Книги по МРТ КТ на английском языке / MR Imaging in White Matter Diseases of the Brain and Spinal Cord - K Sartor Massimo Filippi Nicola De Stefano Vincent Dou
.pdf
232 |
M. Filippi and M. A. Rocca |
Davie et al. 1994; De Stefano et al. 1995a). Recovery of NAA may be related to resolution of edema, increases in the diameter of previously shrunk axons secondary to remyelination and clearance of inflammatory factors and reversible metabolic changes in neurons.
2.Chronic MS lesions are characterized by markedly reduced NAA/Cr peaks. These changes are more pronounced in severely hypointense MS lesions than in isoor mildly hypointense lesions (van Walderveen et al. 1999) and in chronic lesions from patients with SPMS than in those from patients with benign MS (Falini et al. 1998).
3.Cho increase, probably reflecting an altered myelin chemistry or the presence of inflammation, and a decrease in NAA have been also shown in prelesional NAWM (Narayana et al.1998;Sarchielli et al. 1999; Tartaglia et al. 2002).
of the fact that T2-visible lesions represent just a small component of overall brain damage and calls for an accurate assessment of NABT pathology for a better understanding of MS pathophysiology.
3.Metabolite abnormalities, including decrease of NAA and Cho and increase of mI, have also been shown in the cortical GM of MS patients (Kapeller et al. 2001; Sarchielli et al. 2002; Sharma et al. 2001; Chard et al. 2002), since the early phases of the disease (Chard et al. 2002), but not in CIS patients (Kapeller et al. 2002). These changes are more pronounced in patients with SPMS than in those with RRMS (Adalsteinsson et al. 2003). More recently, NAA reduction has also been demonstrated in the thalamus of SPMS (Cifelli et al. 2002) and RRMS patients (Wylezinska et al. 2003).
|
15.4.3 |
15.4.2 |
Correlations with Clinical Manifestations |
1H-MRS Findings in NABT |
and Disability |
1H-MRS also has the potential to provide information on MS pathological changes outside T2-visible lesions. The following are the main findings obtained from the study of the NABT in MS patients using 1H-MRS:
1.Studies of limited and/or selected portions of the brain (Fu et al. 1998; Sarchielli et al. 1999; De Stefano et al. 2002) have shown that NAA reduction is not restricted to MS lesions, but also occurs in the NAWM. These changes are more severe in SPMS and PPMS patients than in those with RRMS (Fu et al. 1998; Suhy et al. 2000); however, they can be detected even in patients with no overt clinical disability (De Stefano et al. 2002) and in those in the early phase of the disease (De Stefano et al. 2001). Diffusely elevated Cho and Cr concentrations have also been described in the NAWM of RRMS (Inglese et al. 2003b) and PPMS (Suhy et al. 2000) patients.
2.The recent development of an unlocalized 1H-MRS sequence for measuring NAA levels in the whole brain (WBNAA) (Gonen et al. 1998) has shown the presence of marked axonal pathology in clinically definite MS (Gonen et al. 2000; Bonneville et al. 2002) and in patients at the earliest clinical phases of MS (Filippi et al. 2003). Interestingly, no correlation has been found between WBNAA concentrations and T2-weighted lesion volumes in patients with RRMS (Bonneville et al. 2002) and in those at presentation with CIS (Filippi et al. 2003a). The lack of such a correlation is likely to be the result
As already mentioned, axonal loss and/or dysfunction is likely to have a major role in the development of fixed clinical disability in MS. In agreement with this notion, a post-mortem study (Bjartmar et al. 2000) has shown a strong correlation between neurological impairment and both axonal density and NAA reduction in the spinal cord of patients with MS. The following are the main in vivo findings obtained from the application of 1H-MRS to the understanding of MS-related disability:
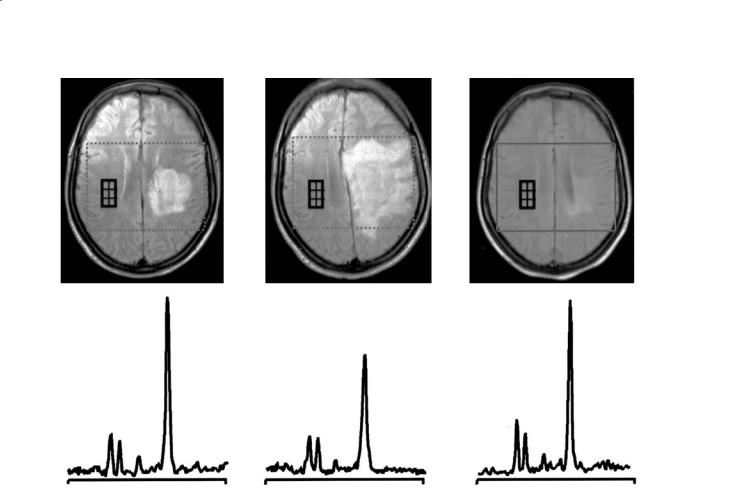
1.Following an acute MS relapse,reversible decreases in NAA have been observed not only in macroscopic lesions responsible for the clinical symptomatology (De Stefano et al. 1995b; Reddy et al. 2000a), but also in the NAWM of the hemisphere contralateral to acute lesions (De Stefano et al. 1999) (Fig. 15.5) and have been correlated with reversal of functional impairment.
2.In patients with RRMS, a longitudinal decrease over time of NAA/Cr in the NAWM correlates strongly with EDSS worsening (De Stefano et al. 1998; Fu et al. 1998), suggesting that progressive axonal damage or loss may be responsible for functional impairment in MS. More recently, it has been demonstrated that brain axonal damage begins in the early stages of MS, develops more rapidly in the earlier clinical stages of the disease and correlates more strongly with disability in patients with mild than in those with more severe disease (De Stefano et al. 2001).