
- •Preface to the First Edition
- •Preface to the Second Edition
- •Contents
- •Diagnostic Challenges
- •Expert Centers
- •Patient Organizations
- •Clinical Trials
- •Research in Orphan Lung Diseases
- •Orphan Drugs
- •Orphanet
- •Empowerment of Patients
- •Conclusions
- •References
- •Introduction
- •Challenges to Overcome in Order to Undertake Quality Clinical Research
- •Lack of Reliable Data on Prevalence
- •Small Number of Patients
- •Identifying Causation/Disease Pathogenesis
- •Disease Complexity
- •Lack of Access to a Correct Diagnosis
- •Delay in Diagnosis
- •Challenges But Not Negativity
- •Some Success Stories
- •The Means to Overcome the Challenges of Clinical Research: Get Bigger Numbers of Well-Characterized Patients
- •The Importance of Patient Organizations
- •National and International Networks
- •End Points for Trials: Getting Them Right When Numbers Are Small and Change Is Modest
- •Orphan Drug Development
- •Importance of Referral Centers
- •Looking at the Future
- •The Arguments for Progress
- •Concluding Remarks
- •References
- •3: Chronic Bronchiolitis in Adults
- •Introduction
- •Cellular Bronchiolitis
- •Follicular Bronchiolitis
- •Respiratory Bronchiolitis
- •Airway-Centered Interstitial Fibrosis
- •Proliferative Bronchiolitis
- •Diagnosis
- •Chest Imaging Studies
- •Pulmonary Function Testing
- •Lung Biopsy
- •Mineral Dusts
- •Organic Dusts
- •Volatile Flavoring Agents
- •Infectious Causes of Bronchiolitis
- •Idiopathic Forms of Bronchiolitis
- •Connective Tissue Diseases
- •Organ Transplantation
- •Hematopoietic Stem Cell Transplantation
- •Drug-Induced Bronchiolitis
- •Treatment
- •Constrictive Bronchiolitis
- •Follicular Bronchiolitis
- •Airway-Centered Interstitial Fibrosis
- •Proliferative Bronchiolitis
- •References
- •Background and Epidemiology
- •Pathophysiology
- •Host Characteristics
- •Clinical Manifestations
- •Symptoms
- •Laboratory Evaluation
- •Skin Testing
- •Serum Precipitins
- •Eosinophil Count
- •Total Serum Immunoglobulin E Levels
- •Recombinant Antigens
- •Radiographic Imaging
- •Pulmonary Function Testing
- •Histology
- •Diagnostic Criteria
- •Historical Diagnostic Criteria
- •Rosenberg and Patterson Diagnostic Criteria
- •ISHAM Diagnostic Criteria
- •Cystic Fibrosis Foundation Diagnostic Criteria
- •General Diagnostic Recommendations
- •Allergic Aspergillus Sinusitis (AAS)
- •Natural History
- •Treatment
- •Corticosteroids
- •Antifungal Therapy
- •Monoclonal Antibodies
- •Monitoring for Treatment Response
- •Conclusions
- •References
- •5: Orphan Tracheopathies
- •Introduction
- •Anatomical Considerations
- •Clinical Presentation
- •Etiological Considerations
- •Idiopathic Subglottic Stenosis
- •Introduction
- •Clinical Features
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Introduction and Clinical Presentation
- •Clinical Features
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Tracheomalacia
- •Introduction
- •Clinical Features
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Tracheobronchomegaly
- •Introduction
- •Clinical Features
- •Pathophysiology
- •Pulmonary Function Studies
- •Imaging Studies
- •Treatment
- •Tracheopathies Associated with Systemic Diseases
- •Relapsing Polychondritis
- •Introduction
- •Clinical Features
- •Laboratory Findings
- •Pulmonary Function and Imaging Studies
- •Treatment
- •Introduction
- •Clinical Features
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Tracheobronchial Amyloidosis
- •Introduction
- •Clinical Features
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Sarcoidosis
- •Introduction
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Orphan Tracheopathies: Conclusions
- •References
- •6: Amyloidosis and the Lungs and Airways
- •Introduction
- •Diagnosis and Evaluation of Amyloidosis
- •Systemic AA Amyloidosis
- •Systemic AL Amyloidosis
- •Amyloidosis Localised to the Respiratory Tract
- •Laryngeal Amyloidosis
- •Tracheobronchial Amyloidosis
- •Parenchymal Pulmonary Amyloidosis
- •Pulmonary Amyloidosis Associated with Sjögren’s Disease
- •Conclusions
- •References
- •Introduction
- •Pathophysiology
- •Genetic Predisposition
- •Immune Dysregulation
- •Epidemiology
- •Incidence and Prevalence
- •Triggering Factors
- •Clinical Manifestations
- •General Symptoms
- •Pulmonary Manifestations
- •Ear, Nose, and Throat (ENT) Manifestations
- •Neurological Manifestations
- •Skin Manifestations
- •Cardiac Manifestations
- •Gastrointestinal Involvement
- •Renal Manifestations
- •Ophthalmological Manifestations
- •Complementary Investigations
- •Diagnosis
- •Diagnostic Criteria
- •Prognosis and Outcomes
- •Phenotypes According to the ANCA Status
- •Treatment
- •Therapeutic Strategies
- •Remission Induction
- •Maintenance Therapy
- •Other Treatments
- •Prevention of AEs
- •Conclusions
- •References
- •8: Granulomatosis with Polyangiitis
- •A Brief Historical Overview
- •Epidemiology
- •Pathogenesis
- •Clinical Manifestations
- •Constitutional Symptoms
- •Ear, Nose, and Throat (ENT) Manifestations
- •Pulmonary Manifestations
- •Kidney and Urological Manifestations
- •Kidney Manifestations
- •Urological Manifestations
- •Neurological Manifestations
- •Peripheral Nervous System (PNS) Manifestations
- •Central Nervous System (CNS) Manifestations
- •Spinal Cord and Cranial Nerve Involvement
- •Skin and Oral Mucosal Manifestations
- •Eye Manifestations
- •Cardiac Involvement
- •Gastrointestinal Manifestations
- •Gynecological and Obstetric Manifestations
- •Venous Thrombosis and Other Vascular Events
- •Other Manifestations
- •Pediatric GPA
- •Diagnosis
- •Diagnostic Approach
- •Laboratory Investigations
- •Biology
- •Immunology
- •Pathology
- •Treatment
- •Glucocorticoids
- •Cyclophosphamide
- •Rituximab
- •Other Current Induction Approaches
- •Other Treatments in GPA
- •Intravenous Immunoglobulins
- •Plasma Exchange
- •CTLA4-Ig (Abatacept)
- •Cotrimoxazole
- •Other Agents
- •Principles of Treatment for Relapsing and Refractory GPA
- •Outcomes and Prognostic Factors
- •Survival and Causes of Deaths
- •Relapse
- •Damage and Disease Burden on Quality of Life
- •Conclusions
- •References
- •9: Alveolar Hemorrhage
- •Introduction
- •Clinical Presentation
- •Diagnosis (Table 9.1, Fig. 9.3)
- •Pulmonary Capillaritis
- •Histology (Fig. 9.4)
- •Etiologies
- •ANCA-Associated Small Vessel Vasculitis: Granulomatosis with Polyangiitis (GPA)
- •ANCA-Associated Small Vessel Vasculitis: Microscopic Polyangiitis
- •Isolated Pulmonary Capillaritis
- •Systemic Lupus Erythematosus
- •Antiphospholipid Antibody Syndrome
- •Anti-Basement Membrane Antibody Disease (Goodpasture Syndrome)
- •Lung Allograft Rejection
- •Others
- •Bland Pulmonary Hemorrhage (Fig. 9.5)
- •Histology
- •Etiologies
- •Idiopathic Pulmonary Hemosiderosis
- •Drugs and Medications
- •Coagulopathy
- •Valvular Heart Disease and Left Ventricular Dysfunction
- •Other
- •Histology
- •Etiologies
- •Hematopoietic Stem Cell Transplantation (HSCT)
- •Cocaine Inhalation
- •Acute Exacerbation of Interstitial Lung Disease
- •Acute Interstitial Pneumonia
- •Acute Respiratory Distress Syndrome
- •Miscellaneous Causes
- •Etiologies
- •Pulmonary Capillary Hemangiomatosis
- •Treatment
- •Conclusions
- •References
- •Takayasu Arteritis
- •Epidemiology
- •Pathologic Features
- •Pathogenesis
- •Clinical Features
- •Laboratory Findings
- •Imaging Studies
- •Therapeutic Management
- •Prognosis
- •Behçet’s Disease
- •Epidemiology
- •Pathologic Features
- •Pathogenesis
- •Diagnostic Criteria
- •Clinical Features
- •Pulmonary Artery Aneurysm
- •Pulmonary Artery Thrombosis
- •Pulmonary Parenchymal Involvement
- •Laboratory Findings
- •Imaging Studies
- •Therapeutic Management
- •Treatment of PAA
- •Treatment of PAT
- •Prognosis
- •References
- •Introduction
- •Portopulmonary Hypertension (PoPH)
- •Epidemiology and Risk Factors
- •Molecular Pathogenesis
- •PoPH Treatment
- •Hepatopulmonary Syndrome (HPS)
- •Epidemiology and Risk Factors
- •Molecular Pathogenesis
- •HPS Treatment
- •Conclusion
- •References
- •12: Systemic Sclerosis and the Lung
- •Introduction
- •Risk factors for SSc-ILD
- •Genetic Associations
- •Clinical Presentation of SSc-ILD
- •Pulmonary Function Tests (PFTs)
- •Imaging
- •Management
- •References
- •13: Rheumatoid Arthritis and the Lungs
- •Introduction
- •Epidemiology
- •Risk Factors for ILD (Table 13.3)
- •Pathogenesis
- •Clinical Features and Diagnosis
- •Treatments
- •Prognosis
- •Epidemiology
- •Risk Factors
- •Clinical Features, Diagnosis, and Outcome
- •Subtypes or RA-AD
- •Obliterative Bronchiolitis
- •Bronchiectasis
- •COPD
- •Cricoarytenoid Involvement
- •Pleural Disease
- •Conclusion
- •References
- •Introduction
- •Systemic Lupus Erythematosus
- •Epidemiology
- •Pathophysiology
- •Pulmonary Manifestations
- •Pleural Disease
- •Shrinking Lung Syndrome
- •Thrombotic Manifestations
- •Interstitial Lung Disease
- •Other Pulmonary Manifestations
- •Prognosis
- •Sjögren’s Syndrome
- •Epidemiology
- •Pathophysiology
- •Pulmonary Manifestations
- •Airway Disorders
- •Lymphoproliferative Disease
- •Interstitial Lung Disease
- •Prognosis
- •Mixed Connective Tissue Disease
- •Epidemiology
- •Pathophysiology
- •Pulmonary Manifestations
- •Pulmonary Hypertension
- •Interstitial Lung Disease
- •Prognosis
- •Myositis
- •Epidemiology
- •Pathophysiology
- •Pulmonary Manifestations and Treatments
- •Interstitial Lung Disease
- •Respiratory Muscle Weakness
- •Other Pulmonary Manifestations
- •Prognosis
- •Other Therapeutic Options in CTD-ILD
- •Lung Transplantation
- •Conclusion
- •References
- •Introduction
- •Diagnostic Criteria
- •Controversies in the Diagnostic Criteria
- •Typical Clinical Features
- •Disease Progression and Prognosis
- •Summary
- •References
- •Introduction
- •Histiocytes and Dendritic Cells
- •Introduction
- •Cellular and Molecular Pathogenesis
- •Pathology
- •Clinical Presentation
- •Treatment and Prognosis
- •Erdheim-Chester Disease
- •Epidemiology
- •Cellular and Molecular Pathogenesis
- •Histopathology and Immunohistochemistry
- •Clinical Presentation
- •Investigation/Diagnosis
- •Chest Studies
- •Cardiovascular Imaging
- •CNS Imaging
- •Bone Radiography
- •Other Imaging Findings and Considerations
- •Disease Monitoring
- •Pathology
- •Management/Treatment
- •Prognosis
- •Rosai-Dorfman Destombes Disease
- •Epidemiology
- •Etiology/Pathophysiology
- •Histopathology and Immunohistochemistry
- •Clinical Presentation
- •Investigation/Diagnosis
- •Management/Treatment
- •Prognosis
- •Conclusions
- •Diagnostic Criteria for Primary Histiocytic Disorders of the Lung
- •References
- •17: Eosinophilic Pneumonia
- •Introduction
- •Eosinophil Biology
- •Physiologic and Immunologic Role of Eosinophils
- •Release of Mediators
- •Targeting the Eosinophil Cell Lineage
- •Historical Perspective
- •Clinical Presentation
- •Pathology
- •Diagnosis
- •Eosinophilic Lung Disease of Undetermined Cause
- •Idiopathic Chronic Eosinophilic Pneumonia
- •Clinical Features
- •Imaging
- •Laboratory Studies
- •Bronchoalveolar Lavage
- •Lung Function Tests
- •Treatment
- •Outcome and Perspectives
- •Clinical Features
- •Imaging
- •Laboratory Studies
- •Bronchoalveolar Lavage
- •Lung Function Tests
- •Lung Biopsy
- •Treatment and Prognosis
- •Eosinophilic Granulomatosis with Polyangiitis
- •History and Nomenclature
- •Pathology
- •Clinical Features
- •Imaging
- •Laboratory Studies
- •Pathogenesis
- •Diagnosis
- •Treatment and Prognosis
- •Long-Term Outcome
- •Hypereosinophilic Syndrome
- •Pathogenesis
- •Clinical and Imaging Features
- •Laboratory Studies
- •Treatment and Prognosis
- •Eosinophilic Pneumonias of Parasitic Origin
- •Tropical Eosinophilia [191]
- •Ascaris Pneumonia
- •Eosinophilic Pneumonia in Larva Migrans Syndrome
- •Strongyloides Stercoralis Infection
- •Eosinophilic Pneumonias in Other Infections
- •Allergic Bronchopulmonary Aspergillosis
- •Pathogenesis
- •Diagnostic Criteria
- •Biology
- •Imaging
- •Treatment
- •Bronchocentric Granulomatosis
- •Miscellaneous Lung Diseases with Associated Eosinophilia
- •References
- •Introduction
- •Pulmonary Langerhans’ Cell Histiocytosis
- •Epidemiology
- •Pathogenesis
- •Diagnosis
- •Clinical Features
- •Extrathoracic Lesions
- •Pulmonary Function Tests
- •Chest Radiography
- •High-Resolution Computed Tomography (HRCT)
- •Bronchoscopy and Bronchoalveolar Lavage (BAL)
- •Lung Biopsy
- •Pathology
- •Treatment
- •Course and Prognosis
- •Case Report I
- •Introduction
- •Epidemiology
- •Clinical Features
- •Histopathological Findings
- •Radiologic Findings
- •Prognosis and Therapy
- •Desquamative Interstitial Pneumonia
- •Epidemiologic and Clinical Features
- •Histopathological Findings
- •Radiological Findings
- •Prognosis and Therapy
- •Conclusion
- •References
- •19: Lymphangioleiomyomatosis
- •Introduction
- •Pathogenesis
- •Presentation
- •Prognosis
- •Management
- •General Measures
- •Parenchymal Lung Disease
- •Pleural Disease
- •Renal Angiomyolipoma
- •Abdominopelvic Lymphatic Disease
- •Pregnancy
- •Tuberous Sclerosis
- •Drug Treatment
- •Bronchodilators
- •mTOR Inhibitors
- •Anti-Oestrogen Therapy
- •Experimental Therapies
- •Interventions for Advanced Disease
- •Oxygen Therapy
- •Pulmonary Hypertension
- •References
- •20: Diffuse Cystic Lung Disease
- •Introduction
- •Lymphangioleiomyomatosis
- •Pathogenesis
- •Pathologic and Radiographic Characteristics
- •Diagnostic Approach
- •Pulmonary Langerhans Cell Histiocytosis (PLCH)
- •Pathogenesis
- •Pathological and Radiographic Characteristics
- •Diagnostic Approach
- •Birt-Hogg-Dubé Syndrome (BHD)
- •Pathogenesis
- •Pathological and Radiographic Characteristics
- •Diagnostic Approach
- •Lymphoproliferative Disorders
- •Pathogenesis
- •Pathological and Radiographic Characteristics
- •Diagnostic Approach
- •Amyloidosis
- •Light Chain Deposition Disease (LCDD)
- •Conclusion
- •References
- •Introduction
- •Lymphatic Development
- •Clinical Presentation of Lymphatic Disorders
- •Approaches to Diagnosis and Management of Congenital Lymphatic Anomalies
- •Generalized Lymphatic Anomaly
- •Etiopathogenesis
- •Clinical Presentation and Diagnosis
- •Course/Prognosis
- •Management
- •Kaposiform Lymphangiomatosis
- •Etiopathogenesis
- •Clinical Presentation and Diagnosis
- •Management
- •Course/Prognosis
- •Gorham Stout Disease
- •Etiopathogenesis
- •Clinical Presentation and Diagnosis
- •Management
- •Course/Prognosis
- •Channel-Type LM/Central Conducting LM
- •Etiopathogenesis
- •Clinical Presentation and Diagnosis
- •Management
- •Course/Prognosis
- •Yellow Nail Syndrome
- •Etiopathogenesis
- •Clinical Presentation and Diagnosis
- •Management
- •Course/Prognosis
- •Summary
- •References
- •Introduction
- •Historical Note
- •Epidemiology
- •Pathogenesis
- •Surfactant Homeostasis in PAP
- •GM-CSF Signaling Disruption
- •Myeloid Cell Dysfunction
- •GM-CSF Autoantibodies
- •Lymphocytosis
- •Clinical Manifestations
- •Clinical Presentation
- •Secondary Infections
- •Pulmonary Fibrosis
- •Diagnosis
- •Pulmonary Function Testing
- •Radiographic Assessment
- •Bronchoscopy and Bronchoalveolar Lavage
- •Laboratory Studies and Biomarkers
- •GM-CSF Autoantibodies
- •Genetic Testing
- •Lung Pathology
- •Diagnostic Approach to the Patient with PAP
- •Natural History and Prognosis
- •Treatment
- •Whole-Lung Lavage
- •Subcutaneous GM-CSF
- •Inhaled GM-CSF
- •Other Approaches
- •Conclusions and Future Directions
- •References
- •Introduction
- •Epidemiology
- •Gastric Contents
- •Pathobiology of GER/Microaspirate in the Lungs of Patients with IPF
- •GER and the Microbiome
- •Diagnosis
- •Clinical History/Physical Exam
- •Investigations
- •Esophageal Physiology
- •Upper Esophageal Sphincter
- •Esophagus and Peristalsis
- •Lower Esophageal Sphincter and Diaphragm
- •Esophageal pH and Impedance Testing
- •High Resolution Esophageal Manometry
- •Esophagram/Barium Swallow
- •Bronchoalveolar Lavage/Sputum: Biomarkers
- •Treatment
- •Anti-Acid Therapy (PPI/H2 Blocker)
- •GER and Acute Exacerbations of IPF
- •Suggested Approach
- •Summary and Future Directions
- •References
- •Introduction
- •Familial Interstitial Pneumonia
- •Telomere Related Genes
- •Genetic
- •Telomere Length
- •Pulmonary Involvement
- •Interstitial Lung Disease
- •Other Lung Disease
- •Hepatopulmonary Syndrome
- •Emphysema
- •Extrapulmonary Manifestations
- •Mucocutaneous Involvement
- •Hematological Involvement
- •Liver Involvement
- •Other Manifestations
- •Treatment
- •Telomerase Complex Agonists
- •Lung Transplantation
- •Surfactant Pathway
- •Surfactant Protein Genes
- •Pulmonary Involvement
- •Treatment
- •Heritable Forms of Pulmonary Fibrosis with Autoimmune Features
- •TMEM173
- •COPA
- •Pulmonary Alveolar Proteinosis
- •GMCSF Receptor Mutations
- •GATA2
- •MARS
- •Lysinuric Protein Intolerance
- •Lysosomal Diseases
- •Hermansky-Pudlak Syndrome
- •Lysosomal Storage Disorders
- •FAM111B, NDUFAF6, PEPD
- •Conclusion
- •References
- •Introduction
- •Pathophysiology
- •Clinical Presentation
- •Epidemiology
- •Genetic Causes of Bronchiectasis
- •Disorders of Mucociliary Clearance
- •Cystic Fibrosis
- •Primary Ciliary Dyskinesia
- •Other Ciliopathies
- •X-Linked Agammaglobulinemia
- •Chronic Granulomatous Disease and Other Disorders of Neutrophil Function
- •Other Genetic Disorders Predisposing to Bronchiectasis
- •Idiopathic Bronchiectasis
- •Diagnosis of Bronchiectasis
- •Management of Patients with Bronchiectasis
- •Airway Clearance Therapy (ACT)
- •Management of Infections
- •Immune Therapy
- •Surgery
- •Novel Therapies for Managing Cystic Fibrosis
- •Summary
- •References
- •Pulmonary Arteriovenous Malformations
- •Background Pulmonary AVMs
- •Anatomy Pulmonary AVMs
- •Clinical Presentation of Pulmonary AVMs
- •Screening Pulmonary AVMs
- •Treatment Pulmonary AVMs
- •Children with Hereditary Hemorrhagic Telangiectasia
- •Pulmonary Hypertension
- •Pulmonary Hypertension Secondary to Liver Vascular Malformations
- •Pulmonary Arterial Hypertension
- •Background HHT
- •Pathogenesis
- •References
- •27: Pulmonary Alveolar Microlithiasis
- •Introduction
- •Epidemiology
- •Pathogenesis
- •Clinical Features
- •Diagnosis
- •Management
- •Summary
- •References
- •Introduction
- •Hermansky-Pudlak Syndrome
- •Telomerase-Associated Pulmonary Fibrosis
- •Lysosomal Storage Diseases
- •Lysinuric Protein Intolerance
- •Familial Hypocalciuric Hypercalcemia
- •Surfactant Dysfunction Disorders
- •Concluding Remarks
- •References
- •Introduction
- •Background
- •Image Acquisition
- •Key Features of Fibrosis
- •Ancillary Features of Fibrosis
- •Other Imaging Findings in FLD
- •Probable UIP-IPF
- •Indeterminate
- •Alternative Diagnosis
- •UIP in Other Fibrosing Lung Diseases
- •Pleuroparenchymal Fibroelastosis (PPFE)
- •Combined Pulmonary Fibrosis and Emphysema
- •Chronic Hypersensitivity Pneumonitis
- •Other Fibrosing Lung Diseases
- •Fibrosing Sarcoidosis
- •CTD-ILD and Drug-Induced FLD
- •Complications
- •Prognosis
- •Computer Analysis of CT Imaging
- •The Progressive Fibrotic Phenotype
- •Other Imaging Techniques
- •Conclusion
- •References
- •Introduction
- •Bronchoalveolar Lavage (BAL)
- •Technique
- •Interpretation
- •Transbronchial Biopsy (TBB)
- •Transbronchial Lung Cryobiopsy (TLCB)
- •References
- •Introduction
- •Overview of ILD Diagnosis
- •Clinical Assessment
- •Radiological Assessment
- •Laboratory Assessment
- •Integration of Individual Features
- •Multidisciplinary Discussion
- •Diagnostic Ontology
- •Conclusions
- •References
- •Introduction
- •Idiopathic Pulmonary Fibrosis
- •Chronic Hypersensitivity Pneumonitis
- •Connective Tissue Disease
- •Drug-Induced Lung Diseases
- •Radiation Pneumonitis
- •Asbestosis
- •Hermansky-Pudlak Syndrome
- •Risk Factors for Progression
- •Diagnosis
- •Pharmacological Management
- •Conclusions
- •References
- •Historical Perspective
- •Epidemiology and Etiologies
- •Tobacco Smoking and Male Sex
- •Genetic Predisposition
- •Systemic Diseases
- •Other Etiological Contexts
- •Clinical Manifestations
- •Pulmonary Function and Physiology
- •Imaging
- •Computed Tomography Characteristics and Patterns
- •Thick-Walled Large Cysts
- •Imaging Phenotypes
- •Pitfalls
- •Pathology
- •Diagnosis
- •CPFE Is a Syndrome
- •Biology
- •Complications and Outcome
- •Mortality
- •Pulmonary Hypertension
- •Lung Cancer
- •Acute Exacerbation of Pulmonary Fibrosis
- •Other Comorbidities and Complications
- •Management
- •General Measures and Treatment of Emphysema
- •Treatment of Pulmonary Fibrosis
- •Management of Pulmonary Hypertension
- •References
- •Acute Interstitial Pneumonia (AIP)
- •Epidemiology
- •Presentation
- •Diagnostic Evaluation
- •Radiology
- •Histopathology
- •Clinical Course
- •Treatment
- •Epidemiology
- •Presentation
- •Diagnostic Evaluation
- •Radiology
- •Histopathology
- •Clinical Course
- •Desquamative Interstitial Pneumonia (DIP)
- •Presentation
- •Diagnostic Evaluation
- •Radiology
- •Histopathology
- •Clinical Course
- •Treatment
- •Epidemiology
- •Presentation
- •Diagnostic Evaluation
- •Radiology
- •Histopathology
- •Clinical Course
- •Treatment
- •References
- •Organizing Pneumonias
- •Epidemiology
- •Pathogenesis
- •Clinical Features
- •Imaging
- •Multifocal Form
- •Isolated Nodular Form
- •Other Imaging Patterns
- •Histopathological Diagnosis of OP Pattern
- •Etiological Diagnosis of OP
- •Treatment
- •Clinical Course and Outcome
- •Severe Forms of OP with Respiratory Failure
- •Acute Fibrinous and Organizing Pneumonia
- •Granulomatous Organizing Pneumonia
- •Acute Interstitial Pneumonia
- •Epidemiology
- •Clinical Picture
- •Imaging
- •Histopathology
- •Diagnosis
- •Treatment
- •Outcome
- •References
- •36: Pleuroparenchymal Fibroelastosis
- •Introduction
- •Epidemiology
- •Clinical Manifestations
- •Laboratory Findings
- •Respiratory Function
- •Radiologic Features
- •Pathologic Features
- •Diagnosis
- •Treatment
- •Prognosis
- •Conclusions
- •References
- •Introduction
- •Acute Berylliosis
- •Chronic Beryllium Disease
- •Exposure
- •Epidemiology
- •Immunopathogenesis and Pathology
- •Genetics
- •Clinical Description and Natural History
- •Treatment and Monitoring
- •Indium–Tin Oxide-Lung Disease
- •Hard Metal Lung
- •Flock Worker’s Disease
- •Asbestosis
- •Nanoparticle Induced ILD
- •Flavoring-Induced Lung Disease
- •Silica-Induced Interstitial Lung Disease
- •Chronic Silicosis
- •Acute and Accelerated Silicosis
- •Chronic Obstructive Disease in CMDLD
- •Simple CMDLD
- •Complicated CMDLD
- •Conclusion
- •References
- •38: Unclassifiable Interstitial Lung Disease
- •Introduction
- •Diagnostic Scenarios
- •Epidemiology
- •Clinical Presentation
- •Diagnosis
- •Clinical Features
- •Radiology
- •Laboratory Investigations
- •Pathology
- •Conclusion
- •References
- •39: Lymphoproliferative Lung Disorders
- •Introduction
- •Nodular Lymphoid Hyperplasia
- •Lymphocytic Interstitial Pneumonia (LIP)
- •Follicular Bronchitis/Bronchiolitis
- •Castleman Disease
- •Primary Pulmonary Lymphomas
- •Primary Pulmonary MALT B Cell Lymphoma
- •Pulmonary Plasmacytoma
- •Follicular Lymphoma
- •Lymphomatoid Granulomatosis
- •Primary Pulmonary Hodgkin Lymphoma (PPHL)
- •Treatment
- •References
- •Introduction
- •Late-Onset Pulmonary Complications
- •Bronchiolitis Obliterans (BO)
- •Pathophysiology
- •Diagnosis
- •Management of BOS
- •Post-HSCT Organizing Pneumonia
- •Other Late-Onset NonInfectious Pulmonary Complications (LONIPCs)
- •Conclusion
- •References
- •Introduction
- •Pulmonary Hypertension Associated with Sarcoidosis (Group 5.2)
- •PH Associated with Pulmonary Langerhans Cell Histiocytosis (Group 5.2)
- •PH in Combined Pulmonary Fibrosis and Emphysema (Group 3.3)
- •PH Associated with Lymphangioleiomyomatosis (Group 3)
- •Hereditary Hemorrhagic Telangiectasia (Group 1.2)
- •Pulmonary Veno-Occlusive Disease (Group 1.5)
- •Small Patella Syndrome (Group 1.2)
- •Conclusion
- •References
- •Introduction
- •Epidemiology
- •Timing, Chronology, Delay Time
- •Route of Administration
- •Patterns of Involvement [3, 4]
- •Drugs and Agents Fallen Out of Favor
- •Drug-Induced Noncardiac Pulmonary Edema
- •Drug-Induced Cardiogenic Pulmonary Edema
- •The “Chemotherapy Lung”
- •Drug-Induced/Iatrogenic Alveolar Hemorrhage
- •Drugs
- •Superwarfarin Rodenticides
- •Transfusion Reactions: TACO–TRALI
- •Acute Eosinophilic Pneumonia
- •Acute Granulomatous Interstitial Lung Disease
- •Acute Organizing Pneumonia (OP), Bronchiolitis Obliterans Organizing Pneumonia (BOOP), or Acute Fibrinous Organizing Pneumonia (AFOP) Patterns
- •Acute Amiodarone-Induced Pulmonary Toxicity (AIPT)
- •Accelerated Pulmonary Fibrosis
- •Acute Exacerbation of Previously Known (Idiopathic) Pulmonary Fibrosis
- •Anaphylaxis
- •Acute Vasculopathy
- •Drug-Induced/Iatrogenic Airway Emergencies
- •Airway Obstruction as a Manifestation of Anaphylaxis
- •Drug-Induced Angioedema
- •Hematoma Around the Upper Airway
- •The “Pill Aspiration Syndrome”
- •Catastrophic Drug-Induced Bronchospasm
- •Peri-operative Emergencies (Table 42.8)
- •Other Rare Presentations
- •Pulmonary Nodules and Masses
- •Pleuroparenchymal Fibroelastosis
- •Late Radiation-Induced Injury
- •Chest Pain
- •Rebound Phenomenon
- •Recall Pneumonitis
- •Thoracic Bezoars: Gossipybomas
- •Respiratory Diseases Considered Idiopathic That May Be Drug-Induced (Table 42.4)
- •Eye Catchers
- •Conclusion
- •References
- •Cancer Mimics of Organizing Pneumonia
- •Lung Adenocarcinoma/Bronchioloalveolar Carcinoma
- •Primary Pulmonary Lymphoma
- •Cancer Mimics of Interstitial Lung Diseases
- •Lymphangitic Carcinomatosis
- •Epithelioid Hemangio-Endothelioma
- •Lymphomatoid Granulomatosis
- •Cystic Tumors
- •Cavitating Tumors
- •Intrathoracic Pseudotumors
- •Respiratory Papillomatosis
- •Pulmonary Langerhans Cell Histiocytosis
- •References
- •Index

Amyloidosis and the Lungs and Airways |
6 |
|
|
Helen J. Lachmann and Jennifer H. Pinney |
|
Introduction
Amyloids occur due to the deposition of soluble plasma proteins within the extracellular space in an abnormal insolublebrillar form. Amyloidosis describes a group of diseases caused by the resulting disruption of the tissue structure and organ function. The diagnosis is often made late in the disease course, frequently as an unexpected histological nding when a failing organ is biopsied. It may be either acquired or inherited, and at least 30 different proteins can form amyloidbrils in humans [1] (Table 6.1). In the right conditions, almost any polypeptide chain can be driven towards misfolding and aggregation but relatively few proteins are amyloidogenic in vivo. There are essentially three circumstances in which amyloid deposition occurs. The rst is when there is a sustained, abnormally high abundance of proteins that are normally present at low levels, such as serum amyloid A protein (SAA) in chronic infammation or beta-2 microglobulin in chronic renal failure. The second is when there is normal abundance of a normal but inherently amyloidogenic protein over an extremely prolonged period, such as transthyretin. The third situation is the presence of an abnormal protein, which is markedly amyloidogenic, such as monoclonal immunoglobulin light chains in AL amyloidosis and genetic variants of transthyretin, apolipoprotein AI, brinogen Aα chain, etc. in autosomal dominant hereditary amyloidosis.
The ultrastructural morphological and histochemical properties of all amyloid brils, regardless of the precursor
H. J. Lachmann (*)
UK National Amyloidosis Centre, Division of Medicine, University College London and Royal Free London NHS Foundation Trust, London, UK
e-mail: h.lachmann@ucl.ac.uk; helen.lachmann@nhs.net
J. H. Pinney
Department of Renal Medicine, University Hospital Birmingham NHS Foundation Trust, Birmingham, UK
protein type, are remarkably similar. Diffraction studies of amyloid brils have con rmed that they all share a common core structure consisting of anti-parallel β-strands lying perpendicular to the long axis of the brils. This extremely abnormal, highly ordered conformation underlies the distinctive physicochemical properties of amyloidbrils. The brils are relatively stable and are resistant to proteolysis. All amyloid brils possess the ability to bind molecules of the dye Congo Red in a spatially organised manner, which results in a pathognomonic apple-green birefringence when viewed under crossed polarised light. Amyloid deposits also always contain the normal plasma glycoprotein, serum amyloid P component (SAP) as a non-brillar constituent. The universal presence of SAP in amyloid deposits refects its speci c binding to an as yet uncharacterised ligand common to all amyloid brils, which forms the basis for diagnostic scintigraphic imaging of amyloids with radiolabelled SAP [2].
The phenotypes associated with amyloid deposition are diverse, ranging from an asymptomatic, small, localised deposit to a systemic, rapidly lethal multi-system disease [3]. Clinically important amyloid deposits accumulate in the extracellular space, progressively disrupting the structure, integrity and function of the tissues and organs. The natural history of amyloidosis is usually of progressive accumulation, although as amyloid deposits are constantly turned over, clinical progression refects the fact that the brillar deposits are laid down faster than they are cleared away [4]. Amyloid deposits can therefore regress if this balance is tipped in favour of clearance; current treatment strategies have focused on reducing the supply of brils by halting the production of the culpable plasma protein. Although treatment is not always effective as many of the conditions that underlie systemic amyloidosis are progressive and unremitting, there are numerous reports describing regression of amyloidosis when associated infammatory and other diseases have been successfully treated.
© Springer Nature Switzerland AG 2023 |
77 |
V. Cottin et al. (eds.), Orphan Lung Diseases, https://doi.org/10.1007/978-3-031-12950-6_6 |
|
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

78 |
|
H. J. Lachmann and J. H. Pinney |
|
||
Table 6.1 Classi cation of the more common types of systemic amyloidoses in humans |
||
|
|
|
Type |
Fibril protein precursor |
Clinical syndrome |
AA |
Serum amyloid A protein |
Reactive systemic amyloidosis associated with chronic |
|
|
infammatory diseases |
AL |
Monoclonal immunoglobulin light chains |
Systemic amyloidosis associated with monoclonal |
|
|
plasma cell dyscrasias |
|
|
|
AH |
Monoclonal immunoglobulin heavy chains |
Systemic amyloidosis associated with monoclonal |
|
|
plasma cell dyscrasias |
Aβ2M |
Normal plasma β2-microglobulin |
Periarticular and, occasionally, systemic amyloidosis |
|
|
associated with long-term dialysis |
Aβ2M |
Variant β2-microglobulin |
Autosomal dominant hereditary systemic amyloidosis |
ATTR |
Normal plasma transthyretin |
Wild-type systemic TTR amyloidosis with prominent |
|
|
cardiac involvement |
|
|
|
ATTR |
Genetically variant transthyretin |
Autosomal dominant systemic amyloidosis |
|
|
Familial amyloid polyneuropathy or cardiomyopathy |
ACys |
Genetically variant cystatin C |
Autosomal dominant Systemic amyloidosis |
|
|
Hereditary cerebral haemorrhage with cerebral and |
|
|
systemic amyloidosis |
|
|
|
AGel |
Genetically variant gelsolin |
Autosomal dominant systemic amyloidosis |
|
|
|
|
|
Predominant cranial nerve involvement with lattice |
|
|
corneal dystrophy |
ALys |
Genetically variant lysozyme |
Autosomal dominant systemic amyloidosis |
|
|
Non-neuropathic with prominent visceral involvement |
AApoAI |
Genetically variant apolipoprotein AI |
Autosomal dominant systemic amyloidosis |
|
|
|
|
|
Predominantly non-neuropathic with prominent viscera |
|
|
involvement |
AApoAII |
Genetically variant apolipoprotein AII |
Autosomal dominant systemic amyloidosis |
|
|
Non-neuropathic with prominent renal involvement |
AApoAIV |
Apolipoprotein AIV |
Sporadic systemic amyloidosis with predominant |
|
|
cardiac and renal involvement |
|
|
|
AApoCII |
Genetically variant apolipoprotein CII |
Autosomal dominant systemic amyloidosis |
|
|
|
|
|
Non-neuropathic with prominent renal involvement |
AApoCIII |
Genetically variant apolipoprotein CIII |
Autosomal dominant systemic amyloidosis |
|
|
Non-neuropathic with prominent renal involvement |
AFib |
Genetically variant brinogen A alpha chain |
Autosomal dominant systemic amyloidosis |
|
|
Non-neuropathic with prominent renal involvement |
ALect 2 |
Leukocyte chemotactic factor 2 |
Sporadic slowly progressive renal amyloid with |
|
|
nephrotic syndrome and liver involvement |
|
|
|
ALys |
Genetically variant lysozyme |
Autosomal dominant systemic amyloidosis |
|
|
Non-neuropathic with prominent renal and hepatic |
|
|
involvement |
Diagnosis and Evaluation of Amyloidosis
As amyloidosis is a remarkably heterogeneous disease, it may present to a variety of different medical specialties. There are several reasons for patients with amyloidosis to present to a respiratory physician. Chronic pulmonary conditions can themselves give rise to systemic amyloidosis, most commonly of the AA type. Although these patients rarely present with symptomatic involvement of the lungs, the underlying pulmonary disease is the driver of the amyloidogenic protein production and it is therefore important to recognise those patients at risk. Patients with systemic amyloidosis may also present with respiratory symptoms as a consequence of the amyloidosis itself, whereby amyloid deposits are found in the lungs as a component of a more
systemic process. Localised, isolated pulmonary and respiratory tract amyloid deposits are also well-described and may either present with symptoms or may be detected incidentally on chest radiography or a biopsy [5]. Finally, it is important to recognise that, especially in the context of AL amyloidosis, pulmonary complications may also arise from treatment.
The diagnostic gold standard of amyloidosis is histological con rmation through Congo Red staining, which produces a red-green birefringence under crossed polarised light [6, 7] (Fig. 6.1). Most tissue specimens, ranging from needle biopsies to open surgical resections, can be studied, although small biopsies are open to sampling errors. A biopsy of any organ can be hazardous in amyloidosis as there is an increased risk of haemorrhage; signi cant bleeds having been reported

6 Amyloidosis and the Lungs and Airways |
79 |
|
|
a |
b |
c |
d |
Fig. 6.1 (a) Bronchial biopsy showing characteristic histological appearance of amorphous amyloid deposits stained with Congo Red. (b) The same section viewed under crossed polarised light demonstrat-
ing an apple-green birefringence. Con rmatory testing is shown by immunohistochemistry using anti-lambda light chain antibodies (c) and anti-transthyretin antibodies (d)
in 5% of liver biopsies [8], although recent data from renal biopsies have been more reassuring [9]. A case report from 1987 described fatal lung haemorrhage following a transbronchial biopsy in a patient with amyloidosis. The postmortem ndings showed that the biopsied blood vessels were in ltrated by amyloids [10]. A further case series by Utz et al. published in 1996 reported no major complications in 11 patients, following transbronchial lung biopsies; however, 2 of the 11 cases were reported to have 100 mL blood loss [11]. More encouragingly, a study from a single centre in 2017 reported no bleeding in 25 cases diagnosed by transbronchial biopsy [12]. Haemorrhage in amyloidosis is due to the increased fragility of the involved blood vessels, reduced elasticity of the amyloidotic tissues and, very occasionally, in the AL type, an acquired de ciency of clotting factors IX or X [13–15]. A less invasive alternative in suspected disease is ne needle aspiration, and this has been successfully used in the respiratory tract [16–18]. Immunohistochemical stains are then used to determine the bril protein type [5, 19].
Suitable antibodies are widely available, but, although immunohistochemistry is usually de nitive in AA amyloidosis, it is non-diagnostic in about 20% of AL deposits [20, 21]. Expertise in the typing of hereditary amyloids is restricted, and de nitive immunohistochemical typing of amyloid deposits cannot always be achieved. Mass spectrometry is extremely useful in those patients who cannot be con dently diagnosed through immunohistochemical typing; its use is limited by current availability but will become more routine in future practice [22].
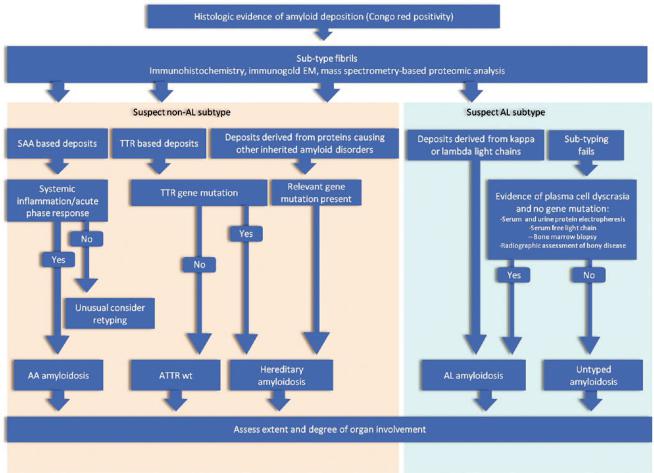
If a genetic variant is suspected, then more detailed analyses examining for mutations in the gene giving rise to the amyloidogenic brils should be performed (Fig. 6.2). In general, sequencing is the preferred modality, and ideally samples should be sent to a reference laboratory with expertise in this area. A web-based repository reviewing all currently known mutations in genes with amyloidogenic potential helps guide these investigations (http://amyloidosismutations.com).
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
80 |
H. J. Lachmann and J. H. Pinney |
|
|
Fig. 6.2 Algorithm for investigations of patients with suspected amyloidosis
Once there is histological con rmation of amyloidosis, the extent of deposits needs to be ascertained. In respiratory tract amyloidosis, this can be challenging and the optimum imaging technique can vary depending upon the distribution of deposits. Plain radiography as an initial assessment can be helpful but may be normal in many cases. Computed tomography (CT) scanning is particularly useful in further de ning interstitial diseases. In combination with positron emission tomography (PET) imaging, it can also help to better de ne the metabolic activity of a solid lesion, thus aiding in differentiation from more typical intrathoracic malignancies or metastases as well as rare entities such as plasmacytomas [23]. In addition, magnetic resonance imaging (MRI) and bronchoscopy may also be useful in combination with comprehensive pulmonary function tests (PFTs). PFTs are an important objective tool to formally establish the severity of clinically relevant diseases and are useful in guiding therapeutic decisions [5, 24]. Evidence of systemic diseases should be sought clinically by performing haematological and biochemical pro les. The plasma cell clones that underlie systemic AL amyloidosis are often subtle and may not be
detected by bone marrow examination or immuno xation of the serum and urine; use of a serum-free light-chain assay increases diagnostic sensitivity [25–28]. Immunoglobulin gene rearrangement studies may identify subtle clones in either the bone marrow or, in the case of localised AL, within the amyloid biopsy material [29] (Fig. 6.1c, d).
Radiolabelled SAP speci cally localises to amyloid deposits in vivo in proportion to the quantity of amyloids present and thereby enables diagnosis, quanti cation and monitoring of the amyloids [2]. SAP scintigraphy is useful in visualising amyloids in solid organs; localisation to the lungs is poor and not routinely used for monitoring amyloid deposits in the respiratory tract (Fig. 6.3a, b). Cardiac amyloidosis is best evaluated by a combination of echocardiography, electrocardiogram (ECG) and cardiac magnetic resonance imaging (CMR). Two-dimensional Doppler echocardiography classically reveals concentric biventricular wall thickening with a restrictive lling pattern [30] (Fig. 6.3b). Amyloidosis causes diastolic dysfunction with preserved contractility until an extremely late stage [31]. The ECG may be normal in patients with substantial cardiac

6 Amyloidosis and the Lungs and Airways |
81 |
|
|
Fig. 6.3 Scintigraphic assessments using 123I-human SAP. An anterior whole body scintigraphic image from a patient obtained following intravenous injection of 123I-human SAP showing abnormal uptake into the amyloid deposits within the spleen, liver and bone marrow (a). An anterior plasmacytoma and deposition in the spleen. An anterior whole body scintigraphic image from a patient with a solitary intrathoracic amyloidoma (b) with corresponding SPECT-CT (c)
a |
b |
c
amyloidosis, but, in advanced disease, it commonly shows small voltages, pathological ‘Q’ waves (a pseudo-infarct pattern) in the anterior chest leads and conduction abnormalities. CMR is extremely useful in identifying cardiac amyloidosis. Typical appearances are of homogeneous late gadolinium enhancement [32]. 99mTc-3,3-diphosphono-1,2- propanodicarboxylic acid (99mTc-DPD) scintigraphy is a speci c test indicative of bril deposition within the heart, with data suggesting that the degree of uptake and pattern of distribution in the extracardiac soft tissue may be speci c for amyloid transthyretin (ATTR) amyloidosis [33, 34].
Elevation of N-terminal pro-brain natriuretic peptide (NT-Pro-BNP) and cardiac troponins can also be helpful in establishing whether a patient has cardiac amyloidosis [35, 36]. These enzymes are not speci c and can be elevated for other reasons such as renal impairment and other forms of cardiomyopathy; however, a normal NT-Pro-BNP can exclude cardiac involvement [37].
The key to effective monitoring of amyloidosis and its treatment is relatively frequent repetition of these investigations, bearing in mind that organ dysfunction may not closely refect amyloid load.
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
