
Книги по МРТ КТ на английском языке / Functional Neuroimaging in Child Psychiatry Ernst 1 ed 2000
.pdf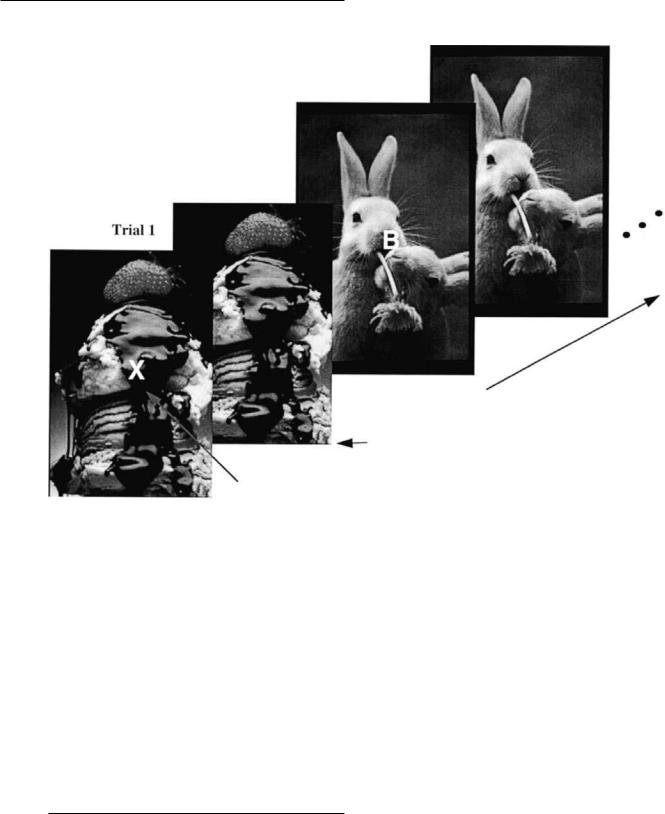
164 B. J. Casey et al.
Trial 2
|
|
|
|
|
|
· |
|
|
|
|
|
· |
|
||
Trial 1 |
|||||||
· |
|
||||||
|
|
|
|
||||
|
|
|
|
|
|||
|
Time
2500 ms interstimulus interval
500 ms stimulus duration
Fig. 9.6. Depiction of stimulus parameters for the emotional n-back task.
negative information and a 1:3 ratio in right-to-left prefrontal activity during the presentation of the negative backgrounds. However, the next three subjects scanned, either activated the hemispheres equally or showed the reverse pattern. Therefore, while the representation of emotional information is in part served by the prefrontal cortex in both children and adults, the laterality of this representation is less straightforward. Given this variability in pattern of activation across subjects, we have since moved to the presentation of face stimuli rather than pictures from the International AVective Picture System. Face stimuli allow for the control of picture complexity, size, familiarity, etc. across all conditions (e.g., positive, negative, and neutral).
Conclusions
In this chapter, a variety of paradigms have been described for use with developmental populations. These tasks, while
limited primarily to the domain of prefrontal functioning, are quite broad with respect to the type of information processed (e.g., verbal, spatial, response, emotional). Across all four empirical studies described, similar locations of prefrontal activity were observed for school-aged children and adults. However, diVerences were observed in the magnitude of the patterns of activity, both in volume (Casey et al., 1997 a,b,c) and in percentage change (Cohen et al., 1994; Casey et al., 1995). These diVerences may be attributed to overall task diYculty. Even when attempts were made to titrate task diYculty across ages, young children performed less well than the adults. Many of these diVerences may be associated with diVerences in how quickly subjects mastered the tasks. Adults became more proWcient in task performance (e.g., the spatial working memory task) as a function of time on-task in the scanner. The children, by comparison, did not increase their performance with time on task in the scanner. Some of the diYculty for children performing tasks in the scanner may
Cognitive and behavioral probes of developmental landmarks |
165 |
|
|
|
|
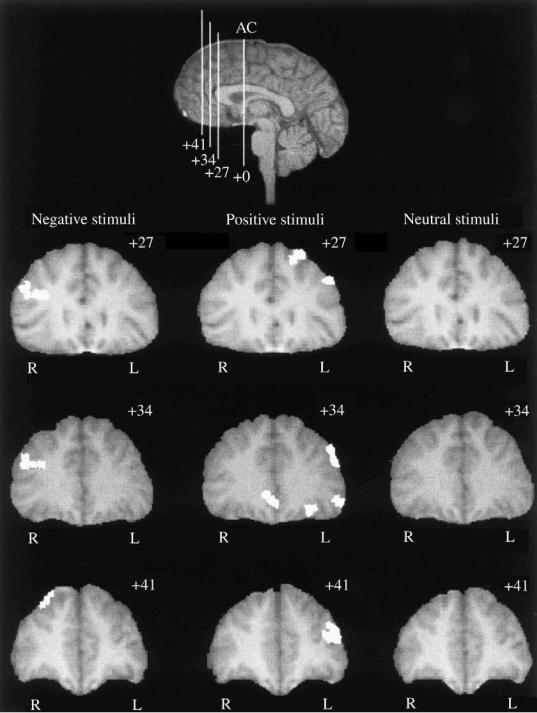
Fig. 9.7. Prefrontal cortical activation during the presentation of negative, positive, and neutral stimuli for coronal images located at 1 27,
1 34, and 1 41mm anterior to the anterior commissure (AC) averaged across two young adults.

166B. J. Casey et al.
be the need to lie down, thus blocking a view of their hands. Their motor skills are not as sophisticated and so even with extended practice the children still beneWt from visual feedback on where their hands and Wngers are with respect to the response-recording device. Alternatively, children and adults may diVer in the speed of acquiring new skills. These issues and others raised in the current chapter are just a few to be addressed as we continue to use functional neuroimaging with children. It is clear that innovative methods like fMRI will transform our current understanding of human brain development and hold signiWcant implications for the study of developmental disorders.
iReferencesi
Baddeley, A. (1986). Modularity, mass-action, and memory. Q. J. Exp. Psychol., 38, 527±33.
Baird, A. A., Gruber, S. A., Fein, D. A. et al. (1999). Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry, 38, 195±9.
Bench, C. J., Frith, C. D., Grasby, P. M. et al. (1993). Investigations of the functional anatomy of attention using the Stroop test.
Neuropsychologia, 31, 907±22.
Benson, R. R., Logan, W. J., Cosgrove, G. R. et al. (1996). Functional MRI localization of language in a 9-year-old child. Can. J. Neurol. Sci., 23, 213±19.
Bonello, C. M., Baird, A. A., Renshaw, P. F., Yurgelun-Todd, D. A. (1998). Structural and functional magnetic resonance imaging in the temporal lobes of children during word production.
Neuroimage, 7, S3.
Booth, J. R., MacWhinney, B.,Thulborn, K. R. et al. (1999). Functional reorganization of activation patterns in children with brain lesions: whole brain fMRI imaging during three diVerent cognitive tasks. Progr. Neuropsychopharmacol. Biol. Psychiatry, 23, 669±82.
Born, P., Rostrup, E., Leth, H., Peitersen, B., Lou, H. C. (1996). Change of visually induced cortical activation patterns during development. Lancet, 347, 543.
Born, P., Rostrup, E., Larsson, H. B. W., Leth, H., Miranda, M., Peitersen, B., Lou, H. C. (1997). Infant visual cortex function evaluated by fMRI. Neuroimage, 5, S171.
Braver, T. S., Cohen, J. D., Nystrom, L. E., Jonides, J., Smith, E. E., Noll, D. C. (1997). A parametric study of prefrontal cortex involvement in human working memory. Neuroimage, 5, 49±62.
Buckner, R. L., Bandettini, P. A., O'Craven, K. M. et al. (1996). Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging.
Proc. Natl. Acad. Sci. USA, 93, 14878±83.
Case, R. (1972). Validation of a neo-Piagetian capacity construct. J. Exp. Child. Psychol., 14, 287±302.
Casey, B. J. and Cohen, J. D. (1996). In reply to: C. T. Morton, Is research in normal and ill children involving radiation exposure ethical? Arch. Gen. Psychiatry, 53, 1059±60.
Casey, B. J., Cohen, J. D., Jezzard P. et al. (1995). Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage, 2, 221±9.
Casey, B. J., Trainor, R., Orendi, J., Schubert, A. (1996). A functional magnetic resonance imaging (fMRI) study of ventral prefrontal cortex mediation of response inhibition. Proc. Soc. Neurosci., 22, 1107.
Casey, B. J., Cohen, J. D., King, S. W. et al. (1997a). A developmental functional MRI study of cortical activation during a spatial working memory task. Neuroimage, 23, S69.
Casey, B. J., Trainor R. J., Orendi, J. L., et al. (1997b). A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J. Cogn. Neurosci., 9, 835±47.
Casey, B. J., Badgaiyan, R. D., Franzen, P. L. et al. (1997c). Prefrontal activation as a function of response set. Neuroimage, 5, S602.
Casey, B. J., Thomas, K. M.,Welsh, T. F. et al. (1998a). A developmental fMRI study of prefrontal organization. Neuroimage, 7, S512.
Casey, B. J., Thomas K. M., Welsh, T. F., Eccard, C. H., Livnat, R. and Pierri, J. N. (1998b). An fMRI study of response inhibition in children with striatal lesions. Neuroimage, 7, S515.
Casey, B. J., Cohen, J. D., Davidson, R. et al. (1998c). Reproducibility of fMRI results across four institutions using a working memory task. Neuroimage, 8, 249±61.
Chugani H. T., Phelps, M. E. and Mazziotta, J. C. (1987). Positron emission tomography study of human brain functional development. Ann. Neurol., 22, 487±97.
Cohen, J. D. and Servan-Schreiber, D. (1992). Context, cortex and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol. Rev., 99, 47.
Cohen, J. D., Forman S. D., Casey, B. J. and Noll, D. C. (1993). Spiralscan imaging of dorsolateral prefrontal cortex during a working memory task. In Proceedings of the 12th Annual Meeting of the Society of Magnetic Resonance in Medicine, p. 1413.
Cohen, J. D., Forman, S. D., Braver, T. S., Casey, B. J., ServanSchreiber, D. and Noll, D. C. (1994). Activation of prefrontal cortex in a nonspatial working memory task with functional MRI. Hum. Brain Map., 1, 293±304.
Cohen, J. D., Perlstein, W. M., Braver, T. S. et al. (1997). Temporal dynamics of brain activation during a working memory task.
Nature, 386, 604±7.
Courtney, S. M., Ungerleider, L. G., Keil, K. and Haxby, J. V. (1997). Transient and sustained activity in a distributed neural system for human working memory. Nature, 386, 608±11.
Dale, A. M. and Buckner, R. L. (1997). Selective arranging of rapidly presented individual trials using fMRI. Hum. Brain Map., 5, 329±40.
Dapretto, M., Bookheimer, S. Y., Cohen, M. S. and Wang, J. (1996). fMRI of language in dyslexic and normally developing children.
Neuroimage, 3, S434.
Davidson, R. J. (1994). Asymmetric brain function, aVective style, and psychopathology: the role of early experience and plasticity.
Dev. Psychopathol., 6, 741±58.
D'Esposito, M., Zarahn, E., Aguirre, G. K., Shin, R. K., Auerbach, P. and Detre, J. A. (1997). The eVect of pacing of experimental stimuli on observed functional MRI activity. Neuroimage, 6, 113±21.
Cognitive and behavioral probes of developmental landmarks |
167 |
|
|
|
|
Donders, F. C. (1969). On the speed of mental processes. In W. G. Koster (Original work published in 1868; reproduced in Attention and performance II (ed. and trans. W. G. Koster). Acta Psychol., 30, 412±31.
Flavell, J. H., Beach, D. R. and Chinsky, J. M. (1966). Spontaneous verbal rehearsal in a memory task as a function of age. Child Dev., 37, 283±99.
Frost, J. A., Binder, J. R., Newby, R. F. et al. (1997). Phonological processing in developmental dyslexia: an fMRI study. Neuroimage,
5, S568.
Fuster, J. M. (1989). The Prefrontal Cortex: Anatomy, Physiology and Neuropsychology of the Frontal Lobe. New York: Raven Press.
George, M. S., Ketter, T. A., Parekh, P. I. et al. (1994). Regional brain activity when selecting a response despite interference. Hum. Brain Map., 1, 194±209.
Goldman-Rakic, P. S. (1987). Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In
Handbook of Physiology. The Nervous System. Higher Functions of the Brain. Sect. 1, Vol. V, Part 1. eds. V. B. Mountcastle, F. Plum and S. R. Geiger, pp. 373±417. Bethesda, MD: American Physiological Society.
Graveline, C., Mikulis, D. J., Crawley, A. P. and Hwang, P. A. (1998). Regionalization evidence of sensorimotor plasticity pre and post hemispherectomy in children with epilepsy. fMRI and clinical studies. Neuroimage, 7, S483.
Hertz-Pannier, L., Gaillard, W. D., Mott, S. et al. (1995). Functional MRI of language tasks: frontal diVuse activation patterns in children. Hum. Brain Map., 1(Suppl.), 231.
Hertz-Pannier, L., Gaillard, W. D., Mott, S. H. et al. (1997). Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study.
Neurology, 48, 1003±12.
Hirsch, J., Kim, K. H. S., Souweidane, M. M. et al. (1997). fMRI reveals a developing language system in a 15-month old sedated infant. Soc. Neurosci. Abst., 23, 2227.
Hirsch, J., Kim, K. H. S., Souweidane, M. M. et al. (1998). Passive listening during fMRI reveals an extensive receptive language system in young and sedated children. Neuroimage, 7, S513.
Hunter, J. V., Liu, G. T., Fletcher, D. W., Brown, L. W. and Haselgrove, J. C. (1998). Functional MRI in children with congenital structural abnormalities of the visual cortex. Neuroimage, 7, S312.
Huttenlocher, P. R. (1990). Morphometric study of human cerebral cortex development. Neuropsychologia, 28, 517±27.
Huttenlocher, P. R. (1997). Regional diVerences in synaptogenesis in human cerebral cortex. J. Comp. Neurol., 387, 167±78.
Joeri, P., Loenneker, T., Huisman, D., Ekatodramis, D., Rumpel, H. and Martin, E. (1996a). fMRI of the visual cortex in infants and children. Neuroimage, 3, S279.
Joeri, P., Huisman, T., Loenneker, T., Ekatodramis, D., Rumpel, H. and Martin, E. (1996b). Reproducibility of fMRI and eVects of pentobarbital sedation of cortical activation during visual stimulation. Neuroimage, 3, S280.
Kato, T., Ohyu, J., Fukumizu, M. and Takashima, S. (1998). Assessment of language lateralization using functional nearinfrared spectroscopy in bedside. Neuroimage, 7, S207.
Keating, D. P. and Bobbitt, B. L. (1978). Individual and developmen-
tal diVerences in cognitive processing components of mental ability. Child Dev., 49, 155±67.
Kiriakopoulos, E. T., Wood, M. L. and Mikulis, D. J. (1996). fMRI in pediatric neurosurgical patients. Neuroimage, 3, S490.
Konishi, S., Nakajima, K., Uchida, I., Sekihara, K. and Miyashita, Y. (1997). Temporally resolved no-go dominant brain activity in the prefrontal cortex revealed by functional magnetic resonance imaging. Neuroimage, 5, S120.
Lang, P. J., Öhman, A. and Vaitl, D. (1988). The International AVective Picture System. [photographic slides] Gainesville, FL: The Center of Research in Psychophysiology, University of Florida.
Logan, W. J. (1998). Functional MRI language localization in children. In Abstracts of the Society of Cognitive Neuroscience, p. 127.
McManis, M. H., Bradley, M. M., Cuthbert, B. N. and Lang, P. J. (1995). Kids have feelings too: children's physiological responses to aVective pictures. Psychophysiology, 32, S53.
Morton, C. T. (1996). Is research in normal and ill children involving radiation exposure ethical? [Letter to the Editor] Arch. Gen. Psychiatry, 53, 1059.
Moses, P., Martinez, A., Roe, K. et al. (1997). Functional MR imaging of children's spatial analysis of hierarchical forms. Neuroimage,
5, S97.
Orendi, J. L., Irwin, W., Ward, R. T. et al. (1997). A fMRI study of cortical activity in children and adults during a spatial working memory task. Neuroimage, 5, S603.
Pascual-Leone, J. A. (1970). A mathematical model for transition in Piaget's developmental stages. Acta Psychol., 32, 301±45.
Popp, C. A., Trudeau, J. D., Durden, D. et al. (1996). Functional MR imaging in children. Neuroimage, 3, S594.
Pugh, K. R., Shaywitz, B. A., Shaywitz, S. E., et al. (1998). The relation between cortical activation proWles and regularity eVects in reading: replications and extensions. Neuroimage, 7, S216.
Rosen, B. R., Buckner, R. I. and Dale, A. M. (1998). Event-related functional MRI: past, present, and future. Proc. Natl. Acad. Sci. USA, 95, 773±80.
Rubia, K., Overmeyer, S., Taylor, E. et al. (1998). Mesial hypofrontality in attention deWcit hyperactivity disorder (ADHD) during motor timing: a study using fMRI. Neuroimage, 7, S114.
Sanders, J. A. and Orrison, W. W. (1993). ANOVA tests for identiWcation of FMRI activation. In Proceedings of the 12th Annual Meeting of the Society of Magnetic Resonance in Medicine, New York, p. 1376.
Savoy, R. L., Bandettini, P. A., O'Craven, K. M. et al. (1995). In
Proceedings of the 3rd ScientiWc Meeting of the Society for Magnetic Resonance, vol. 2, p. 45.
Sergent, J., Zuck, E., Levesque, M. and MacDonald, B. (1992). Positron emission tomography study of letter and object processing: empirical Wndings and methodological considerations.
Cerebr. Cortex, 80, 68±80.
Smith, E. E., Jonides, J. and Koeppe, R. A. (1996). Dissociating verbal and spatial working memory using PET. Cerebr. Cortex, 6, 11±20.
Swedo, S. E., Pietrini, P., Leonard, H. L. et al. (1989). Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch. Gen. Psychiatry, 49, 690±4.

168 B. J. Casey et al.
Thomas, K. M. and Casey, B. J. (1999). Functional MRI in pediatrics. In Medical Radiology: Functional Magnetic Resonance Imaging, eds. P. Bandettini and C. Moonen. New York: Springer-Verlag, in press.
Thomas, K. M., King, S. W., Franzen, P. L. et al. (1999). A developmental fMRI study of spatial working memory. Neuroimage, 10, 327±38.
Truwit, C. L., Le, T. H., Hu, X. et al. (1996). Functional MR imaging of working memory task activation in children: preliminary
Wndings. Neuroimage, 3, S564.
Vaidya, C. J., Gabrieli, J. D. E., Rypma, B. et al. (1997). fMRI of frontal lobe function in children with attention deWcit disorder on and oV Ritalin. Soc. Neurosci. Abst., 23, 859.
Vaidya, C. J., Austin, C., Kirkorian, C. et al. (1998). Selective effects of methylphenidate in attention de®cit hyperactivity disorder: a functional magnetic resonance study. Proc. Natl. Acad. Sci. USA
95, 14494±9.
Vincent, D. J., Bryant, A. E., Worthington, W. C. et al. (1998). When can fMRI replace Wada for language localization? Two years experience. Neuroimage, 7, S147.
Woods, R. P., Cherry, S. R. and Mazziotta, J. C. (1992). Rapid automated algorithm for aligning and reslicing PET images. J. Comp. Assist. Tomogr., 16, 620±33.
Zametkin, A. J., Nordahl, T. E., Gross, M. et al. (1990). Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N. Engl. J. Med., 323, 1361±6.
Zametkin, A. J., Schwartz, D. J., Ernst, M. and Cohen, R. M. (1996). In reply to: C.T. Morton, Is Research in normal and ill children involving radiation exposure ethical? Arch. Gen. Psychiatry, 53, 1060±1.
Zarahn, E., Aguirre, G. and D'Esposito, M. (1998). A trial-based experimental design for fMRI. Neuroimage, 6, 122±38.

Part 4
Psychiatric disorders
Functional neuroimaging methods have only recently begun to be applied to the study of child psychiatric disorders. As with many other areas of psychiatric research, scientiWc hypotheses tested in studies of children frequently have their roots, in part, in knowledge obtained in research on adults. The chapters in this section critically review the application of functional neuroimaging to the study of child psychiatric disorders, drawing on the background of adult neuroimaging research and our knowledge of childhood variations of adult-onset disorders. Theories of the neurobiology of child psychiatric disorders are discussed. Practical issues arising from imaging psychiatrically ill children, methods for addressing these issues, and related interpretative problems are explored. A wide variety of child psychiatric disorders are covered, ranging from those with early (solely childhood) onsets, such as autism, to those whose onset in childhood is rare, e.g., schizophrenia.
Chugani describes the full range of imaging studies of autism, a devastating and chronic disorder with an onset prior to age 3 years (Chapter 10). Jacobsen and Bertolino discuss their unique imaging studies of the rare form of schizophrenia with onset in childhood and the implications of their Wndings for developmental theories of schizophrenia (Chapter 11). Kowatch and his colleagues address childhood unipolar and bipolar depression, as well as neuroimaging paradigms developed to study the biology of mood and its disorders (Chapter 12). Rosenberg and his colleagues assess imaging research on the full spectrum of anxiety disorders, from phobias to panic and obsessive-compulsive disorder (Chapter 13) and Peterson critically evaluates the use of neuroimaging in deWning the neural systems involved in Tourette's disorder (Chapter 14). Given the frequent comorbidity of child psychiatric disorders with speciWc developmental disorders, Wood and Flowers analyze the conceptual models underlying imaging studies of dyslexia and their relevance for the study of child psychiatric disorders (Chapter 15). Schweitzer and colleagues review the progress made in understanding the neuropathophysiology of attention-deWcit hyperactivity disorder (ADHD) by means of functional neuroimaging, as well as the neurobehavioral probes worthy of use in future research on ADHD (Chapter 16) and, Wnally, Chowdhury and colleagues address the eating disorders of anorexia nervosa and bulimia nervosa, as well as those restricted to childhood (Chapter 17).
170 |
Part 4 |
|
|
The reader will gain from this section knowledge of the latest research developments and pathophysiologic theories of speciWc child psychiatric disorders and glean from it information on the current status of brain imaging research in child psychiatry. Indeed, this comprehensive review covers practical challenges involved in scanning children, design issues arising from the heterogeneity of child psychiatry disorders, interpretative dilemmae
posed by various imaging paradigms, advances in neurobiological knowledge gained through the use of neuroimaging, and the future potential of functional neuroimaging in child psychiatry. The identiWcation of neural circuits underlying symptoms and vulnerabilities and of their speciWc disruptions in diVerent disorders emerge as a goal worthy of pursuit given its implications for diagnosis and treatment.

10
Autism
Diane C. Chugani
DeWnition of autism
Autism is a developmental disorder deWned by the presence of a triad of communication, social, and stereotypical behavioral characteristics with onset before 3 years of age. Previous estimates of the incidence of autism were 2±5 cases per 10000 individuals (for review see Wing, 1993). Recent studies show a higher incidence of autism (approximately 1 in 1000), while the three to four times predominance of the disorder in males has remained constant (Bryson, 1996). Autism was Wrst described by Kanner in 1943 in his landmark paper describing a group of children who showed language abnormalities, impairment in social interactions, and restricted interests and preoccupations. One year later, Asperger (1944) described a similar group of children. The term Asperger's syndrome is now used to describe high-functioning individuals with autistic features but relatively normal communication and cognitive skills (Gillberg, 1989; Volkmar et al., 1996).
Underlying the spectrum of autistic behaviors are undoubtedly multiple etiologies, only a small fraction of which have been so far identiWed. The reliance upon this behavioral deWnition is a consequence of a failure to identify biological markers for the majority of individuals with autistic behavior and is a source of diYculty in the design and reproducibility of functional imaging studies. The inexact nature of the diagnosis of autism and other pervasive developmental disorders is also the source of numerous practical problems for the families of autistic children in identifying appropriate medical, behavioral, and educational interventions necessary to promote optimal development of their children. In spite of the fact that there are various etiologies for autistic behavior, the possibility of a common neurochemical mechanistic feature, shared by multiple causes of autism, cannot be excluded. It is upon this premise that functional neuroimaging of groups of
autistic subjects of unknown etiology are compared with nonautistic control groups in search of common biological substrates to deWne and understand autism better.
Clinical versus research criteria for the diagnosis of autism
The accurate diagnosis of autism and careful description of associated features of the subjects are essential criteria for obtaining meaningful functional imaging data and to allow comparison of results among groups (Table 10.1). The majority of the functional imaging studies published to date have utilized clinical diagnoses of autism based upon DSM-III-R (American Psychiatric Association, 1987), DSMIV (American Psychiatric Association, 1994) or ICD-10 (World Health Organization, 1987) criteria. Various additional psychologic instruments, such as the Childhood Autism Rating Scales (CARS; Schopler et al., 1980), have also been employed. There has been a growing concensus in recent years that the Autism Diagnostic InterviewRevised (ADI-R; Lord et al., 1994) and the Autism Diagnostic Observation Scale (ADOS; Lord et al., 1989) developed by Lord and colleagues represent the gold standard for the diagnosis for autism for research purposes. These are excellent instruments with extensive validation for high-functioning autistic subjects over the age of 4 years (Lord et al., 1997). However, these instruments are overinclusive for lower-functioning individuals, who make up 75% of individuals with a clinical diagnosis of autism. The Prelinguistic Autism Diagnostic Observation Schedule (PL-DOS; DiLavore et al., 1995) was developed by the same group to address younger (less than the age of 6 years) children who have not yet developed phrase-level speech. The problem of stability of diagnosis of young autistic children (Lord and Schopler, 1989) can also be addressed by re-eval- uating the children after several years (Lord, 1995).
171

Table 10.1. Summary of functional imaging studies in autistic subjects
Study |
|
Subjects |
|
Controls |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
Number |
Age |
Number |
Age |
Diagnostic |
Testing |
|
||||
|
(sex) |
(years) |
(sex) |
(years) |
criteria |
condition |
Results |
||||
|
|
|
|
|
|
|
|
||||
PET studies with 2-[18F]¯uoro-2-deoxyglucose |
|
|
|
|
|
|
|
||||
Rumsey et al. |
10M |
18±36 |
|
15M |
20±37 |
|
DSM-III |
Rest, eyes covered |
Global hypermetabolism |
||
(1985)a |
|
|
|
|
|
|
|
|
|
|
|
Horwitz et al. |
14M |
18±39 |
|
14M |
20±37 |
|
DSM-III |
Rest, eyes covered |
Fewer positive correlations between frontal and |
||
(1988)a |
|
|
|
|
|
|
|
|
|
|
parietal cortices; lower correlations between |
|
|
|
|
|
|
|
|
|
|
|
thalamus, caudate nucleus, lenticular nucleui, and |
|
|
|
|
|
|
|
|
|
|
|
insula with frontal and parietal regions |
de Volder et al. |
11M, 7F |
2±18 |
|
Normal |
7, 14, 15 |
DSM-III |
Sedated |
Normal global and regional glucose metabolism |
|||
(1987) |
|
|
|
|
Unilateral brain |
9, 12, 12.5 |
|
|
|
||
|
|
|
|
|
pathology |
|
|
|
|
|
|
|
|
|
|
|
Adults, 15 |
Mean 22 |
|
|
|
||
Herold et al. |
16M |
21±25 |
|
6M, 2F |
22±53 |
|
DSM-III-R, |
Listening to music, |
No signiWcant diVerences between groups in cerebral |
||
(1988) |
|
|
|
|
|
|
|
|
ICD-10 |
eyes closed |
blood Xow, oxygen consumption, and glucose |
|
|
|
|
|
|
|
|
|
|
|
metabolism |
Heh et al. |
5M, 2F |
19±36 |
|
7M, 1F |
20±35 |
|
DSM-III, |
Continuous |
No signiWcant diVerence in cerebellar glucose |
||
(1989)a |
|
|
|
|
|
|
|
|
ICDS |
performance task |
metabolism |
Buchsbaum |
5M, 2F |
19±36 |
|
13M |
Mean 24 |
DSM-III, |
Continuous |
Decreased glucose metabolism in right thalamus and |
|||
et al. (1992)a |
|
|
|
|
|
|
|
|
ICDS |
performance task |
putamen; less asymmetry in autistic group |
Siegel et al. |
12M, 4F |
17±38 |
|
19M, 7F |
Mean 27 |
ICDS, |
Continuous |
Normal global glucose metabolism, decreased glucose |
|||
(1992)a |
|
|
|
|
|
|
|
|
DSM-III-R |
performance task |
metabolism in left putamen, increased metabolism in |
|
|
|
|
|
|
|
|
|
|
|
calcarine cortex, reversed asymmetry in rectal gyrus |
Siegel et al. |
12M, 3F |
17±38 |
|
13M, 7F |
19±39 |
|
ICDS, |
Continuous |
Negative correlation of medial frontal glucose |
||
(1995)a |
|
|
|
|
|
|
|
|
DSM-III-R |
performance task |
metabolism with attentional performance |
Schifter et al. |
9M, 4F |
4±11 |
|
No control group |
± |
|
DSM-III-R |
Rest, eyes open |
Regional abnormalities of glucose metabolism by |
||
(1994) |
|
|
|
|
|
|
|
|
|
|
visual assessment in 4 of 13 |
Chugani et al. |
14 |
10 months |
10 |
8 months to |
DSM-IV |
Rest |
Bitemporal glucose hypometabolism, particularly in |
||||
(1996) |
|
|
to 5 years |
|
|
5 years |
|
|
superior temporal gyrus and hippocampus |
||
Haznedar et al. |
5M, 2F |
17±47 |
|
5M, 2F |
20±47 |
|
ADI |
Verbal learning test |
Hypometabolism in right anterior cingulate gyrus |
||
(1997) |
|
|
|
|
|
|
|
|
|
|
|
SPECT blood Xow studies with 133Xe |
|
|
|
|
|
|
|
||||
Sherman et al. |
7M |
18±33 |
|
± |
± |
|
DSM-III |
Rest, eyes open |
Global hypoperfusion |
||
(1984) |
|
|
|
|
|
|
|
|
|
|
|
Zilbovicius |
12M, 9F |
5±11 |
Nonautistic with |
Mean 8.7 |
DSM-III-R |
Sedated (controls |
No global or regional Xow abnormalities in autistic |
et al. (1992) |
|
|
slight language |
|
|
not sedated) |
group compared with language disorder group |
|
|
|
disorder: 10M, 4F |
|
|
|
|
Chiron et al. |
14M, 4F |
4±17 |
5M, 5F |
4±16 |
ADI, ICD-10, |
Sedated (17 of 18); |
Autistic group showed higher blood Xow in right |
(1995) |
|
|
|
|
DSM-III-R |
controls |
hemisphere compared to left; controls showed the |
|
|
|
|
|
|
2 of 10 sedated |
reverse |
Zilbovicius |
3M, 2F |
3±4 and |
5 |
3±4 |
DSM-III-R |
Sedated |
Longitudinal study showed bilateral hypoperfusion in |
et al. (1995) |
|
later at 6±7 |
7 |
6±12 |
|
|
frontal lobes at 3±4 years, but not at 6±7 years |
SPECT blood Xow studies with 99mTC-HMPAO |
|
|
|
|
|
||
Ozbayrak et al. |
1M, 22 y |
|
|
|
Asperger's |
Rest |
Left occipital hypoperfusion |
(1991) |
|
|
|
|
|
|
|
George et al. |
4M |
22±34 |
2M, 2F |
25±32 |
DSM-III-R |
Rest, eyes open |
Global hypoperfusion; focally decreased ¯ow in right |
(1992) |
|
|
|
|
|
|
lateral temporal and bilateral frontal lobes |
McKelvey et al. |
2M, 1F |
14±17 |
|
|
Asperger's |
Rest, eyes open |
Right hypoperfusion (diVuse in 1, temporal in 1, |
(1995) |
|
|
|
|
DSM-III-R, |
|
frontal and occciptal in 1); hypoperfusion of vermis |
|
|
|
|
|
(Wing, 1981; |
|
and right cerebellum in 2 |
|
|
|
|
|
Gillberg, 1989) |
|
|
Mountz et al. |
5M, 1F |
9±21 |
5M, 2F |
6±20 |
DSM-III-R, |
Rest, eyes open |
Bilateral temporal and parietal hypoperfusion, with |
(1995) |
|
|
|
|
ASIEP, ABC |
|
left hemisphere showing greater regional cerebral |
|
|
|
|
|
|
|
blood Xow abnormalities than right |
Functional mapping with H |
15O PET |
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
Happé et al. |
5M |
20±27 |
6M |
24±65 |
Clinical diagnosis |
ªTheory of mindº |
Activation in Brodmann area 9 in autistic group and |
(1996) |
|
|
|
|
of Asperger's |
task |
activation in area 8 in control group |
Müller et al. |
4M, 1F |
18±31 |
5M |
23±30 |
DSM-IV, GARS |
Language and |
Reversed hemispheric dominance during verbal |
(1999)a |
|
|
|
|
|
auditory tasks |
auditory stimulation; reduced cerebellar activation |
|
|
|
|
|
|
|
during nonverbal auditory perception |
Müller et al. |
4M |
18±31 |
5M |
23±30 |
DSM-IV, GARS |
Language and |
Reduced activation in right dentate nucleus and left |
(1998)a |
|
|
|
|
|
auditory tasks |
frontal area 46 and thalamus during expressive |
|
|
|
|
|
|
|
language task |
Neurotransmitter function measured with PET |
|
|
|
|
|||
Ernst et al. |
8M, 6F |
mean 13 |
7M, 3F |
Mean 14 |
DSM-III-R |
Sedated |
[18F]DOPA uptake reduced in medial prefrontal cortex |
(1997) |
|
|
|
|
|
|
|
Chugani et al. |
7M, 1F |
4±11 |
4M, 1F |
8±14 |
DSM IV, CARS, |
Sedated |
Asymmetric '-[11C]-methyltryptophan uptake in |
(1997) |
|
|
|
|
GARS |
|
frontal cortex, thalamus and dentate nucleus of |
|
|
|
|
|
|
|
cerebellum |
