
- •Preface
- •List of contributers
- •History, epidemiology, prevention and education
- •A history of burn care
- •“Black sheep in surgical wards”
- •Toxaemia, plasmarrhea, or infection?
- •The Guinea Pig Club
- •Burns and sulfa drugs at Pearl Harbor
- •Burn center concept
- •Shock and resuscitation
- •Wound care and infection
- •Burn surgery
- •Inhalation injury and pulmonary care
- •Nutrition and the “Universal Trauma Model”
- •Rehabilitation
- •Conclusions
- •References
- •Epidemiology and prevention of burns throughout the world
- •Introduction
- •Epidemiology
- •The inequitable distribution of burns
- •Cost by age
- •Cost by mechanism
- •Limitations of data
- •Risk factors
- •Socioeconomic factors
- •Race and ethnicity
- •Age-related factors: children
- •Age-related factors: the elderly
- •Regional factors
- •Gender-related factors
- •Intent
- •Comorbidity
- •Agents
- •Non-electric domestic appliances
- •War, mass casualties, and terrorism
- •Interventions
- •Smoke detectors
- •Residential sprinklers
- •Hot water temperature regulation
- •Lamps and stoves
- •Fireworks legislation
- •Fire-safe cigarettes
- •Children’s sleepwear
- •Acid assaults
- •Burn care systems
- •Role of the World Health Organization
- •Conclusions and recommendations
- •Surveillance
- •Smoke alarms
- •Gender inequality
- •Community surveys
- •Acknowledgements
- •References
- •Prevention of burn injuries
- •Introduction
- •Burns prevalence and relevance
- •Burn injury risk factors
- •WHERE?
- •Burn prevention types
- •Burn prevention: The basics to design a plan
- •Flame burns
- •Prevention of scald burns
- •Conclusions
- •References
- •Burns associated with wars and disasters
- •Introduction
- •Wartime burns
- •Epidemiology of burns sustained during combat operations
- •Fluid resuscitation and initial burn care in theater
- •Evacuation of thermally-injured combat casualties
- •Care of host-nation burn patients
- •Disaster-related burns
- •Epidemiology
- •Treatment of disaster-related burns
- •The American Burn Association (ABA) disaster management plan
- •Summary
- •References
- •Education in burns
- •Introduction
- •Surgical education
- •Background
- •Simulation
- •Education in the internet era
- •Rotations as courses
- •Mentorship
- •Peer mentorship
- •Hierarchical mentorship
- •What is a mentor
- •Implementation
- •Interprofessional education
- •What is interprofessional education
- •Approaches to interprofessional education
- •References
- •European practice guidelines for burn care: Minimum level of burn care provision in Europe
- •Foreword
- •Background
- •Introduction
- •Burn injury and burn care in general
- •Conclusion
- •References
- •Pre-hospital and initial management of burns
- •Introduction
- •Modern care
- •Early management
- •At the accident
- •At a local hospital – stabilization prior to transport to the Burn Center
- •Transportation
- •References
- •Medical documentation of burn injuries
- •Introduction
- •Medical documentation of burn injuries
- •Contents of an up-to-date burns registry
- •Shortcomings in existing documentation systems designs
- •Burn depth
- •Burn depth as a dynamic process
- •Non-clinical methods to classify burn depth
- •Burn extent
- •Basic principles of determining the burn extent
- •Methods to determine burn extent
- •Computer aided three-dimensional documentation systems
- •Methods used by BurnCase 3D
- •Creating a comparable international database
- •Results
- •Conclusion
- •Financing and accomplishment
- •References
- •Pathophysiology of burn injury
- •Introduction
- •Local changes
- •Burn depth
- •Burn size
- •Systemic changes
- •Hypovolemia and rapid edema formation
- •Altered cellular membranes and cellular edema
- •Mediators of burn injury
- •Hemodynamic consequences of acute burns
- •Hypermetabolic response to burn injury
- •Glucose metabolism
- •Myocardial dysfunction
- •Effects on the renal system
- •Effects on the gastrointestinal system
- •Effects on the immune system
- •Summary and conclusion
- •References
- •Anesthesia for patients with acute burn injuries
- •Introduction
- •Preoperative evaluation
- •Monitors
- •Pharmacology
- •Postoperative care
- •References
- •Diagnosis and management of inhalation injury
- •Introduction
- •Effects of inhaled gases
- •Carbon monoxide
- •Cyanide toxicity
- •Upper airway injury
- •Lower airway injury
- •Diagnosis
- •Resuscitation after inhalation injury
- •Other treatment issues
- •Prognosis
- •Conclusions
- •References
- •Respiratory management
- •Airway management
- •(a) Endotracheal intubation
- •(b) Elective tracheostomy
- •Chest escharotomy
- •Conventional mechanical ventilation
- •Introduction
- •Pathophysiological principles
- •Low tidal volume and limited plateau pressure approaches
- •Permissive hypercapnia
- •The open-lung approach
- •PEEP
- •Lung recruitment maneuvers
- •Unconventional mechanical ventilation strategies
- •High-frequency percussive ventilation (HFPV)
- •High-frequency oscillatory ventilation
- •Airway pressure release ventilation (APRV)
- •Ventilator associated pneumonia (VAP)
- •(a) Prevention
- •(b) Treatment
- •References
- •Organ responses and organ support
- •Introduction
- •Burn shock and resuscitation
- •Post-burn hypermetabolism
- •Individual organ systems
- •Central nervous system
- •Peripheral nervous system
- •Pulmonary
- •Cardiovascular
- •Renal
- •Gastrointestinal tract
- •Conclusion
- •References
- •Critical care of thermally injured patient
- •Introduction
- •Oxidative stress control strategies
- •Fluid and cardiovascular management beyond 24 hours
- •Other organ function/dysfunction and support
- •The nervous system
- •Respiratory system and inhalation injury
- •Renal failure and renal replacement therapy
- •Gastro-intestinal system
- •Glucose control
- •Endocrine changes
- •Stress response (Fig. 2)
- •Low T3 syndrome
- •Gonadal depression
- •Thermal regulation
- •Metabolic modulation
- •Propranolol
- •Oxandrolone
- •Recombinant human growth hormone
- •Insulin
- •Electrolyte disorders
- •Sodium
- •Chloride
- •Calcium, phosphate and magnesium
- •Calcium
- •Bone demineralization and osteoporosis
- •Micronutrients and antioxidants
- •Thrombosis prophylaxis
- •Conclusion
- •References
- •Treatment of infection in burns
- •Introduction
- •Clinical management strategies
- •Pathophysiology of the burn wound
- •Burn wound infection
- •Cellulitis
- •Impetigo
- •Catheter related infections
- •Urinary tract infection
- •Tracheobronchitis
- •Pneumonia
- •Sepsis in the burn patient
- •The microbiology of burn wound infection
- •Sources of organisms
- •Gram-positive organisms
- •Gram-negative organisms
- •Infection control
- •Pharmacological considerations in the treatment of burn infections
- •Topical antimicrobial treatment
- •Systemic antimicrobial treatment (Table 3)
- •Gram-positive bacterial infections
- •Enterococcal bacterial infections
- •Gram-negative bacterial infections
- •Treatment of yeast and fungal infections
- •The Polyenes (Amphotericin B)
- •Azole antifungals
- •Echinocandin antifungals
- •Nucleoside analog antifungal (Flucytosine)
- •Conclusion
- •References
- •Acute treatment of severely burned pediatric patients
- •Introduction
- •Initial management of the burned child
- •Fluid resuscitation
- •Sepsis
- •Inhalation injury
- •Burn wound excision
- •Burn wound coverage
- •Metabolic response and nutritional support
- •Modulation of the hormonal and endocrine response
- •Recombinant human growth hormone
- •Insulin-like growth factor
- •Oxandrolone
- •Propranolol
- •Glucose control
- •Insulin
- •Metformin
- •Novel therapeutic options
- •Long-term responses
- •Conclusion
- •References
- •Adult burn management
- •Introduction
- •Epidemiology and aetiology
- •Pathophysiology
- •Assessment of the burn wound
- •Depth of burn
- •Size of the burn
- •Initial management of the burn wound
- •First aid
- •Burn blisters
- •Escharotomy
- •General care of the adult burn patient
- •Biological/Semi biological dressings
- •Topical antimicrobials
- •Biological dressings
- •Other dressings
- •Exposure
- •Deep partial thickness wound
- •Total wound excision
- •Serial wound excision and conservative management
- •Full thickness burns
- •Excision and autografting
- •Topical antimicrobials
- •Large full thickness burns
- •Serial excision
- •Mixed depth burn
- •Donor sites
- •Techniques of wound excision
- •Blood loss
- •Antibiotics
- •Anatomical considerations
- •Skin replacement
- •Autograft
- •Allograft
- •Other skin replacements
- •Cultured skin substitutes
- •Skin graft take
- •Rehabilitation and outcome
- •Future care
- •References
- •Burns in older adults
- •Introduction
- •Burn injury epidemiology
- •Pathophysiologic changes and implications for burn therapy
- •Aging
- •Comorbidities
- •Acute management challenges
- •Fluid resuscitation
- •Burn excision
- •Pain and sedation
- •End of life decisions
- •Summary of key points and recommendations
- •References
- •Acute management of facial burns
- •Introduction
- •Anatomy and pathophysiology
- •Management
- •General approach
- •Airway management
- •Facial burn wound management
- •Initial wound care
- •Topical agents
- •Biological dressings
- •Surgical burn wound excision of the face
- •Wound closure
- •Special areas and adjacent of the face
- •Eyelids
- •Nose and ears
- •Lips
- •Scalp
- •The neck
- •Catastrophic injury
- •Post healing rehabilitation and scar management
- •Outcome and reconstruction
- •Summary
- •References
- •Hand burns
- •Introduction
- •Initial evaluation and history
- •Initial wound management
- •Escharotomy and fasciotomy
- •Surgical management: Early excision and grafting
- •Skin substitutes
- •Amputation
- •Hand therapy
- •Secondary reconstruction
- •References
- •Treatment of burns – established and novel technology
- •Introduction
- •Partial thickness burns
- •Biological membranes – amnion and others
- •Xenograft
- •Full thickness burns
- •Dermal analogs
- •Keratinocyte coverage
- •Facial transplantation
- •Tissue engineering and stem cells
- •Gene therapy and growth factors
- •Conclusion
- •References
- •Wound healing
- •History of wound care
- •Types of wounds
- •Mechanisms of wound healing
- •Hemostasis
- •Proliferation
- •Epithelialization
- •Remodeling
- •Fetal wound healing
- •Stem cells
- •Abnormal wound healing
- •Impaired wound healing
- •Hypertrophic scars and keloids
- •Chronic non-healing wounds
- •Conclusions
- •References
- •Pain management after burn trauma
- •Introduction
- •Pathophysiology of pain after burn injuries
- •Nociceptive pain
- •Neuropathic pain
- •Sympathetically Maintained Pain (SMP)
- •Pain rating and documentation
- •Pain management and analgesics
- •Pharmacokinetics in severe burns
- •Form of administration [21]
- •Non-opioids (Table 1)
- •Paracetamol
- •Metamizole
- •Non-steroidal antirheumatics (NSAID)
- •Selective cyclooxygenasis-2-inhibitors
- •Opioids (Table 2)
- •Weak opioids
- •Strong opioids
- •Other analgesics
- •Ketamine (see also intensive care unit and analgosedation)
- •Anticonvulsants (Gabapentin and Pregabalin)
- •Antidepressants with analgesic effects
- •Regional anesthesia
- •Pain management without analgesics
- •Adequate communication
- •Psychological techniques [65]
- •Transcutaneous electrical nerve stimulation (TENS)
- •Particularities of burn pain
- •Wound pain
- •Breakthrough pain
- •Intervention-induced pain
- •Necrosectomy and skin grafting
- •Dressing change of large burn wounds and removal of clamps in skin grafts
- •Dressing change in smaller burn wounds, baths and physical therapy
- •Postoperative pain
- •Mental aspects
- •Intensive care unit
- •Opioid-induced hyperalgesia and opioid tolerance
- •Hypermetabolism
- •Psychic stress factors
- •Risk of infection
- •Monitoring [92]
- •Sedation monitoring
- •Analgesia monitoring (see Fig. 2)
- •Analgosedation (Table 3)
- •Sedation
- •Analgesia
- •References
- •Nutrition support for the burn patient
- •Background
- •Case presentation
- •Patient selection: Timing and route of nutritional support
- •Determining nutritional demands
- •What is an appropriate initial nutrition plan for this patient?
- •Formulations for nutritional support
- •Monitoring nutrition support
- •Optimal monitoring of nutritional status
- •Problems and complications of nutritional support
- •Conclusion
- •References
- •HBO and burns
- •Historical development
- •Contraindications for the use of HBO
- •Conclusion
- •References
- •Nursing management of the burn-injured person
- •Introduction
- •Incidence
- •Prevention
- •Pathophysiology
- •Severity factors
- •Local damage
- •Fluid and electrolyte shifts
- •Cardiovascular, gastrointestinal and renal system manifestations
- •Types of burn injuries
- •Thermal
- •Chemical
- •Electrical
- •Smoke and inhalation injury
- •Clinical manifestations
- •Subjective symptoms
- •Possible complications
- •Clinical management
- •Non-surgical care
- •Surgical care
- •Coordination of care: Burn nursing’s unique role
- •Nursing interventions: Emergent phase
- •Nursing interventions: Acute phase
- •Nursing interventions: Rehabilitative phase
- •Ongoing care
- •Infection prevention and control
- •Rehabilitation medicine
- •Nutrition
- •Pharmacology
- •Conclusion
- •References
- •Outpatient burn care
- •Introduction
- •Epidemiology
- •Accident causes
- •Care structures
- •Indications for inpatient treatment
- •Patient age
- •Total burned body surface area (TBSA)
- •Depth of the burn
- •Pre-existing conditions
- •Accompanying injuries
- •Special injuries
- •Treatment
- •Initial treatment
- •Pain therapy
- •Local treatment
- •Course of treatment
- •Complications
- •Infections
- •Follow-up care
- •References
- •Non-thermal burns
- •Electrical injury
- •Introduction
- •Pathophysiology
- •Initial assessment and acute care
- •Wound care
- •Diagnosis
- •Low voltage injuries
- •Lightning injuries
- •Complications
- •References
- •Symptoms, diagnosis and treatment of chemical burns
- •Chemical burns
- •Decontamination
- •Affection of different organ systems
- •Respiratory tract
- •Gastrointestinal tract
- •Hematological signs
- •Nephrologic symptoms
- •Skin
- •Nitric acid
- •Sulfuric acid
- •Caustic soda
- •Phenol
- •Summary
- •References
- •Necrotizing and exfoliative diseases of the skin
- •Introduction
- •Necrotizing diseases of the skin
- •Cellulitis
- •Staphylococcal scalded skin syndrome
- •Autoimmune blistering diseases
- •Epidermolysis bullosa acquisita
- •Necrotizing fasciitis
- •Purpura fulminans
- •Exfoliative diseases of the skin
- •Stevens-Johnson syndrome
- •Toxic epidermal necrolysis
- •Conclusion
- •References
- •Frostbite
- •Mechanism
- •Risk factors
- •Causes
- •Diagnosis
- •Treatment
- •Rewarming
- •Surgery
- •Sympathectomy
- •Vasodilators
- •Escharotomy and fasciotomy
- •Prognosis
- •Research
- •References
- •Subject index

Critical care of thermally injured patient
cess during the first week and reinforces endogenous antioxidant and immune defenses [14].
Major trace element deficiencies have been shown to occur since the 1970s [14]. They are largely explained by the early exudative losses [15, 16]. Copper, which plays an essential role in collagen synthesis, wound repair, and immunity, is strongly depleted in burns [14]: this is unique among medical conditions. Early trace element supplementation is associated with improved wound healing, decreased protein catabolism, decreased pulmonary infection, and shortened ICU stay [15]. Therefore special attention should be given to all patients receiving enteral/ parenteral nutrition. Trace element and vitamin contents of enteral/standard parenteral nutrition are insufficient to cover the increased requirements after major burns. Based on balance studies, early antioxidant trace elements are delivered intravenously at CHUV for 8 to 30 days depending of burn size; the same adult solution is adapted to children per m2 [95]. The trace elements are delivered in addition to basal micronutrient requirements.
into the interstitial space. Serotonin and bradykinin will cause the persistence of this phenomenon during the first 18–24 hours. Due to the loss of plasma, a steep increase in hematocrit is observed. Edema formation follows a biphasic pattern: an early rapid phase and a slower increase during the next 12–36 hours [6]. A slow resolution of the increased permeability will start between 8–12 hours depending on the burn size. During this resolution phase the extravasated plasma proteins and resuscitation fluids will remain sequestered in extra-vascular spaces of non-burned and burned soft tissue. Edema formation exceeds the capacity of the lymph vessels to evacuate fluid. The speed of the edema progression will depend on the quality of the resuscitation: a rapid early delivery of large amounts of resuscitation fluids increases the edema formation and worsens the compromised local microcirculation, worsening ischemia in the injured tissues. The important fluid and protein shifts will modify the capillary hydrostatic (P) and colloid oncotic (CO) pressures of the Starling equation further compromising transmicrovascular fluid flux (Jv).
Fluid and cardiovascular management beyond 24 hours
The observation of an important hemo-concentra- tion after major burns goes back to the late 19th century, but its pathophysiology only became apparent during the 20th century. Shock was recognized early on as a predominantly hypovolemic state, but we now know that shock is a complex process of cardiocirculatory dysfunction which fluid resuscitation alone cannot cure. Immediately after injury inflammatory shock mediators are released from the burned skin. These include histamine, serotonin, bradikinin, nitric oxide, lipid peroxides, prostaglandins, derived oxygen and nitric oxide free radicals, thromboxane, cytokines (interleukins and TNF), and platelet aggregating factor with the subsequent coagulation cascade. The response is proportional to the injury, and systemic effects of these mediators will become obvious with burns exceeding 20–25% TBSA [65]. The massive histamine release will cause the early increase in permeability of local and systemic capillaries initiating a massive capillary leak enabling large molecules such as albumin to escape
Jv = Kf ((Pc-Pif ) – d (COPp – COPif ))
The capillary filtration coefficient (Kf ) increases immediately after burn injury, but does not explain the rapid edema formation [65]. In the interstitial fluid, hydrostatic pressure and oncotic pressure will both increase, while in the capillaries hydrostatic pressure and oncotic pressures decrease become negative, constituting a suction force. Delta, the osmotic reflection coefficient, indicates the relative permeability to proteins: the increased permeability is measurable until 8–12 hours post injury. All this causes increase osmolarity of the extravascular compartment.
Myocardial dysfunction has been repeatedly demonstrated both in humans and animals. Neuroendocrine responses will contribute to the worsening of the hemodynamic condition, by increasing cardiac afterload due to the massive release of endogenous norepinephrine and epinephrine. This is further complicated by the decrease in preload due to the hypovolemic state. Oxygen free radicals and lipid peroxides also play an important role. In burns > 40% BSA intrinsic contractile defects are ob-
205
M. M. Berger et al.
served, which are partly reversed by fluid resuscitation and antioxidants [47]. Troponin I increases in a large proportion of the patients with major burns reflecting intrinsic tissue damage.
Edema in non-burned tissue occurs to some degree in burns affecting 10% TBSA, and is largely explained by the circulating histamine and chemokines. All tissues and organs are affected including the lungs, the splanchnic organs, and the brain.
In addition to the capillary leek, fluid is losses can directly be attributed to the wound exudates (1–2 liters per 10% BSA during the first 24 hours, decreasing thereafter until closer of the wound) and the evaporation. These losses include plasma proteins (30g of protein/10% TBSA/day), minerals (P, Mg) and trace elements (mainly Cu, Se, Zn).
The principles of burn fluid resuscitation were developed in the early fifties. Exudates and edema fluid were shown to be isotonic, containing same amounts of electrolytes and protein as plasma. The development of Parkland formula was based on studies on hemodynamic effects of various regimes, using different proportions of colloids and crystalloids [103]. No single fluid resuscitation formula has proven to be superior, and the Parkland formula has
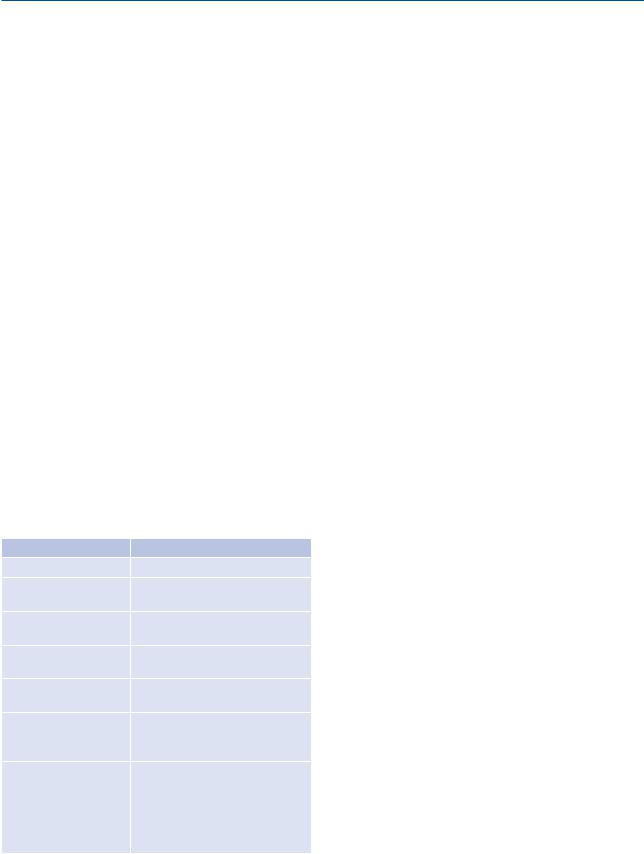
Table 1. Criteria for assessment of under and over-resusci- tation
Under-resuscitation |
Over-resuscitation |
Oliguria > 0.3 ml/kg/h |
Polyuria > 1.0 ml/kg/h |
Hemoglobin |
Decreasing PaO2/FiO2, pul- |
> 180g/l (Ht > 55%) |
monary edema |
Natremia |
Increasing PAPO / PVC |
> 145 mmol/l |
|
Cardiac index |
Rapidly increasing cutaneous |
> 2 L/min/m2 |
edema |
SvO2 > 55% |
Fluid delivery > Ivy index (fluid |
|
delivery > 250 ml/kg BW) |
plasma lacate |
Intra-abdominal P > 20 mmHg |
> 2 mmol/l |
Intra-abdominal hypertension |
or increasing |
leading to |
base excess |
acute renal failure, splanchnic |
> –5 mmol/l |
ischemia, transformation of 2°to |
or decreasing |
3° burns, compartment syndrome |
|
in limbs ( need for fasciotomies), |
|
venous return with hemodynamic |
|
failure |
since remained the most used due to its simplicity. Interestingly, the colloid part of the formula has somehow become forgotten. While under-resusci- tation was the problem in the mid-20th century, overresuscitation has become a major issue since the 90’s [80] with a host of complications: “fluid creep” has become a recognized as a problem over the last decade [87] resulting in general swelling of all organs. Several complications results from this lack of control: abdominal compartment syndrome with renal failure (the most severe) [51], intestinal swelling with ileus, conversion of intermediate burns into deep burns (secondary ischemia), worsening gas exchanges and increasing length of mechanical ventilation [46], and prolonged ICU and hospital length of stay. Today the “Parkland formula” should only be considered as a guide to initiate resuscitation and not absolute fluid requirements.
These complications have forced us to rethink the true aims of resuscitation, which are to stabilize and restore hemodynamic status as soon as possible and to ensure tissue perfusion and oxygenation. In major burns the tools include a combination of fluids (crystalloids and colloids after 8–12hrs), which aim to restore the circulating “volume”, and inotropic and vasopressive agents. Fluids alone are not able to restore adequate tissue perfusion. Invasive cardiovascular monitoring can be used to refine resuscitation, but in absence of such tools a cautious restrictive attitude towards fluids and tight clinical supervision are essential. The initial resuscitation should aim to maintain organ perfusion: urinary output 0.5 ml/hour, lack of tachycardia, maintenance of MAP, normal lactate, and normal base excess will generally reflect this global condition. Nevertheless, one should be cognizant of the difficulty of monitoring the splanchnic compartment.
Among fluids, isotonic crystalloids remain the basic tool. Several burn resuscitation formulae were developed in the 70s, aiming at minimizing fluid delivery and the subsequent edema, which worsens outcomes and further complicates patient care. Hypertonic saline has been studied but the results have been conflicting and currently it’s not part of the mainstay of treatment given the significant increase in acute renal failure [49]. Nevertheless, whatever the formula, the fluid used must contain Na in sufficient concentration to deliver about 0.5 mmol/
206

Critical care of thermally injured patient
kg/%TBSA by 48 hours to prevent water intoxication associated with hypotonic fluids.
Several strategies aimed at reducing edema formation have been proposed, and actually overlap some of those proposed for the control of oxidative stress: 1) topical application of local anesthetic lidocaine/prilocaine cream has some effect in experimental conditions, 2) anti-histamine drugs in the early phase have not reduced the edema formation, 3) use of colloids in fluid resuscitation has proven some efficacy, and 4) antioxidants and particularly vitamin C in high doses.
Early permissive hypovolemia has recently been advocated and seems to reduce organ dysfunction [5], possibly through reduction of organ edema formation and its associated intracellular dysfunction. In the 21st century resuscitation should be guided by the hemodynamic response, and the application of the Parkland formula (even though it’s widely used) should be of historical note.
Upon admission to the ICU, one of the important roles for any intensivist in charge of caring for the thermally injured patient is to ensure hemodynamic stability and to provide restrictive control of fluid balance after the initial resuscitation (which is frequently over-enthusiastic). It begins with reducing the fluid infusion rate as soon as the patient is admitted to the ICU. In the larger burns ( > 40% BSA) this may require some invasive monitoring, and the use of a vasopressor agent such as norepinephrine to maintain tissue perfusion; a mean arterial pressure of 60 mm Hg will generally be sufficient.
Cardiovascular monitoring requirements will depend on the extent of injury and the presence of inhalation injury. An arterial catheter will generally be required for arterial pressure monitoring in patients with burns > 20% TBSA or in those patients requiring intubation due to inhalation injury or burns to face and neck. Blood gas determinations will in addition enable monitoring of arterial lactate and evolution of acid-base status. With increasing severity of burn injury and in elderly patients > 60 years, information about cardiac output becomes necessary. The PiCCO (pulse contour cardiac output) arterial catheter along with a central venous line enables determination of cardiac output without the risks inherent to a pulmonary catheter. Moreover, Holm et al. recently demonstrated that
using the PiCCO for “volume targeted resuscitation” could be more deleterious than the Parkland formula, resulting in increased fluid delivery, worsened oxygenation and prolonged intubation time [46]. The pulmonary catheters may be used in massive burns only or in patients with major cardiopulmonary co-morbidities. Trans-esophageal echocardiography is a further tool that enables a rapid diagnostic workup of the unstable patient [36].
Hemodynamic findings in early invasive cardiovascular monitoring will be variable. Massive burns are generally associated with cardiogenic shock even in the youngest patients, and will invariably be present in elderly during the first 24 hours. The etiology includes increased afterload caused by high levels of stress hormones, direct depression of myocardium by cytokines and lipoperoxides, intravascular hypovolemia with low preload and vasoplegia. The management of shock may require a combination of dobutamine and norepinephrine, in doses titrated to target (cardiac inde× 2.5–3 l/m2, mean arterial pressure >60 mm Hg). Epinephrine will rarely be required. The aim is to restore normal cardiac output, but certainly not supra-normal values or any surrogate of “normal” preload values. Whatever the measurement method used, normal filling pressures should not be the objective goal, as this strategy causes over-resusci- tation and its multitude subsequent complications [3, 5, 45, 51, 80]. Intra-abdominal pressure monitoring is therefore recommended in the burns involving more than 30% TBSA.
After 36–48 hours, the patients generally become spontaneously hyperdynamic. In case of extensive burns, some vasoplegia caused by the massive cytokine release may require norepinephrine (2–10 mcg/min) to permit reducing fluid delivery. Indeed by 24 hours, the fluid delivery should be drastically reduced, to about 30–40% of that infused during the first 24 hours, and the solutions switched to hypotonic, sodium free-poor solutions, in combination with albumin (in case of hypoalbuminemia > 20g/l), or fresh frozen plasma (in case of clotting disorders). The international controversy regarding albumin delivery in critically ill patients has severely complicated burns resuscitations [2]; administration should not be completely liberal, but a very restrictive attitude complicates resuscitation. Albuminemia < 20g/l should certainly be corrected
207
