
- •Preface to the First Edition
- •Preface to the Second Edition
- •Contents
- •Diagnostic Challenges
- •Expert Centers
- •Patient Organizations
- •Clinical Trials
- •Research in Orphan Lung Diseases
- •Orphan Drugs
- •Orphanet
- •Empowerment of Patients
- •Conclusions
- •References
- •Introduction
- •Challenges to Overcome in Order to Undertake Quality Clinical Research
- •Lack of Reliable Data on Prevalence
- •Small Number of Patients
- •Identifying Causation/Disease Pathogenesis
- •Disease Complexity
- •Lack of Access to a Correct Diagnosis
- •Delay in Diagnosis
- •Challenges But Not Negativity
- •Some Success Stories
- •The Means to Overcome the Challenges of Clinical Research: Get Bigger Numbers of Well-Characterized Patients
- •The Importance of Patient Organizations
- •National and International Networks
- •End Points for Trials: Getting Them Right When Numbers Are Small and Change Is Modest
- •Orphan Drug Development
- •Importance of Referral Centers
- •Looking at the Future
- •The Arguments for Progress
- •Concluding Remarks
- •References
- •3: Chronic Bronchiolitis in Adults
- •Introduction
- •Cellular Bronchiolitis
- •Follicular Bronchiolitis
- •Respiratory Bronchiolitis
- •Airway-Centered Interstitial Fibrosis
- •Proliferative Bronchiolitis
- •Diagnosis
- •Chest Imaging Studies
- •Pulmonary Function Testing
- •Lung Biopsy
- •Mineral Dusts
- •Organic Dusts
- •Volatile Flavoring Agents
- •Infectious Causes of Bronchiolitis
- •Idiopathic Forms of Bronchiolitis
- •Connective Tissue Diseases
- •Organ Transplantation
- •Hematopoietic Stem Cell Transplantation
- •Drug-Induced Bronchiolitis
- •Treatment
- •Constrictive Bronchiolitis
- •Follicular Bronchiolitis
- •Airway-Centered Interstitial Fibrosis
- •Proliferative Bronchiolitis
- •References
- •Background and Epidemiology
- •Pathophysiology
- •Host Characteristics
- •Clinical Manifestations
- •Symptoms
- •Laboratory Evaluation
- •Skin Testing
- •Serum Precipitins
- •Eosinophil Count
- •Total Serum Immunoglobulin E Levels
- •Recombinant Antigens
- •Radiographic Imaging
- •Pulmonary Function Testing
- •Histology
- •Diagnostic Criteria
- •Historical Diagnostic Criteria
- •Rosenberg and Patterson Diagnostic Criteria
- •ISHAM Diagnostic Criteria
- •Cystic Fibrosis Foundation Diagnostic Criteria
- •General Diagnostic Recommendations
- •Allergic Aspergillus Sinusitis (AAS)
- •Natural History
- •Treatment
- •Corticosteroids
- •Antifungal Therapy
- •Monoclonal Antibodies
- •Monitoring for Treatment Response
- •Conclusions
- •References
- •5: Orphan Tracheopathies
- •Introduction
- •Anatomical Considerations
- •Clinical Presentation
- •Etiological Considerations
- •Idiopathic Subglottic Stenosis
- •Introduction
- •Clinical Features
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Introduction and Clinical Presentation
- •Clinical Features
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Tracheomalacia
- •Introduction
- •Clinical Features
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Tracheobronchomegaly
- •Introduction
- •Clinical Features
- •Pathophysiology
- •Pulmonary Function Studies
- •Imaging Studies
- •Treatment
- •Tracheopathies Associated with Systemic Diseases
- •Relapsing Polychondritis
- •Introduction
- •Clinical Features
- •Laboratory Findings
- •Pulmonary Function and Imaging Studies
- •Treatment
- •Introduction
- •Clinical Features
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Tracheobronchial Amyloidosis
- •Introduction
- •Clinical Features
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Sarcoidosis
- •Introduction
- •Pulmonary Function Studies
- •Imaging Studies
- •Bronchoscopy
- •Treatment
- •Orphan Tracheopathies: Conclusions
- •References
- •6: Amyloidosis and the Lungs and Airways
- •Introduction
- •Diagnosis and Evaluation of Amyloidosis
- •Systemic AA Amyloidosis
- •Systemic AL Amyloidosis
- •Amyloidosis Localised to the Respiratory Tract
- •Laryngeal Amyloidosis
- •Tracheobronchial Amyloidosis
- •Parenchymal Pulmonary Amyloidosis
- •Pulmonary Amyloidosis Associated with Sjögren’s Disease
- •Conclusions
- •References
- •Introduction
- •Pathophysiology
- •Genetic Predisposition
- •Immune Dysregulation
- •Epidemiology
- •Incidence and Prevalence
- •Triggering Factors
- •Clinical Manifestations
- •General Symptoms
- •Pulmonary Manifestations
- •Ear, Nose, and Throat (ENT) Manifestations
- •Neurological Manifestations
- •Skin Manifestations
- •Cardiac Manifestations
- •Gastrointestinal Involvement
- •Renal Manifestations
- •Ophthalmological Manifestations
- •Complementary Investigations
- •Diagnosis
- •Diagnostic Criteria
- •Prognosis and Outcomes
- •Phenotypes According to the ANCA Status
- •Treatment
- •Therapeutic Strategies
- •Remission Induction
- •Maintenance Therapy
- •Other Treatments
- •Prevention of AEs
- •Conclusions
- •References
- •8: Granulomatosis with Polyangiitis
- •A Brief Historical Overview
- •Epidemiology
- •Pathogenesis
- •Clinical Manifestations
- •Constitutional Symptoms
- •Ear, Nose, and Throat (ENT) Manifestations
- •Pulmonary Manifestations
- •Kidney and Urological Manifestations
- •Kidney Manifestations
- •Urological Manifestations
- •Neurological Manifestations
- •Peripheral Nervous System (PNS) Manifestations
- •Central Nervous System (CNS) Manifestations
- •Spinal Cord and Cranial Nerve Involvement
- •Skin and Oral Mucosal Manifestations
- •Eye Manifestations
- •Cardiac Involvement
- •Gastrointestinal Manifestations
- •Gynecological and Obstetric Manifestations
- •Venous Thrombosis and Other Vascular Events
- •Other Manifestations
- •Pediatric GPA
- •Diagnosis
- •Diagnostic Approach
- •Laboratory Investigations
- •Biology
- •Immunology
- •Pathology
- •Treatment
- •Glucocorticoids
- •Cyclophosphamide
- •Rituximab
- •Other Current Induction Approaches
- •Other Treatments in GPA
- •Intravenous Immunoglobulins
- •Plasma Exchange
- •CTLA4-Ig (Abatacept)
- •Cotrimoxazole
- •Other Agents
- •Principles of Treatment for Relapsing and Refractory GPA
- •Outcomes and Prognostic Factors
- •Survival and Causes of Deaths
- •Relapse
- •Damage and Disease Burden on Quality of Life
- •Conclusions
- •References
- •9: Alveolar Hemorrhage
- •Introduction
- •Clinical Presentation
- •Diagnosis (Table 9.1, Fig. 9.3)
- •Pulmonary Capillaritis
- •Histology (Fig. 9.4)
- •Etiologies
- •ANCA-Associated Small Vessel Vasculitis: Granulomatosis with Polyangiitis (GPA)
- •ANCA-Associated Small Vessel Vasculitis: Microscopic Polyangiitis
- •Isolated Pulmonary Capillaritis
- •Systemic Lupus Erythematosus
- •Antiphospholipid Antibody Syndrome
- •Anti-Basement Membrane Antibody Disease (Goodpasture Syndrome)
- •Lung Allograft Rejection
- •Others
- •Bland Pulmonary Hemorrhage (Fig. 9.5)
- •Histology
- •Etiologies
- •Idiopathic Pulmonary Hemosiderosis
- •Drugs and Medications
- •Coagulopathy
- •Valvular Heart Disease and Left Ventricular Dysfunction
- •Other
- •Histology
- •Etiologies
- •Hematopoietic Stem Cell Transplantation (HSCT)
- •Cocaine Inhalation
- •Acute Exacerbation of Interstitial Lung Disease
- •Acute Interstitial Pneumonia
- •Acute Respiratory Distress Syndrome
- •Miscellaneous Causes
- •Etiologies
- •Pulmonary Capillary Hemangiomatosis
- •Treatment
- •Conclusions
- •References
- •Takayasu Arteritis
- •Epidemiology
- •Pathologic Features
- •Pathogenesis
- •Clinical Features
- •Laboratory Findings
- •Imaging Studies
- •Therapeutic Management
- •Prognosis
- •Behçet’s Disease
- •Epidemiology
- •Pathologic Features
- •Pathogenesis
- •Diagnostic Criteria
- •Clinical Features
- •Pulmonary Artery Aneurysm
- •Pulmonary Artery Thrombosis
- •Pulmonary Parenchymal Involvement
- •Laboratory Findings
- •Imaging Studies
- •Therapeutic Management
- •Treatment of PAA
- •Treatment of PAT
- •Prognosis
- •References
- •Introduction
- •Portopulmonary Hypertension (PoPH)
- •Epidemiology and Risk Factors
- •Molecular Pathogenesis
- •PoPH Treatment
- •Hepatopulmonary Syndrome (HPS)
- •Epidemiology and Risk Factors
- •Molecular Pathogenesis
- •HPS Treatment
- •Conclusion
- •References
- •12: Systemic Sclerosis and the Lung
- •Introduction
- •Risk factors for SSc-ILD
- •Genetic Associations
- •Clinical Presentation of SSc-ILD
- •Pulmonary Function Tests (PFTs)
- •Imaging
- •Management
- •References
- •13: Rheumatoid Arthritis and the Lungs
- •Introduction
- •Epidemiology
- •Risk Factors for ILD (Table 13.3)
- •Pathogenesis
- •Clinical Features and Diagnosis
- •Treatments
- •Prognosis
- •Epidemiology
- •Risk Factors
- •Clinical Features, Diagnosis, and Outcome
- •Subtypes or RA-AD
- •Obliterative Bronchiolitis
- •Bronchiectasis
- •COPD
- •Cricoarytenoid Involvement
- •Pleural Disease
- •Conclusion
- •References
- •Introduction
- •Systemic Lupus Erythematosus
- •Epidemiology
- •Pathophysiology
- •Pulmonary Manifestations
- •Pleural Disease
- •Shrinking Lung Syndrome
- •Thrombotic Manifestations
- •Interstitial Lung Disease
- •Other Pulmonary Manifestations
- •Prognosis
- •Sjögren’s Syndrome
- •Epidemiology
- •Pathophysiology
- •Pulmonary Manifestations
- •Airway Disorders
- •Lymphoproliferative Disease
- •Interstitial Lung Disease
- •Prognosis
- •Mixed Connective Tissue Disease
- •Epidemiology
- •Pathophysiology
- •Pulmonary Manifestations
- •Pulmonary Hypertension
- •Interstitial Lung Disease
- •Prognosis
- •Myositis
- •Epidemiology
- •Pathophysiology
- •Pulmonary Manifestations and Treatments
- •Interstitial Lung Disease
- •Respiratory Muscle Weakness
- •Other Pulmonary Manifestations
- •Prognosis
- •Other Therapeutic Options in CTD-ILD
- •Lung Transplantation
- •Conclusion
- •References
- •Introduction
- •Diagnostic Criteria
- •Controversies in the Diagnostic Criteria
- •Typical Clinical Features
- •Disease Progression and Prognosis
- •Summary
- •References
- •Introduction
- •Histiocytes and Dendritic Cells
- •Introduction
- •Cellular and Molecular Pathogenesis
- •Pathology
- •Clinical Presentation
- •Treatment and Prognosis
- •Erdheim-Chester Disease
- •Epidemiology
- •Cellular and Molecular Pathogenesis
- •Histopathology and Immunohistochemistry
- •Clinical Presentation
- •Investigation/Diagnosis
- •Chest Studies
- •Cardiovascular Imaging
- •CNS Imaging
- •Bone Radiography
- •Other Imaging Findings and Considerations
- •Disease Monitoring
- •Pathology
- •Management/Treatment
- •Prognosis
- •Rosai-Dorfman Destombes Disease
- •Epidemiology
- •Etiology/Pathophysiology
- •Histopathology and Immunohistochemistry
- •Clinical Presentation
- •Investigation/Diagnosis
- •Management/Treatment
- •Prognosis
- •Conclusions
- •Diagnostic Criteria for Primary Histiocytic Disorders of the Lung
- •References
- •17: Eosinophilic Pneumonia
- •Introduction
- •Eosinophil Biology
- •Physiologic and Immunologic Role of Eosinophils
- •Release of Mediators
- •Targeting the Eosinophil Cell Lineage
- •Historical Perspective
- •Clinical Presentation
- •Pathology
- •Diagnosis
- •Eosinophilic Lung Disease of Undetermined Cause
- •Idiopathic Chronic Eosinophilic Pneumonia
- •Clinical Features
- •Imaging
- •Laboratory Studies
- •Bronchoalveolar Lavage
- •Lung Function Tests
- •Treatment
- •Outcome and Perspectives
- •Clinical Features
- •Imaging
- •Laboratory Studies
- •Bronchoalveolar Lavage
- •Lung Function Tests
- •Lung Biopsy
- •Treatment and Prognosis
- •Eosinophilic Granulomatosis with Polyangiitis
- •History and Nomenclature
- •Pathology
- •Clinical Features
- •Imaging
- •Laboratory Studies
- •Pathogenesis
- •Diagnosis
- •Treatment and Prognosis
- •Long-Term Outcome
- •Hypereosinophilic Syndrome
- •Pathogenesis
- •Clinical and Imaging Features
- •Laboratory Studies
- •Treatment and Prognosis
- •Eosinophilic Pneumonias of Parasitic Origin
- •Tropical Eosinophilia [191]
- •Ascaris Pneumonia
- •Eosinophilic Pneumonia in Larva Migrans Syndrome
- •Strongyloides Stercoralis Infection
- •Eosinophilic Pneumonias in Other Infections
- •Allergic Bronchopulmonary Aspergillosis
- •Pathogenesis
- •Diagnostic Criteria
- •Biology
- •Imaging
- •Treatment
- •Bronchocentric Granulomatosis
- •Miscellaneous Lung Diseases with Associated Eosinophilia
- •References
- •Introduction
- •Pulmonary Langerhans’ Cell Histiocytosis
- •Epidemiology
- •Pathogenesis
- •Diagnosis
- •Clinical Features
- •Extrathoracic Lesions
- •Pulmonary Function Tests
- •Chest Radiography
- •High-Resolution Computed Tomography (HRCT)
- •Bronchoscopy and Bronchoalveolar Lavage (BAL)
- •Lung Biopsy
- •Pathology
- •Treatment
- •Course and Prognosis
- •Case Report I
- •Introduction
- •Epidemiology
- •Clinical Features
- •Histopathological Findings
- •Radiologic Findings
- •Prognosis and Therapy
- •Desquamative Interstitial Pneumonia
- •Epidemiologic and Clinical Features
- •Histopathological Findings
- •Radiological Findings
- •Prognosis and Therapy
- •Conclusion
- •References
- •19: Lymphangioleiomyomatosis
- •Introduction
- •Pathogenesis
- •Presentation
- •Prognosis
- •Management
- •General Measures
- •Parenchymal Lung Disease
- •Pleural Disease
- •Renal Angiomyolipoma
- •Abdominopelvic Lymphatic Disease
- •Pregnancy
- •Tuberous Sclerosis
- •Drug Treatment
- •Bronchodilators
- •mTOR Inhibitors
- •Anti-Oestrogen Therapy
- •Experimental Therapies
- •Interventions for Advanced Disease
- •Oxygen Therapy
- •Pulmonary Hypertension
- •References
- •20: Diffuse Cystic Lung Disease
- •Introduction
- •Lymphangioleiomyomatosis
- •Pathogenesis
- •Pathologic and Radiographic Characteristics
- •Diagnostic Approach
- •Pulmonary Langerhans Cell Histiocytosis (PLCH)
- •Pathogenesis
- •Pathological and Radiographic Characteristics
- •Diagnostic Approach
- •Birt-Hogg-Dubé Syndrome (BHD)
- •Pathogenesis
- •Pathological and Radiographic Characteristics
- •Diagnostic Approach
- •Lymphoproliferative Disorders
- •Pathogenesis
- •Pathological and Radiographic Characteristics
- •Diagnostic Approach
- •Amyloidosis
- •Light Chain Deposition Disease (LCDD)
- •Conclusion
- •References
- •Introduction
- •Lymphatic Development
- •Clinical Presentation of Lymphatic Disorders
- •Approaches to Diagnosis and Management of Congenital Lymphatic Anomalies
- •Generalized Lymphatic Anomaly
- •Etiopathogenesis
- •Clinical Presentation and Diagnosis
- •Course/Prognosis
- •Management
- •Kaposiform Lymphangiomatosis
- •Etiopathogenesis
- •Clinical Presentation and Diagnosis
- •Management
- •Course/Prognosis
- •Gorham Stout Disease
- •Etiopathogenesis
- •Clinical Presentation and Diagnosis
- •Management
- •Course/Prognosis
- •Channel-Type LM/Central Conducting LM
- •Etiopathogenesis
- •Clinical Presentation and Diagnosis
- •Management
- •Course/Prognosis
- •Yellow Nail Syndrome
- •Etiopathogenesis
- •Clinical Presentation and Diagnosis
- •Management
- •Course/Prognosis
- •Summary
- •References
- •Introduction
- •Historical Note
- •Epidemiology
- •Pathogenesis
- •Surfactant Homeostasis in PAP
- •GM-CSF Signaling Disruption
- •Myeloid Cell Dysfunction
- •GM-CSF Autoantibodies
- •Lymphocytosis
- •Clinical Manifestations
- •Clinical Presentation
- •Secondary Infections
- •Pulmonary Fibrosis
- •Diagnosis
- •Pulmonary Function Testing
- •Radiographic Assessment
- •Bronchoscopy and Bronchoalveolar Lavage
- •Laboratory Studies and Biomarkers
- •GM-CSF Autoantibodies
- •Genetic Testing
- •Lung Pathology
- •Diagnostic Approach to the Patient with PAP
- •Natural History and Prognosis
- •Treatment
- •Whole-Lung Lavage
- •Subcutaneous GM-CSF
- •Inhaled GM-CSF
- •Other Approaches
- •Conclusions and Future Directions
- •References
- •Introduction
- •Epidemiology
- •Gastric Contents
- •Pathobiology of GER/Microaspirate in the Lungs of Patients with IPF
- •GER and the Microbiome
- •Diagnosis
- •Clinical History/Physical Exam
- •Investigations
- •Esophageal Physiology
- •Upper Esophageal Sphincter
- •Esophagus and Peristalsis
- •Lower Esophageal Sphincter and Diaphragm
- •Esophageal pH and Impedance Testing
- •High Resolution Esophageal Manometry
- •Esophagram/Barium Swallow
- •Bronchoalveolar Lavage/Sputum: Biomarkers
- •Treatment
- •Anti-Acid Therapy (PPI/H2 Blocker)
- •GER and Acute Exacerbations of IPF
- •Suggested Approach
- •Summary and Future Directions
- •References
- •Introduction
- •Familial Interstitial Pneumonia
- •Telomere Related Genes
- •Genetic
- •Telomere Length
- •Pulmonary Involvement
- •Interstitial Lung Disease
- •Other Lung Disease
- •Hepatopulmonary Syndrome
- •Emphysema
- •Extrapulmonary Manifestations
- •Mucocutaneous Involvement
- •Hematological Involvement
- •Liver Involvement
- •Other Manifestations
- •Treatment
- •Telomerase Complex Agonists
- •Lung Transplantation
- •Surfactant Pathway
- •Surfactant Protein Genes
- •Pulmonary Involvement
- •Treatment
- •Heritable Forms of Pulmonary Fibrosis with Autoimmune Features
- •TMEM173
- •COPA
- •Pulmonary Alveolar Proteinosis
- •GMCSF Receptor Mutations
- •GATA2
- •MARS
- •Lysinuric Protein Intolerance
- •Lysosomal Diseases
- •Hermansky-Pudlak Syndrome
- •Lysosomal Storage Disorders
- •FAM111B, NDUFAF6, PEPD
- •Conclusion
- •References
- •Introduction
- •Pathophysiology
- •Clinical Presentation
- •Epidemiology
- •Genetic Causes of Bronchiectasis
- •Disorders of Mucociliary Clearance
- •Cystic Fibrosis
- •Primary Ciliary Dyskinesia
- •Other Ciliopathies
- •X-Linked Agammaglobulinemia
- •Chronic Granulomatous Disease and Other Disorders of Neutrophil Function
- •Other Genetic Disorders Predisposing to Bronchiectasis
- •Idiopathic Bronchiectasis
- •Diagnosis of Bronchiectasis
- •Management of Patients with Bronchiectasis
- •Airway Clearance Therapy (ACT)
- •Management of Infections
- •Immune Therapy
- •Surgery
- •Novel Therapies for Managing Cystic Fibrosis
- •Summary
- •References
- •Pulmonary Arteriovenous Malformations
- •Background Pulmonary AVMs
- •Anatomy Pulmonary AVMs
- •Clinical Presentation of Pulmonary AVMs
- •Screening Pulmonary AVMs
- •Treatment Pulmonary AVMs
- •Children with Hereditary Hemorrhagic Telangiectasia
- •Pulmonary Hypertension
- •Pulmonary Hypertension Secondary to Liver Vascular Malformations
- •Pulmonary Arterial Hypertension
- •Background HHT
- •Pathogenesis
- •References
- •27: Pulmonary Alveolar Microlithiasis
- •Introduction
- •Epidemiology
- •Pathogenesis
- •Clinical Features
- •Diagnosis
- •Management
- •Summary
- •References
- •Introduction
- •Hermansky-Pudlak Syndrome
- •Telomerase-Associated Pulmonary Fibrosis
- •Lysosomal Storage Diseases
- •Lysinuric Protein Intolerance
- •Familial Hypocalciuric Hypercalcemia
- •Surfactant Dysfunction Disorders
- •Concluding Remarks
- •References
- •Introduction
- •Background
- •Image Acquisition
- •Key Features of Fibrosis
- •Ancillary Features of Fibrosis
- •Other Imaging Findings in FLD
- •Probable UIP-IPF
- •Indeterminate
- •Alternative Diagnosis
- •UIP in Other Fibrosing Lung Diseases
- •Pleuroparenchymal Fibroelastosis (PPFE)
- •Combined Pulmonary Fibrosis and Emphysema
- •Chronic Hypersensitivity Pneumonitis
- •Other Fibrosing Lung Diseases
- •Fibrosing Sarcoidosis
- •CTD-ILD and Drug-Induced FLD
- •Complications
- •Prognosis
- •Computer Analysis of CT Imaging
- •The Progressive Fibrotic Phenotype
- •Other Imaging Techniques
- •Conclusion
- •References
- •Introduction
- •Bronchoalveolar Lavage (BAL)
- •Technique
- •Interpretation
- •Transbronchial Biopsy (TBB)
- •Transbronchial Lung Cryobiopsy (TLCB)
- •References
- •Introduction
- •Overview of ILD Diagnosis
- •Clinical Assessment
- •Radiological Assessment
- •Laboratory Assessment
- •Integration of Individual Features
- •Multidisciplinary Discussion
- •Diagnostic Ontology
- •Conclusions
- •References
- •Introduction
- •Idiopathic Pulmonary Fibrosis
- •Chronic Hypersensitivity Pneumonitis
- •Connective Tissue Disease
- •Drug-Induced Lung Diseases
- •Radiation Pneumonitis
- •Asbestosis
- •Hermansky-Pudlak Syndrome
- •Risk Factors for Progression
- •Diagnosis
- •Pharmacological Management
- •Conclusions
- •References
- •Historical Perspective
- •Epidemiology and Etiologies
- •Tobacco Smoking and Male Sex
- •Genetic Predisposition
- •Systemic Diseases
- •Other Etiological Contexts
- •Clinical Manifestations
- •Pulmonary Function and Physiology
- •Imaging
- •Computed Tomography Characteristics and Patterns
- •Thick-Walled Large Cysts
- •Imaging Phenotypes
- •Pitfalls
- •Pathology
- •Diagnosis
- •CPFE Is a Syndrome
- •Biology
- •Complications and Outcome
- •Mortality
- •Pulmonary Hypertension
- •Lung Cancer
- •Acute Exacerbation of Pulmonary Fibrosis
- •Other Comorbidities and Complications
- •Management
- •General Measures and Treatment of Emphysema
- •Treatment of Pulmonary Fibrosis
- •Management of Pulmonary Hypertension
- •References
- •Acute Interstitial Pneumonia (AIP)
- •Epidemiology
- •Presentation
- •Diagnostic Evaluation
- •Radiology
- •Histopathology
- •Clinical Course
- •Treatment
- •Epidemiology
- •Presentation
- •Diagnostic Evaluation
- •Radiology
- •Histopathology
- •Clinical Course
- •Desquamative Interstitial Pneumonia (DIP)
- •Presentation
- •Diagnostic Evaluation
- •Radiology
- •Histopathology
- •Clinical Course
- •Treatment
- •Epidemiology
- •Presentation
- •Diagnostic Evaluation
- •Radiology
- •Histopathology
- •Clinical Course
- •Treatment
- •References
- •Organizing Pneumonias
- •Epidemiology
- •Pathogenesis
- •Clinical Features
- •Imaging
- •Multifocal Form
- •Isolated Nodular Form
- •Other Imaging Patterns
- •Histopathological Diagnosis of OP Pattern
- •Etiological Diagnosis of OP
- •Treatment
- •Clinical Course and Outcome
- •Severe Forms of OP with Respiratory Failure
- •Acute Fibrinous and Organizing Pneumonia
- •Granulomatous Organizing Pneumonia
- •Acute Interstitial Pneumonia
- •Epidemiology
- •Clinical Picture
- •Imaging
- •Histopathology
- •Diagnosis
- •Treatment
- •Outcome
- •References
- •36: Pleuroparenchymal Fibroelastosis
- •Introduction
- •Epidemiology
- •Clinical Manifestations
- •Laboratory Findings
- •Respiratory Function
- •Radiologic Features
- •Pathologic Features
- •Diagnosis
- •Treatment
- •Prognosis
- •Conclusions
- •References
- •Introduction
- •Acute Berylliosis
- •Chronic Beryllium Disease
- •Exposure
- •Epidemiology
- •Immunopathogenesis and Pathology
- •Genetics
- •Clinical Description and Natural History
- •Treatment and Monitoring
- •Indium–Tin Oxide-Lung Disease
- •Hard Metal Lung
- •Flock Worker’s Disease
- •Asbestosis
- •Nanoparticle Induced ILD
- •Flavoring-Induced Lung Disease
- •Silica-Induced Interstitial Lung Disease
- •Chronic Silicosis
- •Acute and Accelerated Silicosis
- •Chronic Obstructive Disease in CMDLD
- •Simple CMDLD
- •Complicated CMDLD
- •Conclusion
- •References
- •38: Unclassifiable Interstitial Lung Disease
- •Introduction
- •Diagnostic Scenarios
- •Epidemiology
- •Clinical Presentation
- •Diagnosis
- •Clinical Features
- •Radiology
- •Laboratory Investigations
- •Pathology
- •Conclusion
- •References
- •39: Lymphoproliferative Lung Disorders
- •Introduction
- •Nodular Lymphoid Hyperplasia
- •Lymphocytic Interstitial Pneumonia (LIP)
- •Follicular Bronchitis/Bronchiolitis
- •Castleman Disease
- •Primary Pulmonary Lymphomas
- •Primary Pulmonary MALT B Cell Lymphoma
- •Pulmonary Plasmacytoma
- •Follicular Lymphoma
- •Lymphomatoid Granulomatosis
- •Primary Pulmonary Hodgkin Lymphoma (PPHL)
- •Treatment
- •References
- •Introduction
- •Late-Onset Pulmonary Complications
- •Bronchiolitis Obliterans (BO)
- •Pathophysiology
- •Diagnosis
- •Management of BOS
- •Post-HSCT Organizing Pneumonia
- •Other Late-Onset NonInfectious Pulmonary Complications (LONIPCs)
- •Conclusion
- •References
- •Introduction
- •Pulmonary Hypertension Associated with Sarcoidosis (Group 5.2)
- •PH Associated with Pulmonary Langerhans Cell Histiocytosis (Group 5.2)
- •PH in Combined Pulmonary Fibrosis and Emphysema (Group 3.3)
- •PH Associated with Lymphangioleiomyomatosis (Group 3)
- •Hereditary Hemorrhagic Telangiectasia (Group 1.2)
- •Pulmonary Veno-Occlusive Disease (Group 1.5)
- •Small Patella Syndrome (Group 1.2)
- •Conclusion
- •References
- •Introduction
- •Epidemiology
- •Timing, Chronology, Delay Time
- •Route of Administration
- •Patterns of Involvement [3, 4]
- •Drugs and Agents Fallen Out of Favor
- •Drug-Induced Noncardiac Pulmonary Edema
- •Drug-Induced Cardiogenic Pulmonary Edema
- •The “Chemotherapy Lung”
- •Drug-Induced/Iatrogenic Alveolar Hemorrhage
- •Drugs
- •Superwarfarin Rodenticides
- •Transfusion Reactions: TACO–TRALI
- •Acute Eosinophilic Pneumonia
- •Acute Granulomatous Interstitial Lung Disease
- •Acute Organizing Pneumonia (OP), Bronchiolitis Obliterans Organizing Pneumonia (BOOP), or Acute Fibrinous Organizing Pneumonia (AFOP) Patterns
- •Acute Amiodarone-Induced Pulmonary Toxicity (AIPT)
- •Accelerated Pulmonary Fibrosis
- •Acute Exacerbation of Previously Known (Idiopathic) Pulmonary Fibrosis
- •Anaphylaxis
- •Acute Vasculopathy
- •Drug-Induced/Iatrogenic Airway Emergencies
- •Airway Obstruction as a Manifestation of Anaphylaxis
- •Drug-Induced Angioedema
- •Hematoma Around the Upper Airway
- •The “Pill Aspiration Syndrome”
- •Catastrophic Drug-Induced Bronchospasm
- •Peri-operative Emergencies (Table 42.8)
- •Other Rare Presentations
- •Pulmonary Nodules and Masses
- •Pleuroparenchymal Fibroelastosis
- •Late Radiation-Induced Injury
- •Chest Pain
- •Rebound Phenomenon
- •Recall Pneumonitis
- •Thoracic Bezoars: Gossipybomas
- •Respiratory Diseases Considered Idiopathic That May Be Drug-Induced (Table 42.4)
- •Eye Catchers
- •Conclusion
- •References
- •Cancer Mimics of Organizing Pneumonia
- •Lung Adenocarcinoma/Bronchioloalveolar Carcinoma
- •Primary Pulmonary Lymphoma
- •Cancer Mimics of Interstitial Lung Diseases
- •Lymphangitic Carcinomatosis
- •Epithelioid Hemangio-Endothelioma
- •Lymphomatoid Granulomatosis
- •Cystic Tumors
- •Cavitating Tumors
- •Intrathoracic Pseudotumors
- •Respiratory Papillomatosis
- •Pulmonary Langerhans Cell Histiocytosis
- •References
- •Index
518 |
T. M. Jacob et al. |
|
|
Complications
In patients with interstitial lung disease, there are several important complications to consider during the assessment of cross-sectional imaging. These include the presence of pulmonary hypertension, lung cancer or evidence of an acute exacerbation of ILD.
Pulmonary hypertension can be suggested on CT by observing a pulmonary artery with a diameter greater than 29 mm [59], straightening or leftward bowing of the interventricular septum of the heart and right ventricular dilatation. The association between main pulmonary artery diameter and the likelihood of pulmonary hypertension has been shown to be maintained in patients with and without lung brosis [60]. Pulmonary hypertension is not an uncommon nding duringbrosing lung disease assessment, with a higher prevalence associated with IPF [61] and CTD-ILD [62].
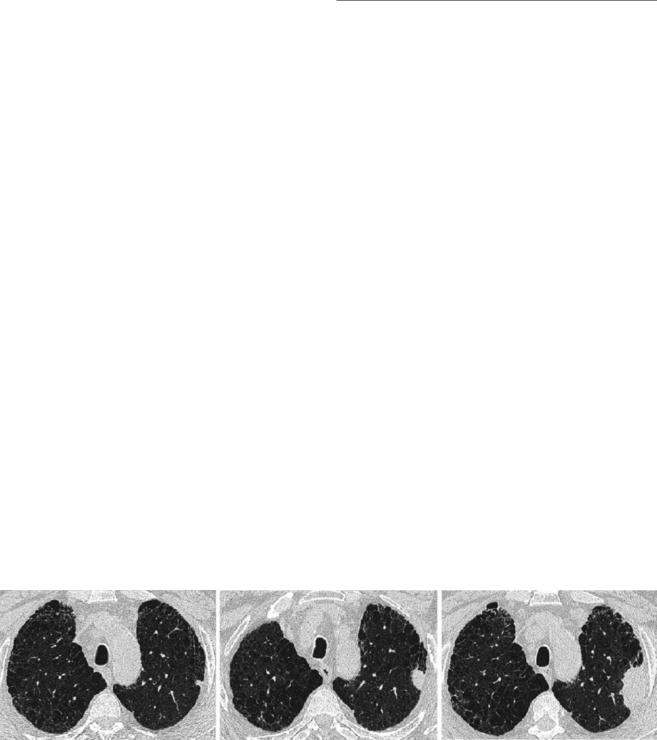
There is an increased risk of lung cancer in patients with ILD, linked to the presence of brosis itself, but also related to the existence of common risk factors, such as smoking and occupational exposures [63]. When lung cancer develops, it is often a solid lesion found peripherally within an area ofbrosis and commonly within the lower lobes [64]. Therefore, the presence of a new nodule on a CT scan requires careful scrutiny and work up (Fig. 29.14).
Although ILDs are typically chronic conditions they can also undergo acute phases of deterioration. Whilst accelerated decline may be secondary to an infection, cardiac failure or pulmonary embolism [65], an acute exacerbation should always be considered. An acute exacerbation typically presents with new bilateral ground glass opacities with or without consolidation which may be peripheral, multifocal or diffuse and has no identi able cause [66]. Acute exacerbations have an incidence of 4–20% [67] per year and have a dismal prognosis with a median survival of 3 months [68]. The presence of an acute clinical deterioration should also
raise the possibility of a complicating pneumothorax or pneumomediastinum.
Prognosis
As well as its utility in formulating a diagnosis, baseline HRCT interpretation can also aid in predicting a patient’s likely prognosis. This is exempli ed in the importance given to the identi cation of a UIP pattern of disease on CT which essentially signi es disease that has a poor outcome. Yet even within a UIP pattern of disease, visual CT scoring can re ne prognostic likelihoods. The combined extents of honeycombing and reticulation have been shown to independently predict mortality in patients with IPF [13]. Honeycombing extent and severity of traction bronchiectasis have also been reported as independent predictors of mortality in both CHP [69] and CTD-ILD [70]. When patients with a variety of brosing lung diseases were examined together, it was found that across the range of aetiologies, the total extent of brosis indicated a poor prognosis [71].
More challenging for visual evaluation is the identi cation of disease worsening on serial CT imaging. Change in ILD extent is the metric most commonly used to determine disease worsening. Yet when brosis occurs in the lungs, damaged parenchyma contracts and reduces in volume, and nonbrotic regions of the lung can hyperexpand to compensate for damage elsewhere. Therefore, when brotic disease increases in extent, as more lung contracts and spared regions hyperexpand, the degree of disease worsening can be underestimated on serial imaging. A consequence is that below a certain threshold of change, it may not be easy to visually quantify an increase in ILD extent on serial CTs (Fig. 29.15).
A further challenge in detecting disease worsening on serial CT imaging involves distinguishing the progression of
a |
b |
c |
Fig. 29.14 Patients with brosing lung diseases, and in particular the subgroup that has a history of smoking have an increased incidence of lung cancer. Careful scrutiny of imaging is necessary to identify new lung nodules that may represent lung cancer. The three axial images show serial computed tomography scans in a patient diagnosed with
idiopathic pulmonary brosis. The nodule in the left upper lobe on therst scan (a) was identi ed but the patient was not medically t for surgery. On follow-up imaging 16 months later (b), the nodule has grown in size signi cantly. When imaged a further 8 months later (c) the nodule has become a large mass

29 Imaging Approach to Interstitial Lung Disease |
|
519 |
|
|
|
a |
b |
c |
d |
e |
f |
Fig. 29.15 Serial computed tomography scans were performed 12 months apart in a patient diagnosed with idiopathic pulmonary brosis. On the initial scan (a–c) there is brosis with reticulation and trac-
tion bronchiectasis visible in the middle and lower zones. At the second time point scan (d–f) it is challenging to distinguish disease maturation from disease progression using visual computed tomography analysis
disease from the maturation of disease. Maturation describes changes in the appearance of a region of brosis as it evolves over time following the reparative processes of the body. Disease maturation may not result in worsening lung damage even though the CT appearances change over time. Disease progression however implies brotic or infammatory involvement of new areas of the lung which may be incorporated into pre-existing regions of damage. Should previously normal lung on the edges of the brotic lung become damaged, maturation and contraction of areas of long-standing damage may result in the entire volume of the involved lung in a region appearing grossly unchanged. In such situations, identifying the encroachment of damage towards adjacent structures (such as vessels) may be an alternative indicator of disease progression (Fig. 29.15).
Computer Analysis of CT Imaging
Quantitative CT (QCT) describes the many computer-based CT image analysis methods developed to measure changes in lung structure in patients with ILD. Most QCT methods employ density or texture-based analysis of varying complexity and offer improved objectivity, speed, reproducibility and scalability compared to visual CT scoring. QCT-derived metrics show potential as prognostic imaging biomarkers with reported utility in the assessment of disease severity at baseline and disease progression on serial CTs.
By simulating human visual perceptual and learning processes, texture-based QCT algorithms can describe CT pat-
terns, previously exclusively within the domain of radiologists [72]. An example of QCT is the Computer Aided Lung Informatics for Pathology Evaluation and Rating (CALIPER) tool developed by the Biomedical Imaging Resource at the Mayo Clinic Rochester, Minnesota, USA. CALIPER characterises HRCT data using morphological and 3D histogram features, enabling voxel volumes to be labelled according to a conventional radiological lexicon: normal lung, ground glass opacity (GGO), reticulation, subtypes of low-attenuation and honeycombing [73]. CALIPER variables were proven more accurate in predicting survival than equivalent visual CT scores, with CALIPER honeycombing extent capable of independently predicting mortality (hazard ratio 1.18; p = 0.002) [74].
A unique attribute of CALIPER is the ability to quantify vessel-related structures. The vessel-related structure corresponds to pulmonary vessels (arteries and veins) and associated structures, for example, perivascular brosis which has no visually scored equivalent. CALIPER vessel-related structure was an independent predictor of mortality in IPF (hazard ratio 1.53; p < 0.0001) and superior to traditional visual CT scores [74]. In the future, vessel-related structures and other emerging, novel QCT imaging biomarkers that are not easily appreciated by the human eye and which identify features with no morphological correlate or radiological descriptor may play a signi cant role in the prognostication of ILD. Longitudinal QCT evaluation on serial HRCT also has the advantage of improved precision and potential for identi cation of patient phenotypes, for example, the progressive brotic phenotype [75].
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

520 |
T. M. Jacob et al. |
|
|
The Progressive Fibrotic Phenotype
As our understanding of FLD has increased it has become increasingly apparent that diagnosis and prognosis do not always go hand in hand. For example, some patients with IPF survive for up to 10 years with no major incremental disability each year. As described earlier, however, some patients with CHP or RA-ILD can have disease trajectories that are indistinguishable from IPF. Assigning a diagnosis to a patient not only allows one to develop a plan of management for a condition, but it also envisions a likely prognosis, gleaned from knowledge about disease trajectories for patients with the same diagnosis. But when related but distinct diseases manifest similar rates of disease progression, it would be logical to ask whether management strategies shown to be successful in one disease might have utility in the related disease.
To answer this question in the FLDs, studies have investigated whether anti brotic medication could curtail further disease progression in patients that have been identi ed as having a progressive form of brosis. The INBULD trial [76] examined the role of anti brotics in patients with a variety of non-IPF FLDs where brosis was found to be progressive using lung function, symptomatic or imaging measures of deterioration. The study demonstrated that nintedanib slowed the rate of disease progression characterised by a reduction in forced vital capacity decline [77]. A consequence of the study is that the treatment of brosing lung disease may become diagnosis-agnostic, with progression becoming the most important disease characteristic that needs identi cation to guide management.
It is also possible that alternative longitudinal measures of disease worsening in the FLDs will be sought in the near- term. The current gold standard measure used to identify disease progression is an annual FVC relative decline of >10% per year. But as awareness of IPF grows, patients are being identi ed at earlier stages of the disease. The institution of lung cancer screening in various countries around the world is also likely to increase the earlier detection of patients with FLD. Anti brotic use in IPF patients, coupled with earlier recognition of patients in their disease course is likely to result in a larger proportion of patients undergoing FVC declines <10% per year. FVC declines of <10% per year are in the range of measurement variation for the test. The coming years are likely to identify larger proportions of IPF patients in whom FVC measurements are unable to distinguish genuine physiological deterioration from measurement variation. Challenges in discerning real from artefactual change are also likely to be encountered in the non-IPF FLDs which often have a smaller rate of annualised FVC decline. Should anti brotic prescription become licensed for use in
non-IPF FLDs, even more patients will routinely have marginal declines in FVC.
Alternatives to FVC decline are being sought, both in terms of patient reported outcome measures, peripheral blood biomarkers and with visual and quantitative CT analysis. Avenues of CT research include identifying variables that might con rm that a marginal FVC decline (5–9.9%) represents real physiological deterioration. This may take the form of a CT measure proven to predict the outcome, that is used to adjudicate marginal FVC declines [78]. More than visual CT analysis, QCT tools hold the potential for identifying disease progression at much shorter intervals than that is possible with FVC decline.
Should change in a QCT metric de nitively identify disease progression over a period of 3–6 months, future drug trials could be shortened and consequently become cheaper. This would in turn improve the feasibility of drug development in FLD. In a future where there are several potential drugs available to treat progressive FLDs, identifying disease progression in a patient taking one drug could provide the evidence to implement a change in therapy. Then, if a patient progresses despite therapy, combination drug therapy could be advocated, or in situations where the disease progresses despite several therapies being trialled, the patient could be referred early for lung transplantation.
Nevertheless, whilst CT analysis, and in particular QCT evaluation hold great promise, the limitations and measurement noise associated with CT acquisitions need further study. Speci cally, measurement inaccuracies that are associated with serial CT imaging can include variability when different CT scanners or reconstruction algorithms are used at consecutive imaging time points in the same patient. FLD patients are often short of breath when scanned, and breathlessness can also contribute to measurement variation as the effort with which patients inspire can differ at different imaging time points. Optimising and limiting these sources of measurement inaccuracy will be crucial to improve the sensitivity and accuracy of QCT disease progression measurement.
Other Imaging Techniques
In addition to HRCT imaging, there are novel applications of existing imaging techniques e.g. magnetic resonance imaging (MRI) and positron emission tomography (PET) that have the potential to allow functional and structural assessment of ILDs [79]. Acquisition of thoracic MRI is inherently challenging due to the low signal-to-noise ratio of air and artefacts that result from cardiac/respiratory motion. These limitations can however be addressed by newer proton MRI methods. For example, ultrashort echo times (UTE) offer an

29 Imaging Approach to Interstitial Lung Disease |
521 |
|
|
enhanced resolution that can identify structural changes in the lung [80]. Hyperpolarised noble gas MRI techniques also offer the potential to assess lung ventilation, microstructure and gas transfer in patients with ILD [81].
PET is rarely employed in clinical practice for the investigation of ILD. However, PET has demonstrated high standard uptake values (SUVs) of 18F-fuorodeoxyglucose (FDG) in regions of lung brosis, questioning the longstanding assumption that these regions are ‘burned out’ and metabolically inactive [82]. There is also evidence of increased FDG uptake in apparently normal lung tissue as determined by visual CT inspection in patients with ILD, suggesting a possible role of PET in the identi cation of subclinical disease. PET also has a key role in characterising/con rming the malignant risk of nodules within areas of brosis, identi ed on routine clinical CT imaging.
Conclusion
Imaging techniques are an essential part of the diagnostic pathway in ILD. Chest radiography is frequently the initial indicator of ILD, with HRCT playing a key role in diagnosis and differentiation between speci c ILDs. Typical imaging features, including those for UIP-IPF as described in this chapter, are well described in international consensus guidelines. In addition to aiding diagnosis, HRCT may be employed to exclude important complications and determine progression. Prediction of ILD prognosis is possible using traditional visual HRCT scoring methods and newer QCT analyses. Quanti cation of lung density changes and parenchymal textural features by advanced QCT algorithms has the potential to standardise and enhance the role of HRCT in ILD. Whilst the role of MRI and PET in ILD remains exploratory, there is signi cant potential for these techniques to complement the structural information derived from HRCT with measures of functional damage to the lung. Comprehensive assessment of lung structure-function changes in ILD, for example, using HRCT alongside hyperpolarised xenon MRI, as well as application of emerging QCT-derived imaging biomarkers may enable more accurate diagnosis, monitoring of treatment response and prognostication of ILD in the future.
Acknowledgements Joseph Jacob is a recipient of a Wellcome Trust Clinical Research Career Development Fellowship Award: 209553/Z/17/Z and is supported by the NIHR UCLH Biomedical Research Centre.
Disclosures Joseph Jacob has received fees from Boehringer Ingelheim, GlaxoSmithKline, Takeda, NHSX and Roche and Roche unrelated to the current submission.
References
1.\Weibel ER. Morphometry of the human lung. Berlin: Academic Press; 1963. p. 1–151.
2.\Flaherty KR, King TE Jr, Raghu G, Lynch JP III, Colby TV, Travis WD, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170:904–10.
3.\Demedts M, Wells AU, Antó JM, Costabel U, Hubbard R, Cullinan P, et al. Interstitial lung diseases: an epidemiological overview. Eur Respir J. 2001;18(32 Suppl):2s–16s.
4.\B.T. SOCIETY, S. O. COMMITTEE. The diagnosis, assessment and treatment of diffuse parenchymal lung disease in adults. Thorax. 1999;54(Suppl 1):S1–S28.
5.\Ryu JH, Daniels CE, Hartman TE, Yi ES. Diagnosis of interstitial lung diseases. Mayo Clin Proc. 2007;82(8):976–86.
6.\Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary brosis. An of cial ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–68.
7.\Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An of cial ATS/ERS/JRS/ALAT statement: idiopathic pulmonary brosis—evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824.
8.\Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonarybrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6(2):138–53.
9.\Silva CI, Churg A, Muller NL. Hypersensitivity pneumonitis: spectrum of high-resolution CT and pathologic ndings. Am J Roentgenol. 2007;188(2):334–44.
10.\Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722.
11.\Jacob J, Hansell DM. HRCT of brosing lung disease. Respirology. 2015;20(6):859–72.
12.\Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–44.
13.\Lynch DA, Godwin JD, Safrin S, et al. High-resolution computed tomography in idiopathic pulmonary brosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172:488.
14.\Lee JS, Im JG, Ahn JM, Kim YM, Han MC. Fibrosing alveolitis: prognostic implication of ground-glass attenuation at high-
resolution CT. Radiology. 1992;184:451–4. |
|
15.\Remy-Jardin M, Remy J, Giraud F, Wattinne L, |
Gosselin |
B. Computed tomography (CT) assessment of ground-glass |
|
opacity: semiology and signi cance. J Thorac |
Imaging. |
1993;8:249–64. |
|
16.\Remy-Jardin M, Giraud F, Remy J, Copin MC, Gosselin B, Duhamel A. Importance of ground-glass attenuation in chronic diffuse in ltrative lung disease: pathologic-CT correlation. Radiology. 1993;189:693–8.
17.\Akira M, Sakatani M, Ueda E. Idiopathic pulmonary brosis: progression of honeycombing at thin-section CT. Radiology. 1993;189(3):687–91.
18.\Kim TS, Han J, Chung MP, Chung MJ, Choi YS. Disseminated dendriform pulmonary ossi cation associated with usual interstitial pneumonia: incidence and thin-section CT-pathologic correlation. Eur Radiol. 2005;15(8):1581–5.
19.\Egashira R, Jacob J, Kokosi MA, Brun A-L, Rice A, Nicholson AG, et al. Diffuse pulmonary ossi cation in brosing interstitial lung diseases: prevalence and associations. Radiology. 2017;284(1):255–63.
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
522 |
T. M. Jacob et al. |
|
|
20.\Silva CI, Muller NL, Lynch DA, Curran-Everett D, Brown KK, Lee KS, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary brosis and nonspeci c interstitial pneumonia by using thin-section CT. Radiology. 2008;246(1):288–97.
21.\Yagihashi K, Huckleberry J, Colby TV, Tazelaar HD, Zach J, Sundaram B, et al. Radiologic–pathologic discordance in biopsy-proven usual interstitial pneumonia. Eur Respir J. 2016;47(4):1189–97.
22.\Mastora I, Remy-Jardin M, Sobaszek A, Boulenguez C, Remy J, Edme JL. Thin-section CT nding in 250 volunteers: assessment of the relationship of CT ndings with smoking history and pulmonary function test results. Radiology. 2001;218:695–702.
23.\Churg A, Muller NL, Flint J, Wright JL. Chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2006;30(2):201–8.
24.\Raghu G, Remy-Jardin M, Ryerson CJ, Myers JL, Kreuter M, Vasakova M, et al. Diagnosis of hypersensitivity pneumonitis in adults. An of cial ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2020;202(3):e36–69.
25.\Barnett J, Molyneaux PL, Rawal B, Abdullah R, Hare SS, Vancheeswaran R, et al. Variable utility of mosaic attenuation to distinguish brotic hypersensitivity pneumonitis from idiopathic pulmonary brosis. Eur Respir J. 2019;54(1):1900531.
26.\Flaherty KR, Thwaite EL, Kazerooni EA, Gross BH, Toews GB, Colby TV, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax. 2003;58:143–8.
27.\Gruden JF, Panse PM, Leslie KO, Tazelaar HD, Colby TV. UIP diagnosed at surgical lung biopsy, 2000-2009: HRCT patterns and proposed classi cation system. Am J Roentgenol. 2013;200(5):W458–W67.
28.\Chung JH, Chawla A, Peljto AL, Cool CD, Groshong SD, Talbert JL, et al. CT scan ndings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest. 2015;147(2):450–9.
29.\Raghu G, Lynch D, Godwin JD, Webb R, Colby TV, Leslie KO, et al. Diagnosis of idiopathic pulmonary brosis with high-resolution CT in patients with little or no radiological evidence of honeycombing: secondary analysis of a randomised, controlled trial. Lancet Respir Med. 2014;2(4):277–84.
30.\Vourlekis JS, Schwarz MI, Cherniack RM, Curran-Everett D, Cool CD, Tuder RM, et al. The effect of pulmonary brosis on survival in patients with hypersensitivity pneumonitis. Am J Med. 2004;116:662–8.
31.\Salisbury ML, Gu T, Murray S, Gross BH, Chughtai A, Sayyouh M, et al. Hypersensitivity pneumonitis: radiologic phenotypes are associated with distinct survival time and pulmonary function trajectory. Chest. 2019;155(4):699–711.
32.\Jacob J, Hirani N, van Moorsel CHM, Rajagopalan S, Murchison JT, van Es HW, et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur Respir J. 2019;53(1):1800869.
33.\Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, Van Uden JH, et al. Usual interstitial pneumonia in rheumatoid arthritis- associated interstitial lung disease. Eur Respir J. 2010;35(6):1322–8.
34.\Frankel SK, Cool CD, Lynch DA, Brown KK. Idiopathic pleuroparenchymal broelastosis: description of a novel clinicopathologic entity. Chest. 2004;126(6):2007–13.
35.\Azoulay E, Paugam B, Heymann M, Kambouchner M, Haloun A, Valeyre D, et al. Familial extensive idiopathic bilateral pleuralbrosis. Eur Respir J. 1999;14(4):971–3.
36.\von der Thusen JH, Hansell DM, Tominaga M, Veys PA, Ashworth MT, Owens CM, et al. Pleuroparenchymal broelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Mod Pathol. 2011;24(12):1633–9.
37.\Lauretis AD, Basra H, Hakim W, Jacob J, Visca D, Kokosi M, et al. Pleuroparenchymal broelastosis (PPFE) predicts survival in idiopathic pulmonary brosis (IPF). In: American Thoracic Society conference 2016. p. A1142-A.
38.\Jacob J, Odink A, Brun AL, Macaluso C, de Lauretis A, Kokosi M, et al. Functional associations of pleuroparenchymal broelastosis and emphysema with hypersensitivity pneumonitis. Respir Med. 2018;138:95–101.
39.\Jacob J, Bartholmai B, Rajagopalan S, Kokosi M, Maher T, Nair A, et al. Functional and prognostic effects when emphysema complicates idiopathic pulmonary brosis. Eur Respir J. 2017;50:1700379.
40.\Jacob J, Song JW, Yoon H, Cross G, Barnett J, Woo WL, et al. Prevalence and effects of emphysema in never-smokers with rheumatoid arthritis interstitial lung disease. EBiomedicine. 2018;28:303–10.
41.\Cottin V, Hansell DM, Sverzellati N, Weycker D, Antoniou KM, Atwood M, et al. Effect of emphysema extent on serial lung function in patients with idiopathic pulmonary brosis. Am J Respir Crit Care Med. 2017;196(9):1162–71.
42.\Cottin V, Le PJ, Prevot G, Mal H, Humbert M, Simonneau G, et al. Pulmonary hypertension in patients with combined pulmonary brosis and emphysema syndrome. Eur Respir J. 2010;35(1):105–11.
43.\Koo HJ, Do K-H, Lee JB, Alblushi S, Lee SM. Lung cancer in combined pulmonary brosis and emphysema: a systematic review and meta-analysis. PLoS One. 2016;11(9):e0161437.
44.\Antoniou KM, Margaritopoulos GA, Goh NS, Karagiannis K, Desai SR, Nicholson AG, et al. Combined pulmonary brosis and emphysema in scleroderma lung disease has a major confounding effect on lung physiology and screening for pulmonary hypertension. Arthritis Rheumatol. 2015;68(4):1004–12.
45.\Lynch DA, Rose CS, Way D, King TE Jr. Hypersensitivity pneumonitis: sensitivity of high-resolution CT in a population-based study. AJR. 1994;3:469–72.
46.\Johannson KA, Elicker BM, Vittinghoff E, Assayag D, de Boer K, Golden JA, et al. A diagnostic model for chronic hypersensitivity pneumonitis. Thorax. 2016;71(10):951–4.
47.\Ryerson CJ, Urbania TH, Richeldi L, Mooney JJ, Lee JS, Jones KD, et al. Prevalence and prognosis of unclassi able interstitial lung disease. Eur Respir J. 2013;42(3):750–7.
48.\Ryerson CJ, Corte TJ, Lee JS, Richeldi L, Walsh SLF, Myers JL, et al. A standardized diagnostic ontology for brotic interstitial lung disease. An international working group perspective. Am J Respir Crit Care Med. 2017;196(10):1249–54.
49.\Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An of cial American Thoracic Society/ European Respiratory Society statement: update of the international multidisciplinary classi cation of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48.
50.\Silva CI, Muller NL, Hansell DM, Lee KS, Nicholson AG, Wells AU. Nonspeci c interstitial pneumonia and idiopathic pulmonarybrosis: changes in pattern and distribution of disease over time. Radiology. 2008;247(1):251–9.
51.\Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, et al. An of cial European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46:976.
52.\Abehsera M, Valeyre D, Grenier P, Jaillet H, Battesti JP, Brauner MW. Sarcoidosis with pulmonary brosis: CT patterns and correlation with pulmonary function. Am J Roentgenol. 2000;174(6):1751–7.
53.\Criado E, Sanchez M, Ramirez J, Arguis P, de Caralt TM, Perea RJ, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010;30(6):1567–86.
54.\Padley SPG, Padhani AR, Nicholson A, Hansell DM. Pulmonary sarcoidosis mimicking cryptogenic brosing alveolitis on CT. Clin Radiol. 1996;51:807–10.
55.\Hassan WU, Keaney NP, Holland CD, Kelly CA. High resolution computed tomography of the lung in lifelong non-smoking patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54(4):308–10.
29 Imaging Approach to Interstitial Lung Disease |
523 |
|
|
56.\Remy-Jardin M, Remy J, Wallaert B, Bataille D, Hatron PY. Pulmonary involvement in progressive systemic sclerosis: sequential evaluation with CT, pulmonary function tests, and bronchoalveolar lavage. Radiology. 1993;188:499–506.
57.\Mino M, Noma S, Taguchi Y, Tomii K, Kohri Y, Oida K. Pulmonary involvement in polymyositis and dermatomyositis: sequential evaluation with CT. AJR. 1997;169(1):83–7.
58.\Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018;7:E356.
59.\Tan RT, Kuzo R, Goodman LR, Siegel R, Haasler GB, Presberg KW. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest. 1998;113(5):1250–6.
60.\Chin M, Johns C, Currie BJ, Weatherley N, Hill C, Elliot C, et al. Pulmonary artery size in interstitial lung disease and pulmonary hypertension: association with interstitial lung disease severity and diagnostic utility. Front Cardiovasc Med. 2018;5:53.
61.\Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary brosis. Chest. 2006;129(3):746–52.
62.\Handa T, Nagai S, Fushimi Y, Miki S, Ohta K, Niimi A, et al. Clinical and radiographic indices associated with airfow limitation in patients with sarcoidosis. Chest. 2006;130(6):1851–6.
63.\Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48(3):889–902.
64.\Oh SY, Kim MY, Kim J-E, Kim S-S, Park TS, Kim DS, et al. Evolving early lung cancers detected during follow-up of idiopathic interstitial pneumonia: serial CT features. Am J Roentgenol. 2015;204(6):1190–6.
65.\Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE. Acute exacerbations of idiopathic pulmonary brosis. Am J Respir Crit Care Med. 2007;176:636.
66.\Akira M, Kozuka T, Yamamoto S, Sakatani M. Computed tomography ndings in acute exacerbation of idiopathic pulmonary brosis. Am J Respir Crit Care Med. 2008;178(4):372–8.
67.\Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute exacerbation of idiopathic pulmonary brosis. An international working group report. Am J Respir Crit Care Med. 2016;194(3):265–75.
68.\Ryerson CJ, Cottin V, Brown KK, Collard HR. Acute exacerbation of idiopathic pulmonary brosis: shifting the paradigm. Eur Respir J. 2015;46(2):512–20.
69.\Walsh SL, Sverzellati N, Devaraj A. Chronic hypersensitivity pneumonitis: high resolution computed tomography patterns and pulmonary function indices as prognostic determinants. Eur Radiol. 2012;22:1672.
70.\Walsh SL, Sverzellati N, Devaraj A, Keir GJ, Wells AU, Hansell DM. Connective tissue disease related brotic lung disease: high
resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax. 2014;69(3):216–22.
71.\Edey AJ, Devaraj AA, Barker RP, Nicholson AG, Wells AU, Hansell DM. Fibrotic idiopathic interstitial pneumonias: HRCT ndings that predict mortality. Eur Radiol. 2011;21(8):1586–93.
72.\Rosas IO, Yao J, Avila NA, Chow CK, Gahl WA, Gochuico BR. Automated quanti cation of high-resolution CT scanndings in individuals at risk for pulmonary brosis. Chest. 2011;140(6):1590–7.
73.\Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, et al. Automated quantitative computed tomography versus visual computed tomography scoring in idiopathic pulmonary brosis: validation against pulmonary function. J Thorac Imaging. 2016;31(5):304–11.
74.\Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, et al. Mortality prediction in idiopathic pulmonarybrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J. 2017;49(1):1601011.
75.\Wells AU, Brown KK, Flaherty KR, Kolb M, Thannickal VJ, Group IPFCW. What’s in a name? That which we call IPF, by any other name would act the same. Eur Respir J. 2018;51(5):1800692.
76.\Flaherty KR, Brown KK, Wells AU, Clerisme-Beaty E, Collard HR, Cottin V, et al. Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive brosing interstitial lung disease. BMJ Open Respir Res. 2017;4(1):e000212.
77.\Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive brosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–27.
78.\Jacob J, Aksman L, Mogulkoc N, Procter AJ, Gholipour B, Cross G, et al. Serial CT analysis in idiopathic pulmonary brosis: comparison of visual features that determine patient outcome. Thorax. 2020;75(8):648–54.
79.\Weatherley ND, Eaden JA, Stewart NJ, Bartholmai BJ, Swift AJ, Bianchi SM, et al. Experimental and quantitative imaging techniques in interstitial lung disease. Thorax. 2019;74(6):611–9.
80.\Ohno Y, Koyama H, Yoshikawa T, Seki S, Takenaka D, Yui M, et al. Pulmonary high-resolution ultrashort TE MR imaging: comparison with thin-section standardand low-dose computed tomography for the assessment of pulmonary parenchyma diseases. J Magn Reson Imaging. 2016;43(2):512–32.
81.\Kaushik SS, Freeman MS, Yoon SW, Liljeroth MG, Stiles JV, Roos JE, et al. Measuring diffusion limitation with a perfusion- limited gas—hyperpolarized 129Xe gas-transfer spectroscopy in patients with idiopathic pulmonary brosis. J Appl Physiol (1985). 2014;117(6):577–85.
82.\Groves AM, Win T, Screaton NJ, Berovic M, Endozo R, Booth H, et al. Idiopathic pulmonary brosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/ CT. J Nucl Med. 2009;50(4):538–45.
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
