Psychiatry, 34, 566±78.
Reiman, E. M., Lane, R. D., Ahern, G. L. et al. (1997). Neuroanatomical correlates of externally and internally generated human emotion. Am. J. Psychiatry, 154, 918±25.
Renshaw, P. F., Lafer, B., Babb, S. M. et al. (1997). Basal ganglia
choline levels in depression and response to Xuoxetine treatment: an in vivo proton magnetic resonance spectroscopy study.
Biol. Psychiatry, 41, 837±43.
Rie, H. E. (1966). Depression in childhood. A survey of some pertinent contributions. J. Am. Acad. Child Psychiatry, 5, 653±85.
Robbins, D. R., Alessi, N. E., Yanchyshyn, G. W. and Colfer, M. (1982). Preliminary report on the dexamethasone suppression test in adolescents. Am. J. Psychiatry, 139, 942±3.
Rogeness, G. A., Javors, M. A. and Pliszka, S. R. (1992). Neurochemistry and child and adolescent psychiatry. J. Am. Acad. Child Adolesc. Psychiatry, 31, 765±81.
Rubinow, D. R. and Post, R. M. (1992). Impaired recognition of aVect in facial expression in depressed patients. Biol. Psychiatry,
31, 947±53.
Rush, A., Stewart, R., Garver, D. and Waller, D. (1998). Neurobiological bases for psychiatric disorders. In
Comprehensive Neurology, eds. R. Rosenberg and D. Pleasure, pp. 887±919. New York: Wiley.
Ryan, N. D., Birmaher, B., Perel, J. M. et al. (1992). Neuroendocrine response to l-5-hydroxytryptophan challenge in prepubertal major depression. Depressed vs. normal children. Arch. Gen. Psychiatry, 49, 843±51.
Ryan, N. D., Dahl, R. E., Birmaher, B. et al. (1994). Stimulatory tests of growth hormone secretion in prepubertal major depression: depressed versus normal children. J. Am. Acad. Child Adolesc. Psychiatry, 33, 824±33.
Sackeim, H. A., Prohovnik, I., Moeller, J. R. et al. (1990). Regional cerebral blood Xow in mood disorders. I. Comparison of major depressives and normal controls at rest. Arch. Gen. Psychiatry,
47, 60±70.
Schatzberg, A. F., Orsulak, P. J., Rosenbaum, A. J. et al. (1982). Toward a biochemical classiWcation of depressive disorders, V: heterogeneity of unipolar depression. Am. J. Psychiatry, 139, 471±5.
Schlaepfer, T. E., Pearlson, G. D., Wong, D. F., Marenco, S. and Dannals, R. F. (1997). PET study of competition between intravenous cocaine and [11C] raclopride at dopamine receptors in human subjects. Am. J. Psychiatry, 154, 1209±13.
Schneider, F., Gur, R. C., Jaggi, J. L. and Gur, R. E. (1994). DiVerential eVects of mood on cortical cerebral blood Xow: a 133-xenon clearance study. Psychiatry Res., 52, 215±36.
Schneider, F., Gur, R. E., Alavi, A. et al. (1996). Cerebral blood Xow changes in limbic regions induced by unsolvable anagram tasks.
Am. J. Psychiatry, 153, 206±12.
Schneider, F., Grodd, W., Weiss, U. et al. (1997). Functional MRI reveals left amygdala activation during emotion. Psychiatry Res.,
76, 75±82.
Sergent, J., Ohta, S. and Macdonald, B. (1992). Functional neuroanatomy of face and object processing: a positron emission tomography study. Brain, 115, 15±36.
ShaVer, D., Fisher, P., Dulcan, M. K. et al. (1996). The NIMH diagnostic interview schedule for children version 2.3 (DISC-2.3): description, acceptability, prevalence rates, and performance in the MECA study. Methods for the epidemiology of child and adolescent mental disorders study. J. Am. Acad. Child Adolesc. Psychiatry, 35, 865±77.
Pediatric mood disorders and neuroimaging |
223 |
|
|
Sharma, R., Venkatasubramanian, P. N., Barany, M. and Davis, J. M. (1992). Proton magnetic resonance spectroscopy of the brain in schizophrenic and aVective patients. Schizophr. Res., 8, 43±9.
Soares, J. C. and Mann, J. J. (1997a). The anatomy of mood disorders ± review of structural neuroimaging studies. Biol. Psychiatry, 41, 86±106.
Soares, J. C. and Mann, J. J. (1997b). The functional neuroanatomy of mood disorders. J. Psychiatr. Res., 31, 393±432.
Steingard, R. J., Renshaw, P. F., Yurgelun-Todd, D. et al. (1996). Structural abnormalities in brain magnetic resonance images of depressed children. J. Am. Acad. Child Adolesc. Psychiatry, 35, 307±11.
Steingard, R., Renshaw, P., Yurgelun-Todd, D. and Wald, L. (1998). Proton MRS studies of the orbitofrontal cortex of depressed adolescents. Biol. Psychiatry, 43, 28S.
Stoll, A. L., Renshaw, P. F., Sachs, G. S. et al. (1992). The human brain resonance of choline-containing compounds is similar in patients receiving lithium treatment and controls: an in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatry,
32, 944±9.
Strober, M., Lampert, C., Schmidt, S. and Morrell, W. (1993). The course of major depressive disorder in adolescents. I. Recovery
and risk of manic switching in a follow-up of psychotic and nonpsychotic subtypes. J. Am. Acad. Child Adolesc. Psychiatry, 32, 34±42.
Sweeney, J. A., Wetzler, S., Stokes, P. and Kocsis, J. (1989). Cognitive functioning in depression. J. Clin. Psychol., 45, 836±42.
Swerdlow, N. and Koob, G. (1987). Dopamine, schizophrenia, mania, and depression: toward a uniWed hypothesis of cortico- striato-pallido-thalamic function. Behav. Brain Sci., 10, 197±245.
Tucker, D. (1988). Neuropsychological Mechanisms of AVective Selfregulation, pp. 1±22.Washington, DC: American Psychiatric Press. van Praag, H. M. (1977). Depression and Schizophrenia: A Contribution on Their Chemical Pathologies. NewYork: Spectrum. Willner, P. (1985). Depression: A Psychobiological Synthesis. New
York: Wiley.
Wozniak, J., Biederman, J., Kiely, K. et al. (1995). Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J. Am. Acad. Child Adolesc. Psychiatry, 34, 867±76.
Zubenko, G. S. Moossy, J. and Kopp, U. (1990). Neurochemical correlates of major depression in primary dementia. Arch. Neurol.,
47, 209±14.
