
- •Contents
- •Preface
- •Contributors
- •01 Neuroanatomy
- •03 Seizures and Epilepsy
- •04 Disorders of Myelination
- •05 Tumors of the Nervous System
- •06 Headache and Pain Disorders
- •I. Episodic Headache
- •A. Episodic Headaches Lasting More than Four Hours
- •07 Behavioral Neurology
- •08 Movement Disorders
- •09 Diseases of the Nerves
- •10 Diseases of the Muscles
- •I. Bacteria
- •I. Diabetes Mellitus (DM)
- •Index
1
Neuroanatomy
Note: Significant diseases are indicated in bold and syndromes in italics.
I. Cerebral Cortex
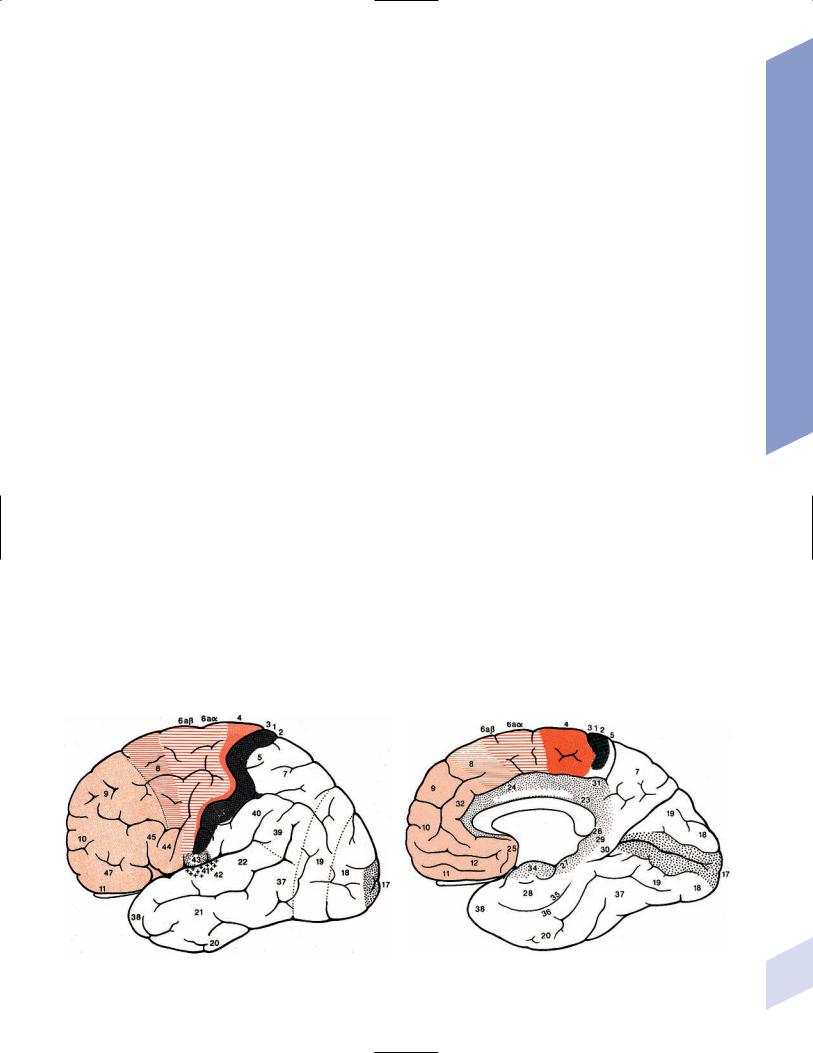
1.Important gyri, lobes, and Brodmann areas (Fig. 1–1)
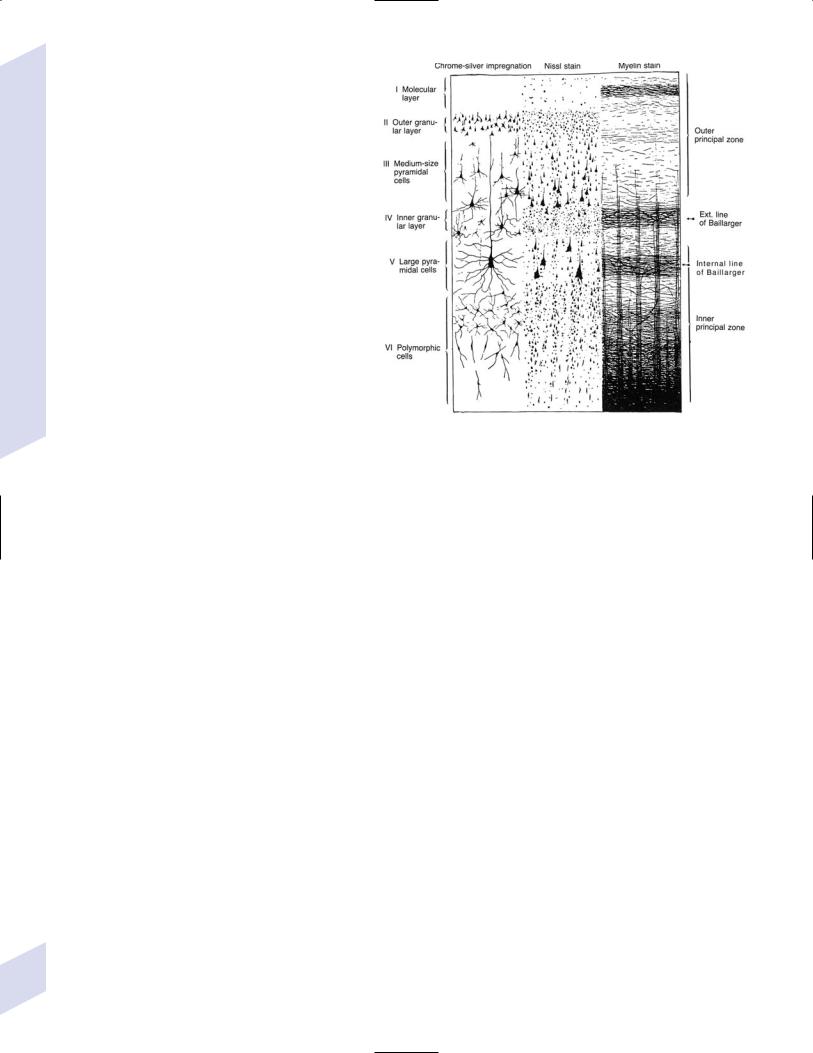
2.Types of cortical neurons (Fig. 1–2)
a.projection neurons: pyramidal and fusiform neurons
b.interneurons: stellate and granule cells, horizontal cells of Cajal
3.Chemoanatomy
a.afferents
i.corticocortical fibers and thalamocortical fibers (glutamate, aspartate)
ii.projections from the nucleus basalis of Meynert (acetylcholine), hypothalamus (histamine, orexin/hypocretin), and brainstem (norepinephrine, serotonin, dopamine)
b.efferents
i.all use glutamate and aspartate
4.Subtypes of cortex
a.cytoarchitectonic subtypes
i.isocortex: exhibits the six typical layers
(1)homotypical isocortex: the six layers are proportionate
(2)heterotypical isocortex: certain layers are enlarged, for example, the
(a)primary motor cortex (Brodmann area 4) exhibits an enlarged layer 5 due to oversized pyramidal cells that project into the corticospinal tract {Betz cells}
A B
Cerebral Cortex
Figure 1–1 Important gyri, lobes, and Brodmann areas. (A) lateral surface; (B) medial surface. (From Duus P, Topical Diagnosis in Neurology. |
1 |
Stuttgart, Germany: Georg Thieme; 1998:276, Fig. 8.23. Reprinted by permission.) |
1 Neuroanatomy
(b)primary visual cortex (Brodmann area 17) exhibits a pronounced outer line of Baillarger in layer 4 {band of Gennari} that is thalamocortical afferents
ii.mesocortex: exhibits five or six poorly defined layers, located at transition between isocortex and allocortex (e.g., parahippocampal gyrus)
iii.allocortex: exhibits only three layers; includes the inferior and mesial temporal lobes (hippocampal formation)
b. general functional subtypes
i.primary motor and sensory areas
ii.monomodal association areas
(1)monomodal motor association areas: receive input from the primary and monomodal association sensory areas, and project to the primary motor area
(2)monomodal sensory association areas: receive input from the primary sensory area, and project to the heteromodal association areas and primary motor area
Figure 1–2 Types of cortical neurons. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:262, Fig. 8.11. Reprinted by permission.)
iii.heteromodal association areas: receive
input from monomodal association areas; project to other heteromodal association and paralimbic areas, and the basal ganglia
iv.limbic and paralimbic areas
A. Motor Systems
1.Primary motor cortex (Brodmann area 4)
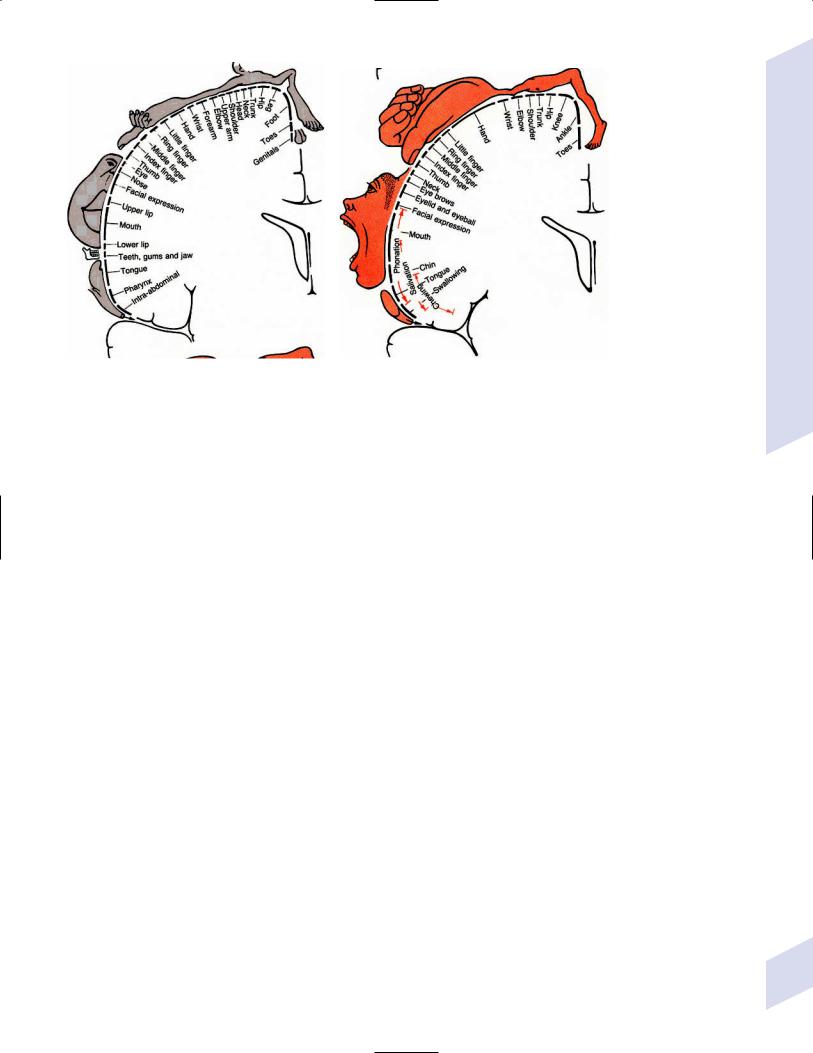
a.somatotopic organization (Fig. 1–3)
b.lesions strictly limited to Brodmann area 4 produce focal paralysis with hypotonia, mild hyperreflexia, and a partial Babinski sign (i.e., upgoing first toe only)
i.extending the lesion into the premotor area (lateral surface of Brodmann area 6) produces the typical “clasp knife” spasticity of the antigravity muscles (upper extremity flexors and lower extremity extensors), pronounced hyperreflexia, and a complete Babinski sign (i.e., upgoing first toe with fanning of the other toes)
c.stimulation causes simple flick-like focal movements involving a few agonist and antagonist muscles
2.Monomodal motor association areas: share the precentral sulcus with the primary motor cortex, and extend rostrally onto the superior, middle, and inferior frontal gyri
a.premotor area (lateral surface of Brodmann area 6): prepares the body posture for subsequent complex limb movements; acts via the rubrospinal, tectospinal, and reticulospinal tracts
i.shares the homunculus of the primary motor cortex: the frontal eye fields (Brodmann area 8) are located anterior to head representation
b.supplementary motor area (medial surface of Brodmann area 6): prepares and initiates complex limb movements; likely exhibits its own homunculus independent of that of the premotor area and primary motor cortex
2 |
i. lesions cause |
A B
Figure 1–3 Sensory (A) and motor (B) homunculi. Note the proximity of the thumb to the forehead. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:273, Fig. 8.20. Reprinted by permission.)
(1)global akinesia with speech arrest that develops into hypokinesia (contralateral ipsilateral), reduced speech, and reduced facial expression; chronically, patients will exhibit only poor hand coordination
(2)ideomotor apraxia, which may be associated with a contralateral alien hand phenomenon characterized by unconscious reflexive movements
ii.stimulation causes bilateral posturing movements, contralateral head and body rotation, and vocalizations {salutary seizures}
3.Subcortical projections
a.corticobulbar tract: the ventral division travels with the corticospinal tract and synapses in the facial nucleus; the dorsal division travels closer to the medial lemniscus and synapses in other cranial nerve nuclei and the reticular formation
b.corticospinal tract: composed of fibers not only from the primary motor cortex (25%) and monomodal motor association cortex (30%) but also the primary somatosensory cortex (30%) and superior parietal lobule (Brodmann areas 5 and 7; 15%)
i.terminates in the cervical spinal cord (55%), thoracic spinal cord (20%), and lumbosacral spinal cord (25%)
ii.the majority (80%) of the corticospinal tract decussates at cervicomedullary junction (upper extremity fibers decussate rostral to the lower extremity fibers)
(1)the rest of the corticospinal tract does not decussate until it arrives at its terminal cervical and upper thoracic spinal cord levels (ventral corticospinal tract)
B.Somatosensory Systems
1.Primary sensory cortex (Brodmann areas 3-1-2) a. subdivided according to sensory modality
i.Brodmann area 3a receives deep sensation from muscle spindles and Golgi tendon organs
ii.Brodmann area 3b receives superficial sensation from exteroceptors and cutaneous receptors
Cerebral Cortex
3
1 Neuroanatomy
iii.Brodmann areas 1 and 2 receive deep and superficial sensation including subcutaneous tissues
b.somatotopic organization: similar to the motor homunculus, but less well defined and may extend caudally into Brodmann areas 5 and 7
i.face and body are bilaterally represented; limbs are unilaterally represented
c.lesions cause loss of proprioception (but not vibration sensation), poor localization of stimuli {topagnosia}, difficulty with two-point discrimination, reduced object recognition through touch {astereognosis}, tactile hallucinations, rapid fatigability of prolonged sensory stimulation, and paresthesias
i.primary modality sensory loss is pronounced with postcentral gyrus lesions that involve the posterior insula and underlying white matter
d.stimulation produces numbness and paresthesias, and often a sensation of movement
2.Secondary sensory cortex (supramarginal gyrus; Brodmann area 40): Posterior to the inferior part of the primary somatosensory cortex
a.exhibits bilateral inputs with contralateral predominance, and is somatotopically organized into a separate homunculus that may be related to pain sensation
3.Unimodal somatosensory association area (Brodmann area 5): Involved in recognition of object shape by tactile sensation {stereognosis}
4.Subcortical afferents: anterolateral system/spinothalamic tract, dorsal columns, and trigeminal pathways terminate in the ventroposteromedial and ventroposterolateral thalamic nuclei that then project to the primary sensory cortex
C. Special Sensory Systems
1.Vision (see p. 37) and hearing (see p. 48)
2.Vestibular sensation (see p. 50)
3.Taste (Brodmann area 43): Adjacent to the primary somatosensory tongue representation and the insular cortex motor areas for salivation and gastrointestinal motility
a.distorted taste perception {dysgeusia} is poorly localized and usually is caused by zinc or vitamin A deficiency
4.Olfaction: represented by bilateral projections from the olfactory nerves to the piriform cortex and amygdala via lateral olfactory stria, and to the orbitofrontal cortex overlying the septal nuclei via the medial olfactory stria; no olfactory bulb projections enter the anterior perforated substance in humans
a.distorted odor perception {parosmia} is usually caused by injury to the olfactory nerves from head trauma or by psychiatric illness (e.g., depression)
b.olfactory hallucinations can be caused either by injury to the olfactory nerves or by seizures in the mesial temporal lobe or uncus that involve the amygdala (Box 1.1)
D. Heteromodal Association Areas
1.Frontal heteromodal association area/prefrontal cortex: involves all the cortex located anterior to the monomodal motor association areas
a. unlike other heteromodal association areas, the prefrontal cortex
i.projects to the amygdala and caudate
ii.receives indirect projections from the hippocampus through the dorsomedial thalamus and direct projections from the cingulate gyrus
4 |
and hypothalamus |
Box 1.1
Other Types of Hallucinations
Visual—Formed hallucinations from posterior temporal lobe or mesencephalon (i.e., peduncular hallucinosis) dysfunction or in reaction to vision loss {Bonnet’s syndrome}, which have hallucinations in the area of vision loss; unformed hallucinations with occipital lobe dysfunction
Auditory—Formed hallucinations from superior temporal gyrus dysfunction; unformed hallucinations from pontine dysfunction, usually in the presence of hearing loss
Gustatory—from frontoparietal cortex dysfunction
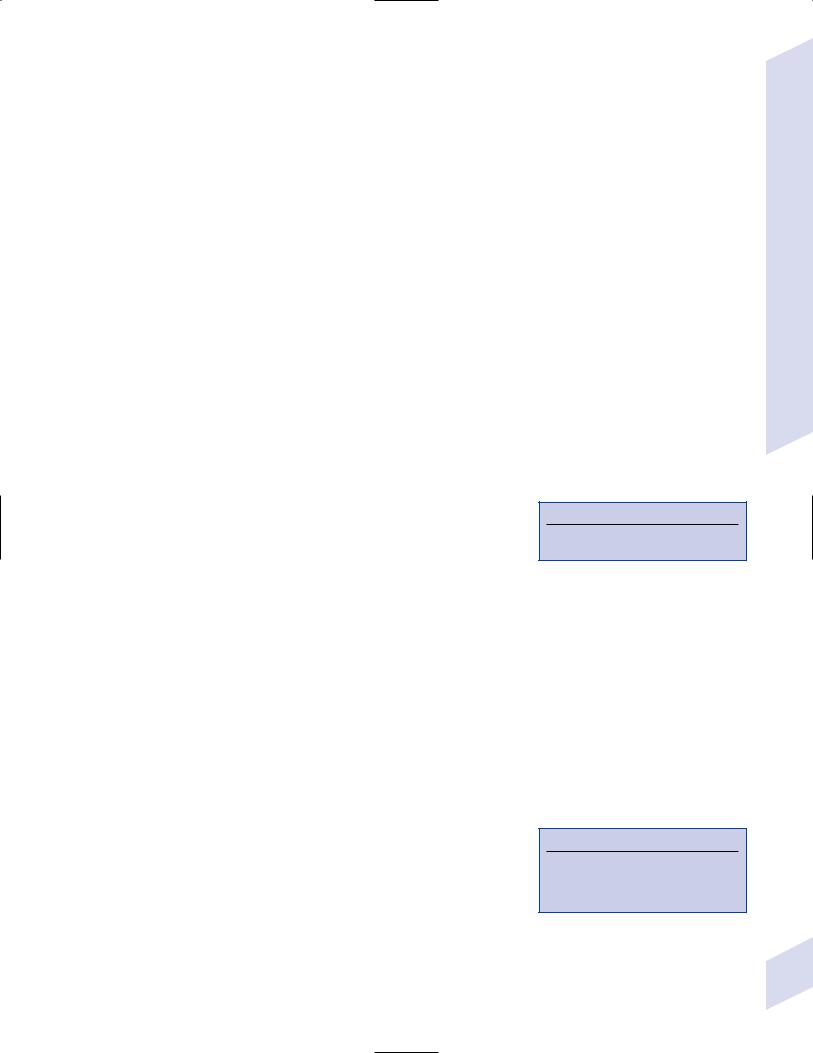
Table 1–1 Frontal Lobe Syndromes
Prefrontal |
|
Effects of |
cortex region |
Effects of unilateral damage |
bilateral damage |
|
|
|
Dorsolateral |
Dominant: ↓ verbal intellect and |
Global intellectual |
|
memory; perseveration |
impairment (dementia) |
|
Nondominant: ↓ visuospatial intellect |
|
|
and memory |
|
Ventromedial |
Dominant: labile emotions, sociopathy, |
Sociopathy, environmental |
|
environmental dependency (i.e., the |
dependency |
|
presence of an object induces its use); |
|
|
speech apraxia, gait apraxia |
|
|
Nondominant: sociopathy, |
|
|
environmental dependency |
|
Anterior cingulate |
Inability to perform tasks or make |
Akinetic mutism |
|
decisions {abulia} |
|
Orbitofrontal |
Disinhibited behavior |
|
|
|
|
b.lesions: in addition to the injured part of the prefrontal cortex, the depth of the lesion and the age at which the patient was injured will also affect the symptoms (Table 1–1)
i.Bruns’ frontal lobe ataxia/marché à petits pas of Dejerine—wide-based, slow, short-stepped gait with flexed posture and frequent freezes; can progress to inability to stand
(1)caused by lesions anywhere in frontal lobes that are often bilateral and multifocal
2.Parietal heteromodal association areas: Located in the superior and inferior parietal lobules
a.dominant-sided lesions produce (Box 1.2)
i.alexia
ii.Gerstmann’s syndrome—classically involves agraphia, finger agnosia, acalculia, and left-right confusion; lesions are located on the angular gyrus (Brodmann area 39)
iii.contralateral tactile neglect and spatial inattention
iv.somatotopic ideomotor apraxia
b.nondominant-sided lesions produce
i.contralateral tactile neglect and spatial inattention, which may be so severe that the patient does not recognize the deficit {anosognosia}
ii.constructional and dressing apraxias
iii.spatial disorientation
iv.impersistence; inattention; static confusional state/encephalopathy
E. Language Areas
1.Dominant hemisphere language areas
a.Broca’s area: located in the inferior frontal gyrus, and includes the pars opercularis (Brodmann area 44; a monomodal motor association area) (Box 1.3) and the pars triangularis (Brodmann area 45; a heteromodal association area)
i.lesions produce
(1)Broca’s aphasia—classically involves Broca’s area, the mouth representations of the primary motor and somatosensory cortices, and the underlying white matter
(a)symptoms include nonfluent speech that is slow and agrammatic as well as dysarthric, and impaired repetition; writing is minimal and agrammatic
Cerebral Cortex
Box 1.2
Large dominant-sided inferior parietal lobule lesions can cause an acquired illiteracy.
Box 1.3
Bilateral damage of Brodmann area 44 that extends into the inferior parietal lobe causes loss of voluntary eye, face, oropharynx, and tongue movements (an apraxia).
5

1 Neuroanatomy
6
(2)aphemia—a pure anarthria caused by lesions strictly limited to Broca’s area (i.e., not involving the primary motor and somatosensory cortices or the underlying white matter); may also be caused by bilateral lesions of the corticobulbar tracts
b.Wernicke’s area
i.includes posterior superior and middle temporal gyri, planum temporale (Brodmann area 42; a monomodal auditory association area), and heteromodal association areas of the parietal lobe (angular and supramarginal gyri; Brodmann areas 39 and 40)
ii.lesions cause Wernicke’s aphasia
(1)characterized by excessive speech of normal cadence and prosody associated with impaired naming, comprehension, and repetition, and the presence of paraphasias and neologisms; writing is similarly excessive with paraphasias and neologisms
(2)impairment of comprehension can be particularly pronounced with specific subjects, thereby giving the false appearance of a fluctuant deficit
c.secondary language areas (i.e., the cortex surrounding Broca’s and Wernicke’s areas): lesions produce the transcortical aphasias because they isolate the primary language areas without directly injuring them; all have preservation of repetition
i.subtypes of transcortical aphasias
(1)transcortical motor aphasia—generally caused by lesions anterior or superior to Broca’s area, or by lesions in the supplementary motor area
(2)transcortical sensory aphasia—can be caused by lesions surrounding Wernicke’s area, but otherwise is usually poorly localized
(3)mixed transcortical aphasia—caused by large areas of dominant hemisphere injury that spare the perisylvian regions (e.g., as in watershed infarction or dementias)
d.subcortical connections of Broca’s and Wernicke’s areas: lesions produce
i.conduction aphasia—caused by disconnection of Broca’s and Wernicke’s areas, most commonly the result of a subcortical white matter lesion that interrupts the arcuate fasciculus (in the superior longitudinal fasciculus; and/or the extreme capsule
(1)symptoms: isolated loss of repetition with occasional paraphasic errors
ii.anomia—generally has no localizing value when mild, but significant anomia may reflect lesions in the basal temporal lobe that interrupt hippocampal projections to Wernicke’s area
2.Nondominant hemisphere language areas
a.areas related to the variation of pitch, intonation, melody, rhythm, and loudness of speech {prosody}
i.affective prosody—involves the expression of mood and emotion in speech; caused by lesions in the nondominant hemisphere in areas that are anatomical and functional parallels to Broca’s and Wernicke’s areas of the dominant hemisphere
(1)affective prosody is most commonly observed in psychiatric diseases (e.g., motor and sensory aprosodies in schizophrenia, motor hyperprosody in mania)
ii.propositional prosody—defines a phrase as a question or a statement; can occur after lesions of either hemisphere
b.areas related to gesturing that accompanies speech {kinesia}: poorly localized
c.areas related to singing: an acquired inability to sing {amelodia} is caused by lesions of the posterior inferior frontal lobe or anterior parietal lobe
i.amelodia/amusia, which is the inability to recognize music (e.g., a melody); amusia is usually caused by superior temporal gyrus lesions on either side
F.Paralimbic Cortex
1.Paralimbic cortex: collects inputs from heteromodal association areas, monomodal association areas, and limbic areas (hippocampal formation and amygdala); projects primarily to the hippocampal formation
2.Paralimbic cortex includes
a.anterior and posterior cingulate gyrus
b.parahippocampal gyrus (entorhinal cortex and piriform/periamygdaloid cortex)
c.insular and orbitofrontal cortex
d.temporal poles: lesions cause
i.Kluver-Bucy syndrome—occurs only after bilateral anterior temporal cortex destruction, usually from temporal lobectomy; does not have to involve the amygdala or hippocampus to produce all of its symptoms
(1)symptoms (Box 1.4)
(a)placidity
(b)hypersexuality
(c)hyperorality and increased manual exploration, which likely reflects an inability to recognize objects by vision alone {visual agnosia}
(d)memory impairment, dementia
(e)hyperphagia
Box 1.4
Placidity, hypersexuality, and hyperorality are the classic triad that occurs in animal experiments.
ii.rage—caused by bilateral anterior and inferior temporal lesions, usually due to trauma or herpes encephalitis
G. Limbic Areas
1.Hippocampal formation: includes the hippocampus, dentate gyrus, and subiculum
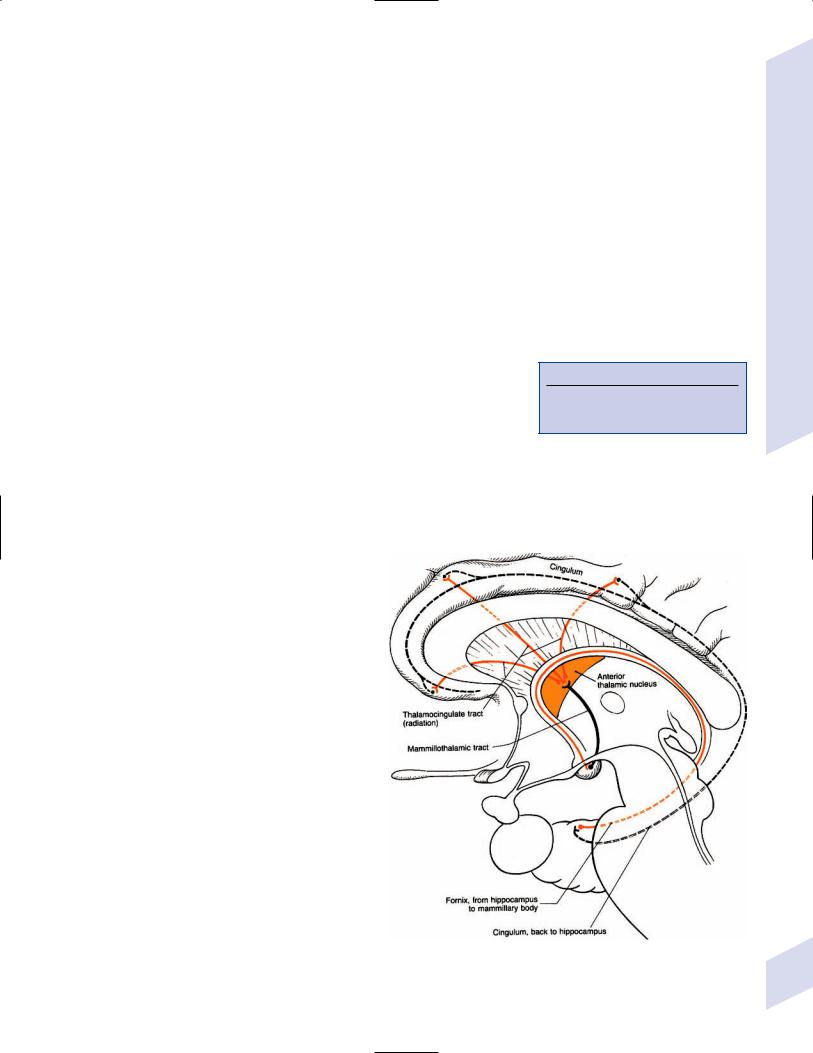
a.afferent and efferent connections: the circuit of Papez (Fig. 1–4)
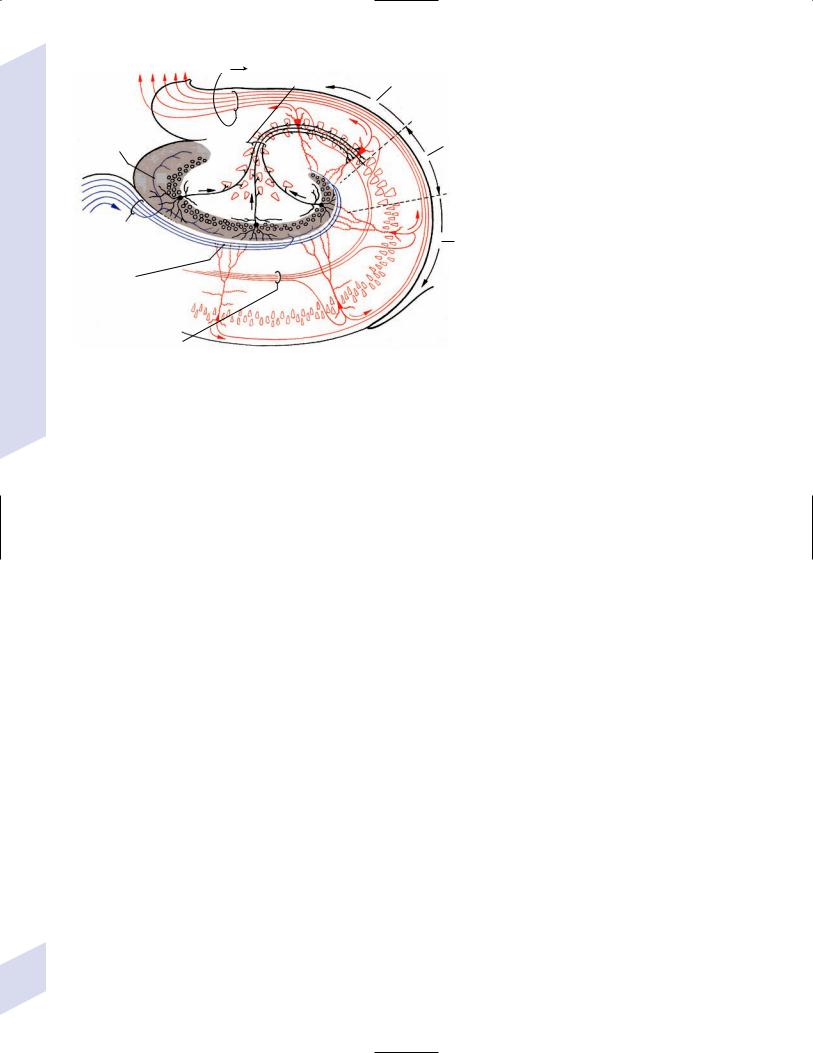
b.intrinsic hippocampal connections (Fig. 1–5)
c.functions: memory formation, learning, and regulation of emotional behavior
d.pathophysiology—transient global amnesia
i.an unknown type of neurological dysfunction, likely involving bilateral mesial temporal lobes
ii.epidemiology: common only in patients50 years old; associated with a history of migraine
iii.symptoms: short-term anterograde and retrograde memory loss with preserved immediate recall and long-term memory; often develops acutely after exertion or excitement
(1) patient has a normal level of arousal and attention, and no of other neurological signs
iv.diagnostic testing: none are necessary to establish the diagnosis
Figure 1–4 The circuit of Papez. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:206, Fig. 5.20. Reprinted by permission.)
Cerebral Cortex
7
1 Neuroanatomy
8
Alveus |
fimbria |
CA3 |
|
Mossy fibers |
|
|
field |
|
|
|
|
Projections to |
|
|
granular cells of |
|
CA2 |
the dentate gyrus |
|
|
|
field |
|
|
|
Perforating |
CA1 |
|
tract |
||
field |
||
|
||
Dendritic |
|
|
branches |
|
|
of pyramidal |
|
|
neurons |
|
|
Schaffer |
|
|
collaterals |
|
Figure 1–5 Intrinsic hippocampal connections. (From Kahle W, Color Atlas/Text of Human Anatomy, Vol. 3: Nervous System and Sensory Organs. 4th ed. Stuttgart, Germany: Georg Thieme; 1993:221, Fig. A. Reprinted by permission.)
(1)electroencephalogram (EEG): can rule out nonconvulsive temporal lobe seizures
(2)magnetic resonance imaging (MRI) may exhibit increased diffusionweighted imaging signal in the mesial temporal lobes
v.treatment: none specific
vi.prognosis: gradual return of memory over 12–24 hours; recurs in 20% of patients
2.Amygdala
a. subdivisions
i.corticomedial amygdala: interconnected with the olfactory bulb via the lateral olfactory tract, the hypothalamus, and the septum via the stria terminalis
ii.basolateral amygdala: interconnected with the hippocampal formation, ventral basal ganglia (limbic loop, see Table 1–2), and basal forebrain
iii.central amygdala: interconnected reciprocally with brainstem autonomic centers (e.g., solitary nucleus)
Table 1–2 The Five Major Basal Ganglia—Thalamocortical Loops
|
|
|
|
Thalamic relay |
|
Loop* |
Cerebral cortex input |
Input nuclei |
Output nuclei |
nuclei |
Function |
|
|
|
|
|
|
Motor |
Primary motor, monomodal |
Putamen (posterior) |
GPe,i, SNR |
Ventral anterior |
Initiating voluntary |
|
motor association, primary |
|
|
and ventral |
movement, postural |
|
somatosensory |
|
|
lateral |
reflexes, muscle tone |
Ocular motor |
Frontal eye field (Brodmann |
Caudate, putamen |
Gpi, SNR superior |
Dorsomedial |
Generation of voluntary |
|
area 8) |
(anterior) |
colliculus |
|
saccades |
Dorsolateral |
Dorsolateral prefrontal |
Caudate (head) |
GPe,i |
Dorsomedial |
? Cognitive functions |
prefrontal |
|
|
|
|
|
Orbitofrontal |
Orbitofrontal, ventromedial, |
Caudate, putamen |
GPe,i |
Dorsomedial |
? Cognitive functions |
|
frontal |
(anterior) |
|
|
|
Limbic |
Cingulate gyrus, hippocampus, |
Nucleus accumbens |
GPe,i |
Dorsomedial |
Mood, emotional |
|
amygdala |
|
|
|
behavior, motivation |
|
|
|
|
|
|
*All loops receive dopamine from the substantia nigra pars compacta except for the limbic loop, which receives dopamine from the ventral tegmental area.
Abbreviations: GPe, globus pallidus externus; GPi, globus pallidus internus; SNR, substantia nigra reticulata.
b.functions
i.olfactory sensory processing and integration of olfactory-visceral reflexes
ii.coordination of emotional behavior and autonomic nervous system responses
iii.association of memories with their emotional content
c.lesions generally produce a loss of aggressive behavior; stimulation produces fear or defense reactions
II. Subcortical White Matter
1.Corona radiata/Centrum semiovale
a.pathophysiology
i.leukoaraiosis: can be part of, but is not identical to, vascular dementia or Binswanger’s disease
(1)lesion types include (Fig. 1–6)
(a)irregular white matter abnormalities, typically scattered about randomly
(i)histology: resembles ischemia
(b)periventricular white matter caps and halos
(i)histology: resembles demyelination (not ischemia), with subependymal gliosis and breakdown of the underlying ependyma of the ventricles
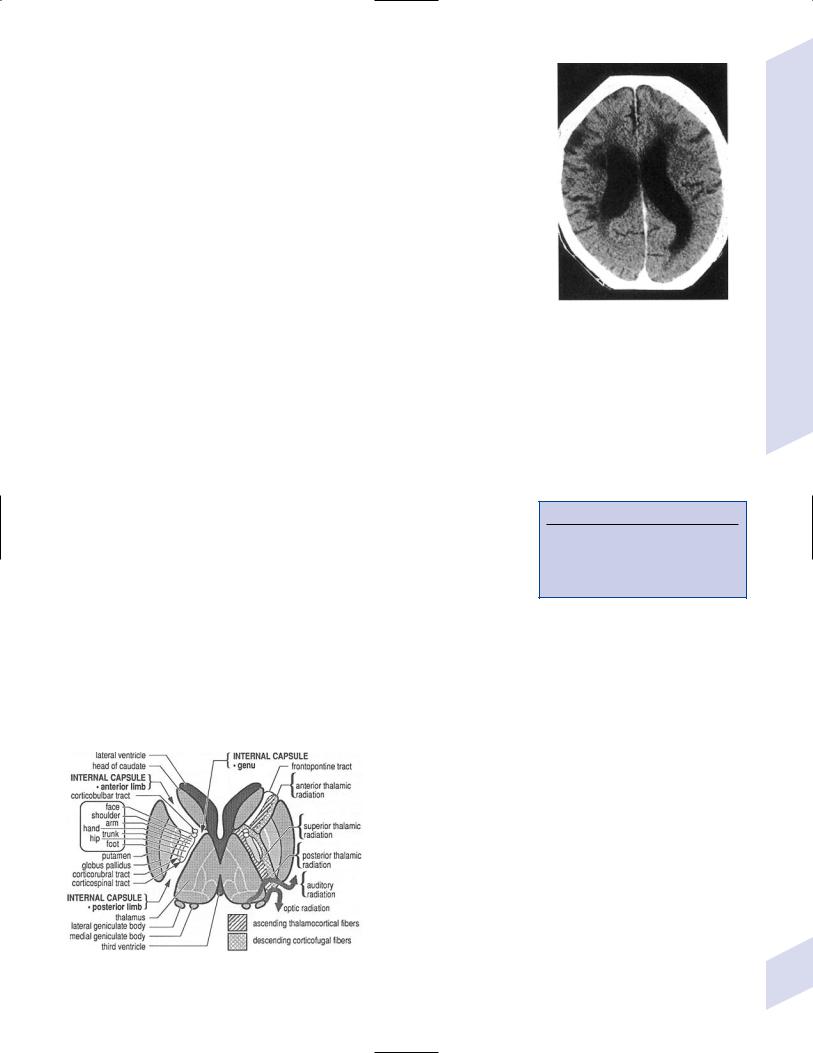
b.internal capsule (Fig. 1–7)
i.pathophysiology: the striatocapsular syndrome, typically caused by infarction from occlusion of a lenticulostriate artery; symptoms include
(1)hemiparesis (95%), which is often the only symptom {pure motor syndrome} (Box 1.5)
(2)hemi-sensory loss (60%)
(3)aphasia or neglect (60%)
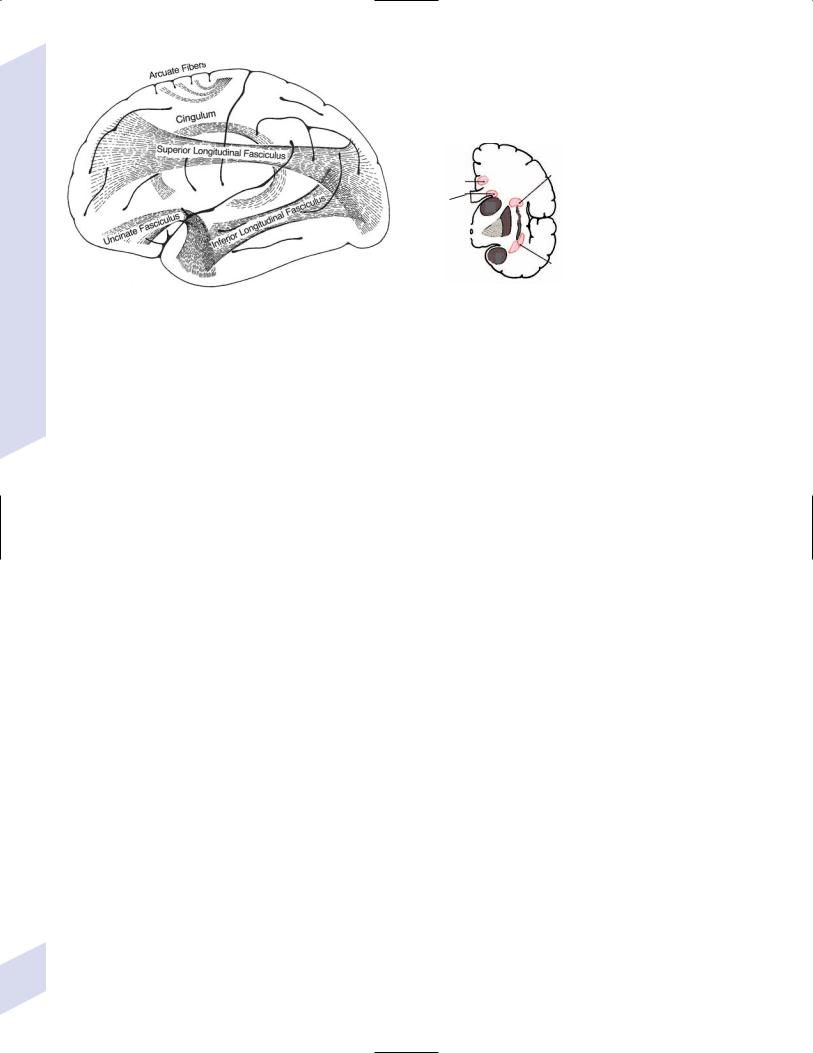
c.association fibers
i.short association fibers/“U” fibers: short-range cortical–cortical projections
ii.long association fibers (Fig. 1–8)
(1)cingulum: connects the temporal and prefrontal heteromodal cortices with the cingulate gyrus
(2)uncinate fasciculus: connects the temporal and prefrontal heteromodal cortices
Figure 1–6 Leukoaraiosis. (From Hosten N, Liebig T, CT of the Head and Spine. Stuttgart, Germany: Georg Thieme; 2002:31, Fig. 1.33b. Reprinted by permission.)
Box 1.5
Differentiate striatocapsular syndrome from primary motor cortex lesions that may allow reflex movements of the apparently paralyzed limb (mediated by monomodal motor association areas).
Subcortical White Matter
Figure 1–7 Internal capsule. (From Greenberg MS, Handbook of Neurosurgery. 3rd ed. Greenberg |
9 |
Press; 1994:114, Fig. 23.12. Reprinted by permission.) |
1 Neuroanatomy
|
|
Superior |
|
|
Cingulum |
longitudinal |
|
|
fasciculus |
|
|
|
|
|
|
|
Superior |
|
|
|
frontooccipital |
|
|
|
fasciculus |
|
|
|
|
Inferior |
|
|
|
frontooccipital |
|
A |
|
fasciculus |
B |
Figure 1–8 Long association fibers. (A) Lateral view; (B) transected hemi- |
1992:49, Fig. 18 and right, from Kahle W, Color Atlas/Text of Human |
||
sphere at the level of the insula. (Left, from Mumenthaler M, Neurological |
Anatomy, Vol. 3: Nervous System and Sensory Organs. 4th ed. Stuttgart, |
||
Differential Diagnosis. 2nd ed. Stuttgart, Germany: Georg Thieme; |
Germany: Georg Thieme; 1993:247, Fig. D. Reprinted by permission.) |
||
(3)superior longitudinal fasciculus: connects the parietal and temporal lobes with the frontal lobe; includes the arcuate fasciculus, lesions of which cause conduction aphasia
(4)inferior longitudinal fasciculus: connects the occipital cortex with the inferior temporal and fusiform gyri; important for the ventral occipitotemporal neural network, which is involved in face and object recognition (the “what” pathway)
(5)superior occipitofrontal fasciculus: interconnects all the lobes; important for the dorsal parietofrontal neural network, which is involved in spatial orientation (the “where” pathway)
(6)inferior occipitofrontal fasciculus: connects the inferior temporal, fusiform, and lingual gyri with the frontal lobe
d. commissures
i.corpus callosum: regions of the cortex project through the corpus callosum to symmetric regions in the contralateral hemisphere, and occasionally to other regions; fibers originate from cortex layer 3
(1)pathophysiology
(a)transection of the corpus callosum: symptoms are generally detectable only by specific testing, and include
(i)inability to name objects or read words presented in the visual hemifield of the nondominant hemisphere
(ii)inability to name objects felt by the nondominant hand
(iii)inability to write with the nondominant hand
(iv)impaired spatial construction skills with the dominant hand
(v)callosal alien hand syndrome—discoordination of simultaneous voluntary hand movements
ii.anterior commissure: connects the caudal orbitofrontal, anterior temporal, and parahippocampal cortices as well as the contralateral olfactory nerve to the piriform cortex for bilateral olfactory representation
iii.posterior commissure: carries fibers of the medial longitudinal fasciculus, posterior thalamus, pretectal nuclei, mesencephalic accessory ocular motor nuclei, and superior colliculi
iv.hippocampal commissure: minimal size and function in humans
10
Putamen
Caudate |
|
Internal |
Cingulate |
capsule |
gyrus |
|
Globus |
|
pallidus |
Extreme capsule
underneath Superior the insula temporal
gyrus
Claustrum
Anterior commissure |
Olfactory cortex |
|
(see enlargement) |
Septum pellucidum above the fornices
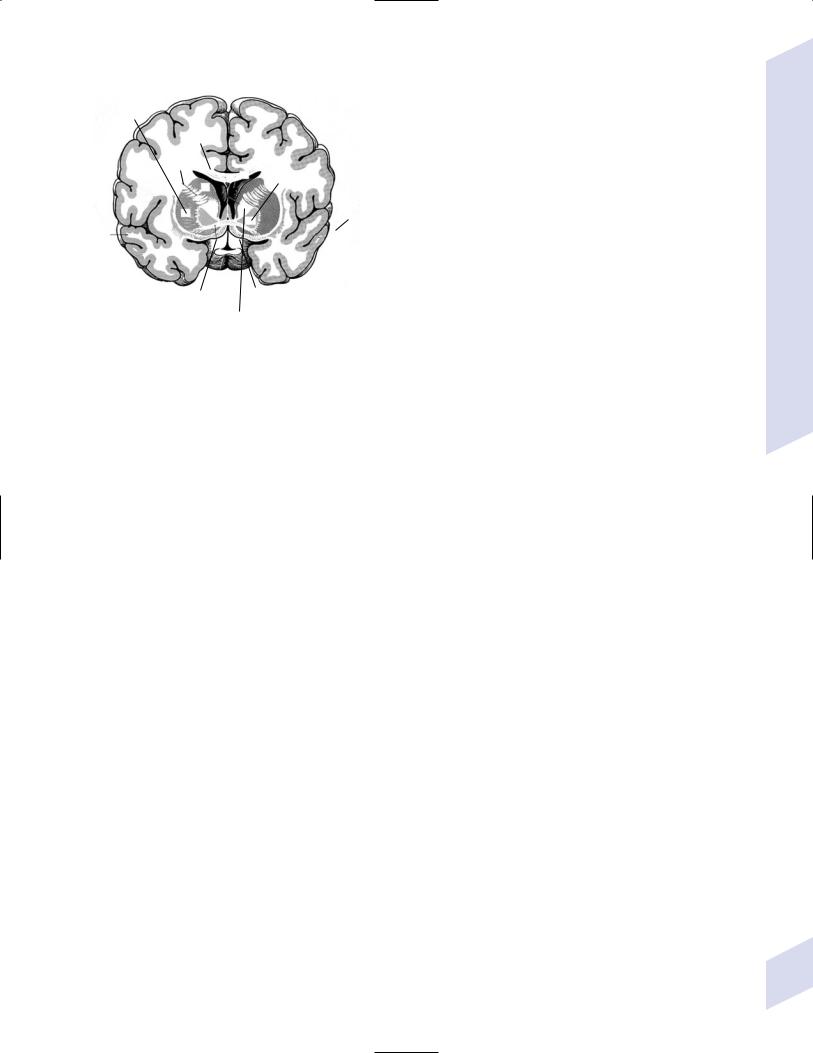
Figure 1–9 The basal forebrain posterior to the nucleus accumbens demonstrating the olfactory cortex, septal nuclei, and ventral basal ganglia (inferior to the anterior commissure). (From Kahle W, Color Atlas/Text of Human Anatomy, Vol. 3: Nervous System and Sensory Organs. 4th ed. Stuttgart, Germany: Georg Thieme; 1993:201, Fig. B; 211. Reprinted by permission.)
III. Basal Forebrain (Fig. 1–9)
1.Septal region: Includes
a.septal nuclei: the lateral septal nucleus receives input from the medial olfactory stria, the hypothalamus, and the hippocampus via the fimbria-fornix, and relays that information to the medial septal nucleus; projections from the medial nucleus (acetylcholinergic as well as GABAergic) go to the hippocampus via the fornix and to the brainstem via the medial forebrain bundle
b.nucleus of the diagonal band of Broca, which provides acetylcholinergic innervation to the hippocampus as do the septal nuclei
c.nucleus accumbens: the equivalent of the caudate and putamen for a limbic basal ganglia loop (Table 1–2); receives dopaminergic afferents from the ventral tegmental area and is involved in positive reinforcement and craving behaviors
d.islets of Calleja, which have a poorly defined function
2.Nucleus basalis of Meynert: Similar to the septal nuclei but provides acetylcholinergic innervation to the cortex (particularly pronounced in limbic and paralimbic areas) and extrinsic cholinergic innervation to the striatum (although 80% of striatal acetylcholine is intrinsic) and not to the hippocampus; functions in memory formation and retrieval
IV. Basal Ganglia (Fig. 1–10)
1.Striatum: Includes the caudate, putamen, and nucleus accumbens; some also include the claustrum
a.neuron types: the motor striatum (caudate and putamen) and the limbic striatum (nucleus accumbens) are cytologically identical
i.spiny neurons: project from the striatum to the globus pallidus and substantia nigra
(1)nondopaminergic inputs to the spiny neurons terminate on the tips of their spines; dopaminergic inputs terminate on the spine bases, thereby modulating the efficiency of the nondopaminergic inputs
Basal Ganglia
11
1 Neuroanatomy
12
(2)subtypes
(a)type I spiny neurons: contain GABA and enkephalin, and express D2 receptors; act in the indirect pathway from the putamen
(b)type II spiny neurons: contain GABA, substance P, and dynorphin, and express D1 receptors; act in the direct pathway from the putamen
ii.aspiny neurons: the interneurons of the striatum
(1)subtypes
(a)type I aspiny neurons: contain GABA
(b)type II (large) aspiny neurons: contain acetylcholine
(c)type III aspiny neurons: contain somatostatin and neuropeptide Y
b.effects of lesions
|
Caudate |
|
Septum |
|
Stria |
pellucidum |
|
terminalis |
|
|
and |
|
|
thalamo- |
|
|
striate |
Fornices |
|
vein |
|
|
|
|
Thalamus |
Habenula |
Postcentral |
|
at the end |
gyrus |
Pineal |
of the stria |
|
|
medullaris |
Superior and inferior colliculi
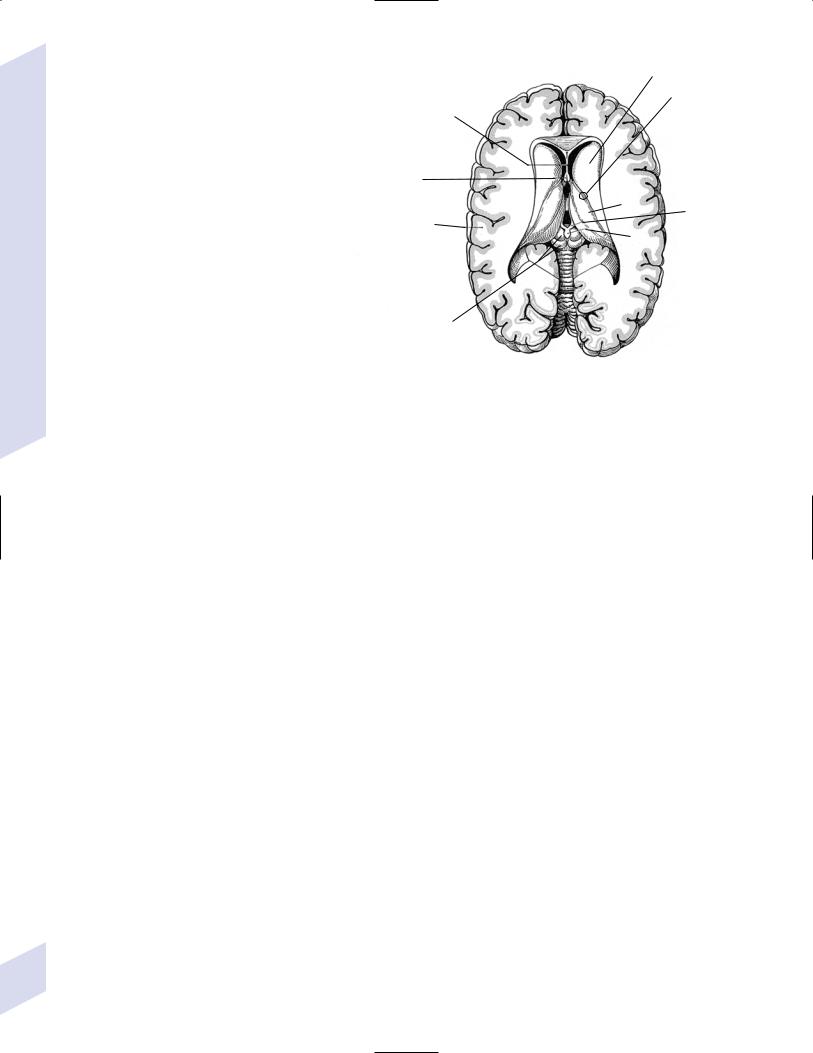
Figure 1–10 Superficial view of the diencephalic roof. (From Kahle W, Color Atlas/Text of Human Anatomy, Vol. 3: Nervous System and Sensory Organs. 4th ed. Stuttgart, Germany: Georg Thieme; 1993:207, Fig. B. Reprinted by permission.)
i.caudate lesions cause contralateral choreoathetosis, abulia, and behavioral disinhibition
ii.putamen lesions cause
(1)contralateral hemidystonia (70%) hemichorea or hemiparkinsonism
(2)hypophonic dysarthria with a transcortical motor aphasia-like language impairment due to bradykinesia
(3)impairment of short-term memory
(4)contralateral tilting and falling (in lesions that involve the globus pallidus)
2.Globus pallidus: contains large motor-type neurons and interneurons
a.unilateral lesions cause contralateral hemidystonia and/or hemiparkinsonism, abulia, and short-term memory loss; bilateral lesions may also cause akinetic mutism
3.Substantia nigra: lesions produce contralateral parkinsonism; subdivisions include
a.pars compacta (dopaminergic): has a predominantly afferent function in the basal ganglia loops; projects to the striatum via the nigrostriatal dopaminergic pathway
b.pars reticulata (GABAergic): has a predominantly efferent function in the basal ganglia loops; projects to the ventral anterior and ventral lateral nuclei of the thalamus
4.Ventral tegmental area: projects to the nucleus accumbens via the mesolimbic dopaminergic pathway; analogous to the substantia nigra for the limbic striatum
5.Subthalamic nucleus
a.lesions cause hypotonia and continuous flailing movements of the extremities sparing the face due to uncontrollable contractions of the proximal musculature {ballismus}
i.ballismus typically involves the contralateral half of the body {hemiballismus}, and may involve only a single extremity {monoballismus}
ii.ballismus is a violent type of chorea, and it may resolve over time into chorea
iii.ballismus may occur with lesions elsewhere in the basal ganglia, thalamus, or motor cortex, although these forms are generally milder than that caused by subthalamic nucleus lesions
Figure 1–11 Coronal section of the thalamus at the level of the mammillary bodies. (From Kahle W, Color Atlas/Text of Human Anatomy, Vol. 3: Nervous System and Sensory Organs. 4th ed. Stuttgart, Germany: Georg Thieme; 1993:163, Fig. B. Reprinted by permission.)
(1)transient dysfunction of the basal ganglia may underlie a rapidly reversible form of hemiballismus that occurs during hyperglycemia
iv.treatment: antipsychotic medications that have antagonist properties on D2 receptors (i.e., not clozapine); stereotactic lesioning or deep brain stimulation of the internal globus pallidus or ventral anterior– ventral lateral thalamus
6.General circuitry: See p. 12 (Table 1–2)
a.output projections (GABAergic) from the globus pallidus and substantia nigra reticulata to the (Box 1.6)
i.thalamus via ansa lenticularis and the lenticular and thalamic fascicles
ii.subthalamic nucleus
Box 1.6
Ansa lenticularis also carries ascending cerebellar projections to ventral lateral thalamic nucleus.
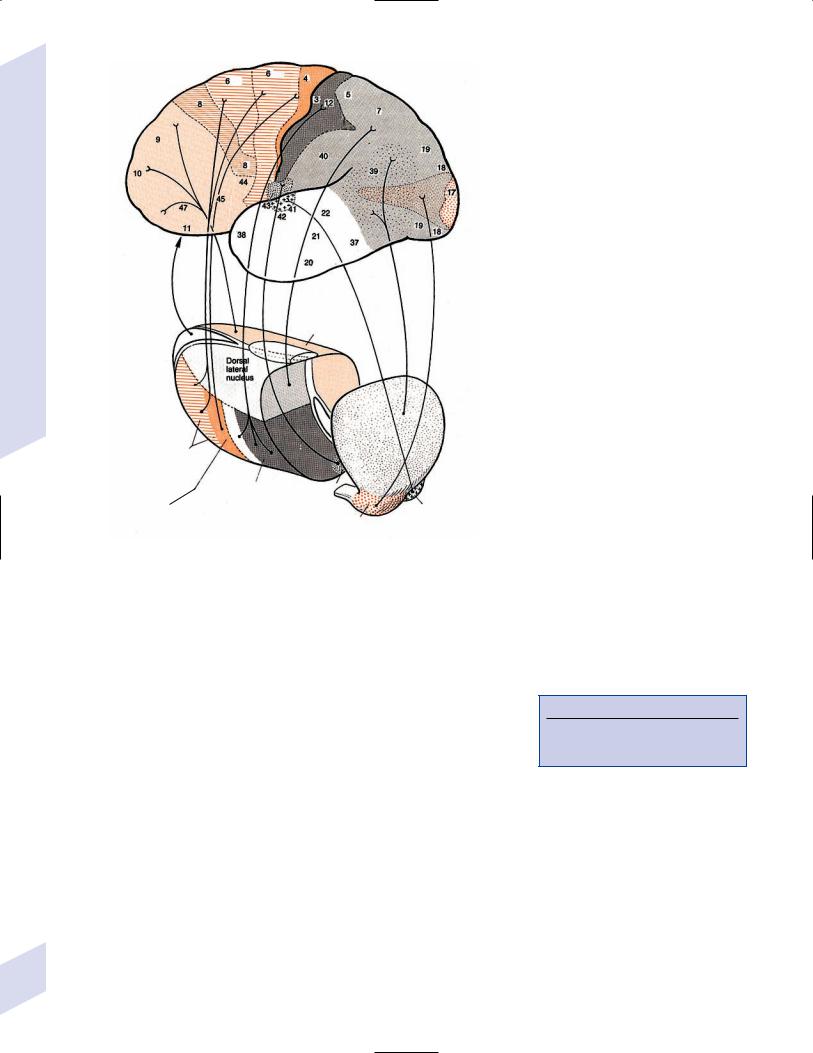
V. Thalamus (Fig. 1–11)
1.Functional divisions (Fig. 1–12) a. cortical relay nuclei
i.anterior nuclei group (AG): functions in memory processing, learning, and attention
(1)subcortical afferents: hippocampus and mammillary bodies via mammillothalamic tract (part of the circuit of Papez)
(2)bilateral lesions cause
(a)anterograde amnesia, when they occur in conjunction with bilateral lesions of the dorsomedial thalamic nuclei
(b)anteroand retrograde amnesia {Korsakoff’s syndrome}, when they occur in conjunction with bilateral mammillary body and mammillothalamic tract lesions
ii.ventral nuclei group (Box 1.7) (Box 1.8)
(1)ventral anterior nuclei
(2)ventral lateral nuclei: afferents involve not only globus pallidus and substantia nigra reticulata, but cerebellum as well
(a)lesions cause contralateral
Box 1.7
Subcortical Aphasias
Caused by dominant-side lesions of the anterior limb of internal capsule head of caudate nucleus and/or putamen
Thalamic nuclei: dorsomedial nucleus or ventral nuclei group, which usually produces a mixed transcortical aphasia sparing reading and writing.
Box 1.8
Ventral anterior nuclei and ventral lateral nuclei are the motor nuclei of ventral nuclei group.
Thalamus
13
1 Neuroanatomy
14
To cingulate gyrus
|
Dorsomedial |
Anterior nucleus |
nucleus |
|
Anterior ventral and
lateral nucleus |
|
Pulvinar |
|
|
(superior colliculus) |
||
(from globus pallidus |
|
||
|
|
|
|
and substantia nigra |
|
|
|
pars reticulata) |
|
|
|
|
Ventroposterolateral |
Taste |
|
From cerebellum |
nucleus, from |
|
Medial |
medial lemniscus, |
|
||
|
spinothalamic tract |
Lateral geniculate |
geniculate body |
|
|
body (optic nerve) |
(inferior colliculus) |
Figure 1–12 Functional divisions of the thalamus. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:275, Fig. 8.22. Reprinted by permission.)
(i)hemiataxia, usually with hemiparesis and/or sensory loss from involvement of nearby structures; develops acutely after injury
(ii)intention tremor: develops several weeks after injury
(iii)dystonia: develops several months or years after injury
(3)lateral geniculate (see p. 14) (Box 1.9)
(4)medial geniculate
(5)ventral posterior nuclei
(a)ventral posterolateral nucleus has distinct areas for proprioception (i.e., the shell of the nucleus) and cutaneous (i.e., the core) inputs from body
(b)ventral posteromedial nucleus receives trigeminal inputs for facial sensation and inputs from the solitary tract nucleus via the solitary tract conveying taste
(c)lesions cause
(i)chiro-oral syndrome—symptoms include the contralateral loss of all sensory modalities and paresthesias of the mouth, tongue, and hand
1.face, trunk, and proximal extremities are bilaterally represented (with a contralateral preference) in the ventral posterior thalamus, and so their sensation is preserved in unilateral lesions
Box 1.9
Lateral geniculate, medial geniculate, and posterior lateral nuclei: sensory nuclei of the ventral nuclei group
(ii)tactile neglect—symptoms include contralateral neglect to touch that can be so severe with nondominant thalamus lesions that it approximates an anosognosia
(iii)Dejerine-Roussy syndrome/anesthesia dolorosa (Box 1.10)
1.generally develops weeks or months after the initial lesion; occasionally develops acutely after injury
2.symptoms: burning pain that may be continuous or triggered by innocuous stimuli, and that is aggravated by emotional stress; sensory loss (proprioception other modalities; deep sensation cutaneous sensation)
a.threshold for pain is paradoxically increased in the affected regions
3.treatment: thalamotomy of the intralaminar thalamic nuclei
b.association nuclei
i. dorsomedial nucleus (Box 1.11)
(1)specific afferents are from limbic structures (ventral basal ganglia, amygdala, hippocampal formation) and hypothalamus
(2)bilateral lesions cause
(a)hypersomnolence with reduced non-REM sleep
(b)akinetic mutism, coma
(c)anterograde amnesia, when they occur in conjunction with bilateral anterior thalamic lesions
(d)Klein-Levin syndrome—symptoms include recurrent episodes lasting several days or weeks of compulsive eating, sexual disinhibition, hypersomnia with normal sleep structure, and impaired memory
(i)likely involves some simultaneous dysfunction of the posterior hypothalamus
(ii)more common in adolescent boys, and spontaneously remits in adulthood
(iii)treatment: lithium, valproate
Box 1.10
Dejerine-Roussy syndrome rarely occurs with lesions in the primary somatosensory cortex.
Box 1.11
The dorsomedial nucleus is injured during Wernicke–Korsakoff syndrome even more than the mammillary bodies.
ii.pulvinar has poorly defined visual and language functions
c.intralaminar thalamic nuclei (ITN): connections involve
i.rostral nuclei: reticular formation → ITNr → entire telencephalon and diencephalon
ii.caudal nuclei: reticular formation → ITNc → motor telencephalon and diencephalon
d.reticular thalamic nucleus (RTN): forms a thin shell on the lateral surface of the thalamus
i.connections: reticular formation, collaterals of thalamocortical projections
(particularly from intralaminar thalamus group), and cortex → RTN → all thalamic nuclei and reticular formation
2.Chemoanatomy: glutamate and aspartate are released from all thalamic nuclei except the reticular thalamic nucleus, which is GABAergic
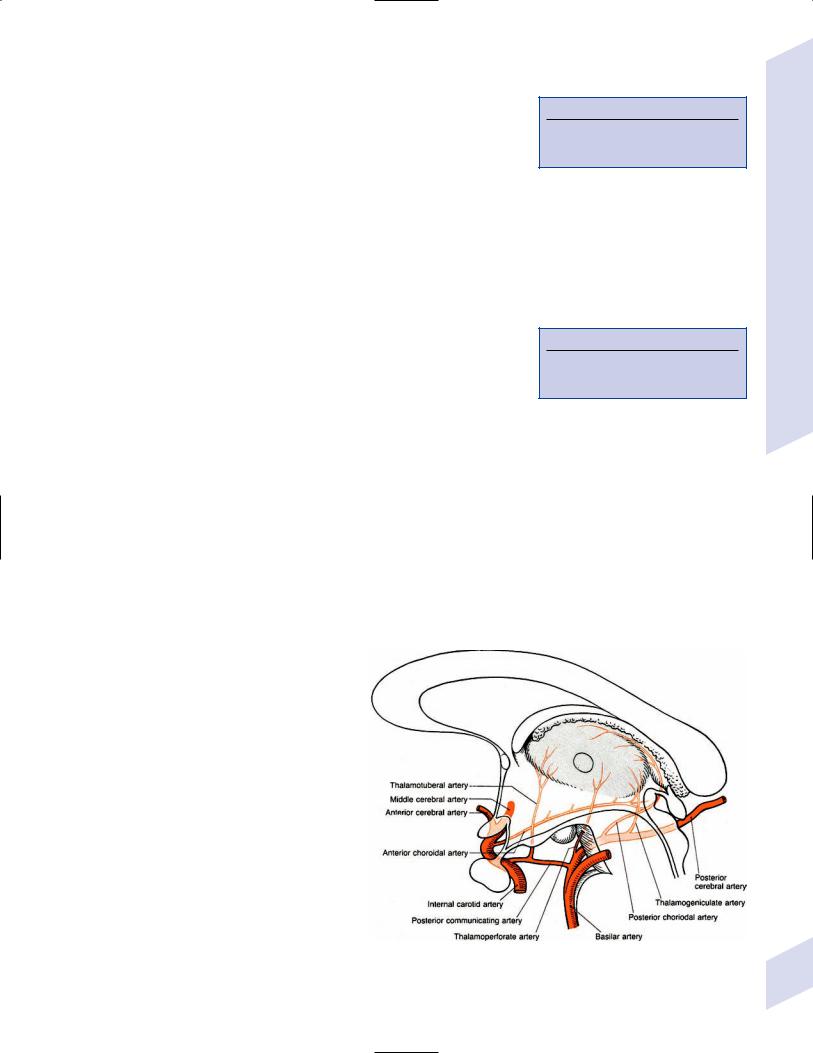
3.Vascular anatomy (Fig. 1–13)
Figure 1–13 Vascular anatomy of the thalamus. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:188, Fig. 5.8. Reprinted by permission.)
Thalamus
15

1 Neuroanatomy
A
Figure 1–14 The hypothalamus in sagittal (A) and coronal (B) views. Panel A shows only the medial hypothalamus. Arrows in panel A correspond to the cross-sections in panel B. (From Duus P, Topical Diagnosis
B
in Neurology. Stuttgart, Germany: Georg Thieme; 1998:194, Fig. 5.13; 195, Fig.5.14. Reprinted by permission.)
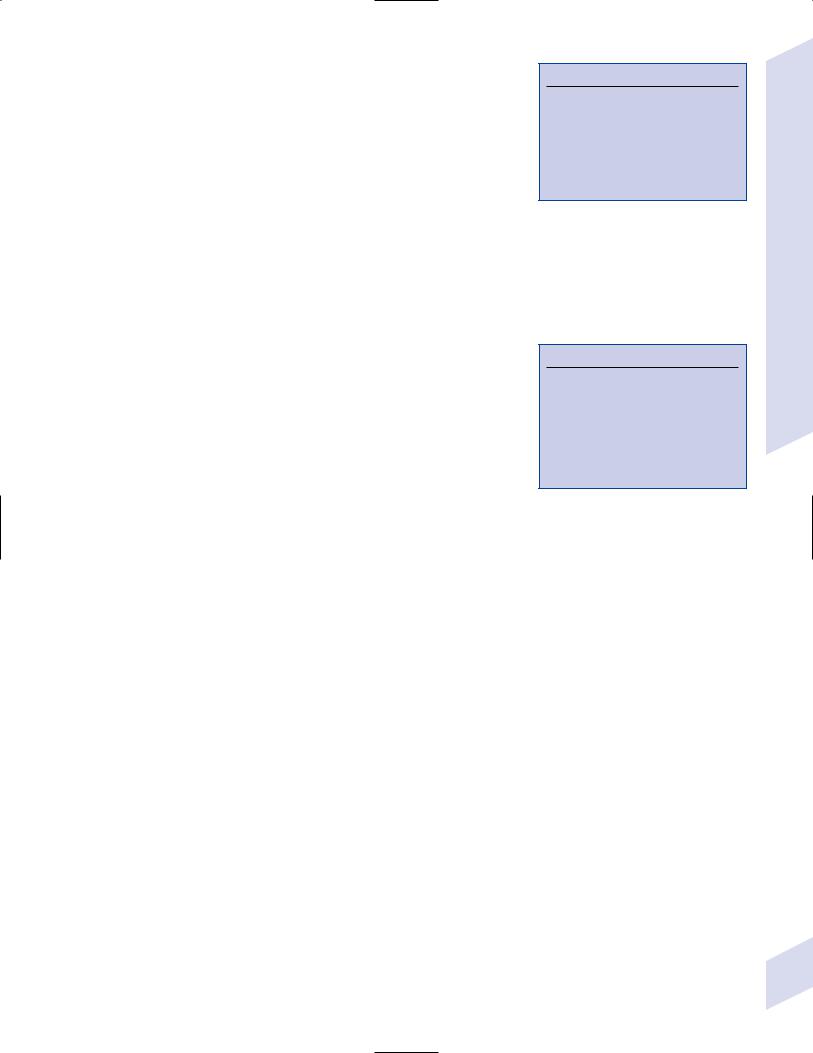
VI. Hypothalamus
1.General functions
a.controls the autonomic nervous system and the endocrine system (Box 1.12)
b.maintains visceral activity, metabolic activity, and body temperature
c.participates in goal-directed behavior and motivation, affective behavior (particularly aggressive behaviors), and sleep–wake cycling
2.Subdivisions (Fig. 1–14)
a.lateral hypothalamus: a “reward” center that likely mediates pleasurable sensations; regulates thirst (by means of vasopressin secretion from the paraventricular and supraoptic nuclei of the supraoptic region) and hunger and food-motivated behaviors (Table 1–3)
b.medial hypothalamus: divided into
i.preoptic region
(1)median preoptic nucleus: a sexually dimorphic nucleus
Table 1–3 Neurogenic Syndromes of Water Imbalance
Box 1.12
Autonomic Effects of Stimulation
Parasympathetic effects: Preoptic region (medial hypothalamus), supraoptic region (medial hypothalamus)
Sympathetic effects: Mammillary region (medial hypothalamus), lateral hypothalamus
Syndrome |
Abnormality |
Symptoms and signs |
Cause |
Treatment |
|
Syndrome of |
↑ ADH |
Hypotonic hyponatremia, ↓ |
|
inappropriate ADH |
|
Urination, ↑ |
|
secretion (SIADH) |
|
Urine osmol and [Na ] |
|
Cerebral salt |
-↑ Sympathetic activity? |
Hypotonic hyponatremia, |
|
wasting syndrome |
-Circulating natriuretic |
Polydipsia, ↑ urination, ↓ urine |
|
|
factors? |
osmol, ↑ urine [Na ], ↓ vascular |
|
|
|
volume |
|
Diabetes insipidus |
↓ ADH |
Polydipsia,↑ urination; weight loss |
|
(central type) |
|
with fluid restriction; urine does not |
|
|
|
concentrate |
|
Psychogenic |
Psychiatric |
Polydipsia,↑ urination; rarely |
16 |
polydipsia |
|
hyponatremia; urine concentrates |
|
|
|
AIDS (35%), any type of |
Water restriction, |
surgery, hypothalamic |
hypertonic saline, |
injury, systemic tumor |
demeclocycline |
Nonspecific neurological |
Salt supplements, |
injury |
fludrocortisone |
Hypothalamic injury |
Allow free access to |
(50%), including trauma; |
water; desmopressin, |
idiopathic (50%) |
vasopressin; thiazides |
Schizophrenia, chronic |
Clozapine |
alcoholism |
|
(2)medial preoptic nucleus: regulates body temperature and febrile responses (Box 1.13)
(3)lateral preoptic nucleus: promotes sleep onset, and inhibits brain regions that are necessary for maintaining arousal by GABAergic and galanin projections
ii.supraoptic region
(1)neurohormonal/magnocellular nuclei (paraventricular and supraoptic nuclei): project to the posterior pituitary where they directly release hormones
(2)suprachiasmatic nucleus: the pacemaker of circadian rhythms (e.g., sleep–wake cycle, body temperature, cortisol and growth hormone release)
(a)receives direct input from the retina; projects predominantly to the preoptic region and neurohormonal nuclei
(b)lesions cause irregular timing of sleep–wake behaviors; otherwise a normal amount of time is spent in each sleep phase
iii.tuberal region
(1)ventromedial nucleus: inhibits feeding behaviors together with the arcuate nucleus; opposed by the lateral hypothalamus (Box 1.14)
(2)hormonal/parvocellular nuclei, which includes the ventromedial and arcuate nuclei: project to the median eminence where they release hormones into the portal system that then influence anterior pituitary hormone release (Table 1–4)
(3)dorsomedial nucleus, tuberal nucleus
iv.mammillary region
(1)mammillary bodies: receives projections from the hippocampus via the postcommissural fornix
(a)serves in the circuit of Papez for memory formation; one of the chief sites of atrophy in Wernicke–Korsakoff syndrome
(2)posterior nucleus: a “punishment” center that likely mediates negative affect; also involved in
(a)inducing and maintaining wakefulness by diffuse histamine projections
(b)initiating rage and fighting behaviors
(c)regulation of the sympathetic nervous system
Table 1–4 Hypothalamic and Anterior Pituitary Hormones
Hypothalamic |
Effect on pituitary |
Pituitary hormone |
Target tissue |
hormone |
hormones |
target |
hormones |
|
|
|
|
Thyrotropin-releasing |
( ) TSH |
Thyroid |
T3, T4 |
hormone (TRH) |
|
|
|
Growth hormone releasing |
( ) GH |
Whole body |
n/a |
hormone (GHRH) |
|
|
|
Corticotropin releasing |
( ) ACTH |
Adrenal cortex |
Glucocorticoids, |
hormone (CRH) |
|
|
adrenocorticoids |
Gonadotropic-releasing |
( ) LH |
Testes, ovaries |
Sex steroids |
hormone (GnRH) |
( ) FSH |
|
|
Somatostatin |
( ) GH |
Whole body |
n/a |
|
( ) Prolactin |
Breast |
|
Dopamine |
( ) Prolactin |
Breast |
n/a |
Oxytocin |
n/a |
Smooth muscle in |
n/a |
|
|
uterus and |
|
|
|
mammary gland |
|
Vasopressin/antidiuretic |
n/a |
Distal renal tubule |
n/a |
hormone (ADH) |
|
|
|
|
|
|
|
Box 1.13
Responses involved in body temperature regulation include:
Heat loss (preoptic region): vasodilation; sweating, panting; decreased metabolism
Heat production (posterior nucleus): vasoconstriction; shivering, piloerection; increased metabolism
Box 1.14
Froehlich’s Syndrome
Symptoms—Obesity, failure of sexual development, and polyuria, occurring mostly in boys
Cause—Hypothalamic lesions producing multiple releasing hormone deficiencies
Differential diagnosis—Prader–Willi syndrome
Hypothalamus
17

1 Neuroanatomy
18
(3)posterolateral nucleus: induces and maintains wakefulness by diffuse orexin/hypocretin projections
3.Connections (Fig. 1–15)
4.Vascular supply
a.anterior hypothalamus: recurrent artery of Huebner
b.middle hypothalamus: thalamotuberal branches from posterior communicating artery
c.posterior hypothalamus: thalamoperforating branches of posterior cerebral artery
VII. Cerebellum
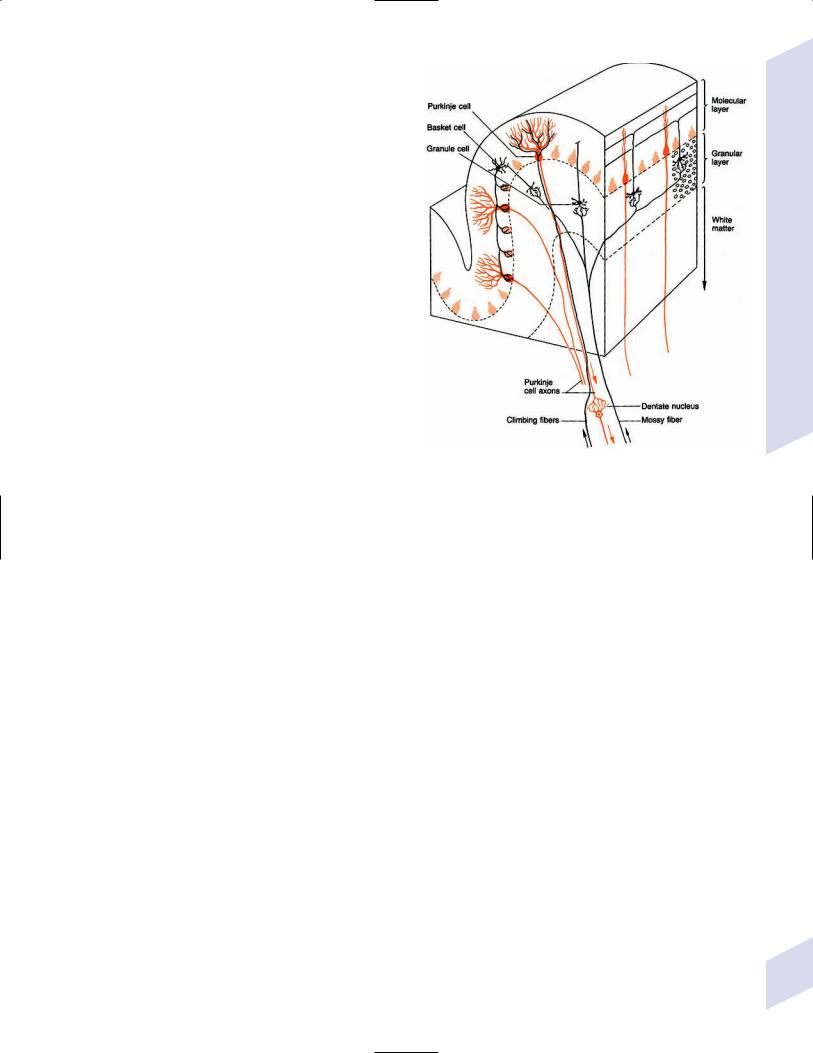
A
1. Types of cerebellar neurons (Fig. 1–16) |
|
||
a. |
molecular layer |
|
|
|
i. |
Purkinje neurons: have a flat den- |
|
|
|
dritic field that is organized per- |
|
|
|
pendicular to the long axis of folia |
|
|
|
(1) receive excitatory inputs from |
|
|
|
granular cells, which send |
|
|
|
parallel fibers that pass per- |
|
|
|
pendicularly through the den- |
|
|
|
dritic field of the Purkinje |
|
|
|
neurons |
|
|
|
(2) send inhibitory GABAergic pro- |
|
|
|
jections to the deep cerebellar |
|
|
|
nuclei and directly to the lat- |
|
|
|
eral vestibular nucleus |
|
|
ii. |
interneurons (stellate cells, basket |
|
|
|
cells): all are GABAergic and in- |
|
|
|
hibitory on the Purkinje neurons; |
B |
|
|
all are excited by parallel fibers |
Figure 1–15 Afferents (A) and efferents (B) of the hypothalamus. (Refer to Figure |
|
|
from the granular cells |
1–14 for nuclei labels.) (From Duus P, Topical Diagnosis in Neurology. Stuttgart, |
|
iii. |
Bergman glia: form shells around |
Germany: Georg Thieme; 1998:196, Figs. 5.15, 5.16. Reprinted by permission.) |
|
|
||
|
|
the Purkinje cell bodies, exposing |
|
|
|
only synaptic sites; also serve to guide granule cell migration during |
|
|
|
development |
|
b. |
granular layer |
|
|
i.granular cells: each granular cell receives input from a single mossy fiber in a multisynaptic complex {glomerulus} that also involves inhibitory Golgi neuron inputs
ii.Golgi neurons: receive input from parallel fibers, but unlike Purkinje neurons they exhibit a three-dimensional dendritic field that extends into the molecular layer
(1)send inhibitory projections to the glomeruli
2.General afferent fibers
a. cerebellar relay nuclei
i.inferior olivary complex: sends glutamatergic projections to Purkinje neurons and Golgi cell dendrites {climbing fibers} (Box 1.15)
ii.pontine nuclei and arcuate nuclei (ectopic pontine nuclei), which in turn receive projections from the motor cortex {corticopontine tract}
Box 1.15
Pontine nuclei arcuate nuclei, and all sensory inputs form mossy fiber inputs (glutamate) to the granular layer glomeruli
b.sensory inputs
i.spinal cord via spinocerebellar tracts
ii.medial and inferior vestibular nuclei
iii.vestibular division of CN VIII directly
iv.tactile, auditory, and visual inputs by poorly defined routes
c.modulatory inputs: pedunculopontine tegmental nucleus (acetylcholine); locus coeruleus (norepinephrine); ventral tegmental area (dopamine); medullary raphe nuclei (serotonin)
3.Efferents from the deep cerebellar nuclei: outputs are glutamatergic except for some of the projection to the inferior olivary nucleus, which is GABAergic
a.fastigial nucleus: contralateral ipsilateral projections to the vestibular nuclei and pontomedullary reticular formation {uncinate fasciculus} via the inferior cerebellar peduncle (juxtarestiform body)
b.interposed nuclei (globose, emboliform): contralateral projections to the red nucleus via the superior cerebellar peduncle
c.dentate nucleus: contralateral projections via the superior cerebellar peduncle to the thalamus (ventrolateral nuclei and a small part of the ventroposterolateral nucleus), the ocular motor and accessory ocular motor nuclei, and the pontomedullary reticular formation
4.Efferents from direct projections: The flocculus and the vermis of the anterior cerebellar lobule project directly to vestibular nuclei
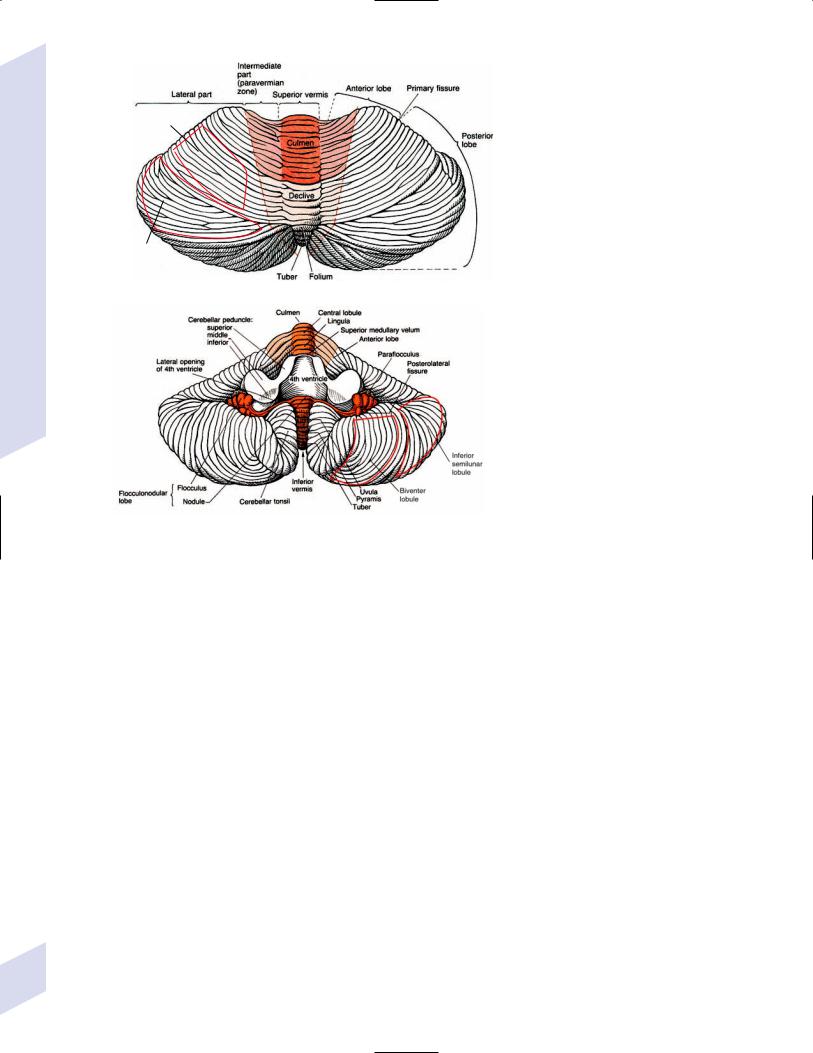
5.Subdivisions based on function (Fig. 1–17)
Figure 1–16 Types of cerebellar neurons and basic connections of the cerebellum. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:166, Fig. 4.3. Reprinted by permission.)
a.vestibulocerebellum (includes the flocculus, nodulus, and uvula): regulates postural reflexes, equilibrium, and autonomic nervous system activity
i.principal afferents are from the vestibular nuclei and the vestibular part of CN VIII
ii.output is to the fastigial nucleus; the flocculus also has a direct output to the vestibular nuclei
b.spinocerebellum (includes the anterior cerebellar lobe, simple lobule, and vermis and paravermal areas of the biventer lobule): involved in the maintenance of muscle tone
i.principal afferents are from the spinocerebellar tracts and precerebellar nuclei of the reticular formation (see p. 29)
ii.output is to the interposed nuclei (emboliform and globose nuclei); the vermis of anterior cerebellar lobe also has direct output to the vestibular nuclei
c.pontocerebellum (includes the superior and inferior semilunar lobules of the cerebellar hemispheres): involved in the coordination of voluntary movements
i.principal afferents are from the pontine nuclei
ii.output is to the dentate nucleus
6.Vascular supply (Fig. 1–18)
7.Pathophysiology: the classic cerebellar syndromes
a.rostral vermis syndrome—symptoms include gait ataxia with some limb ataxia (mostly in the lower extremities), but rarely hypotonia, nystagmus, or dysarthria
i.typical cause: atrophy from chronic alcoholism
Cerebellum
19
1 Neuroanatomy
20
Simple lobule
Superior semilunar lobule
A
B
Figure 1–17 Dorsal (A) and ventral (B) views of the cerebellum. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:165, Figs. 4.1, 4.2. Reprinted by permission.)
b.caudal vermis syndrome—symptoms include gait ataxia without limb ataxia, axial disequilibrium, nystagmus, and a rotated head posture
i.typical cause: midline tumors, posterior fossa surgery
c.hemispheric syndrome—symptoms include ipsilateral limb ataxia and dysarthria; does not have nystagmus, but may have ocular dysmetria
i.typical cause: infarction
d.pancerebellar syndrome—symptoms include bilateral symptoms of the other three types of cerebellar syndromes
i.typical cause: infection, paraneoplastic syndromes
8.Pathophysiology: the uncommon cerebellar syndromes
a.symptoms include an oropharyngeal apraxia causing dysphagia, mutism, and an inability to open the eyes; also may exhibit urinary retention
i.typical cause: surgical removal of midline cerebellar tumors
(1)typically develops 1–3 days after surgery
(2)symptoms generally improve but may leave residual dysarthria
b.cerebellar fits/diencephalic autonomic seizures—symptoms include transient decerebrate rigidity occurring most commonly in comatose patients with intracranial mass lesions located in the posterior fossa; often are mistaken for seizures
i.typical cause: transient episodes of increased intracranial pressure, which likely cause periods of mesencephalic dysfunction
c.cerebellar cognitive-affective syndrome—symptoms include slowed decision making, personality changes (disinhibited or blunted), and impaired spatial and language abilities
i.typical cause: pancerebellar diseases

A
B
Figure 1–18 Vascular anatomy of the cerebellum. (A) Midline view; (B) Inferior view. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:173, Figs. 4.8, 4.9. Reprinted by permission.)
VIII. Brainstem
A. Mesencephalon
1.Motor output centers
a.eye movements: oculomotor nucleus, trochlear nucleus
b.red nucleus
i.has connections with (Box 1.16)
(1)motor cortices
(2)cerebellar deep nuclei: dentate nucleus (parvocellular red nucleus); interposed nuclei (magnocellular red nucleus)
(3)inferior olivary complex
(4)spinal cord
ii.pathophysiology: palatal myoclonus
(1)classically develops several months after lesions of the central tegmental tract, although 25% of cases are idiopathic; lesions elsewhere in the triangle of Mollaret (Box 1.17) just cause intention tremor
Box 1.16
Cerebellar Peduncles
Superior cerebellar peduncle/brachium conjunctivum:
Afferents from the ventral spinocerebellar tract, red nucleus, tectum, and modulatory inputs
Efferents to the red nucleus (from interposed nuclei) and thalamus (from dentate nucleus)
Middle cerebellar peduncle: Exclusively carries afferents from the pontine nuclei
Inferior cerebellar peduncle:
Restiform body: Carries afferents from the inferior olivary nucleus, dorsal spinocerebellar tract from Clarke’s column (exteroceptors from below T1), lateral cuneate nucleus from fasciculus cuneatus (exteroceptors from above T1), and arcuate nuclei
Juxtarestiform body: Carries bidirectional connections with the vestibular nuclei
Box 1.17
Triangle of Mollaret—Bidirectional connections between ipsilateral red nucleus, ipsilateral inferior olivary complex, and contralateral dentate nucleus
Brainstem
21
1 Neuroanatomy
22
(2)symptoms
(a)bilateral palatal myoclonus and the perception that is a clicking sound at 2–3 Hz from the eustachian tube opening caused by tensor veli palatini contractions (innervated by CN V3); both are continuous during sleep, unlike virtually all other movement disorders
(b)pendular nystagmus (30%) that is often vertical and asymmetric; head and/or extremity tremor (10%); uncontrollable vocalizations and spasmodic dysphonia caused by laryngeal myoclonus
(3)diagnostic testing: MRI may show hypertrophy of inferior olivary nucleus more than 3 weeks after injury to the central tegmental tract
2.Other centers
a.accessory ocular motor nuclei
b.dopaminergic nuclei
i.substantia nigra pars compacta: forms the nigrostriatal dopaminergic pathway to the caudate and putamen
ii.ventral tegmental area: forms
(1)mesocortical dopaminergic pathway to the frontal lobe, anterior cingulate gyrus, and mesial temporal lobe
(2)mesolimbic dopaminergic pathway to the nucleus accumbens, amygdala, and septal nuclei
c.substantia nigra pars reticulata
d.mesencephalic reticular formation
e.inferior colliculus
3.Pathophysiology: The anterior mesencephalic syndromes (Fig. 1–19)
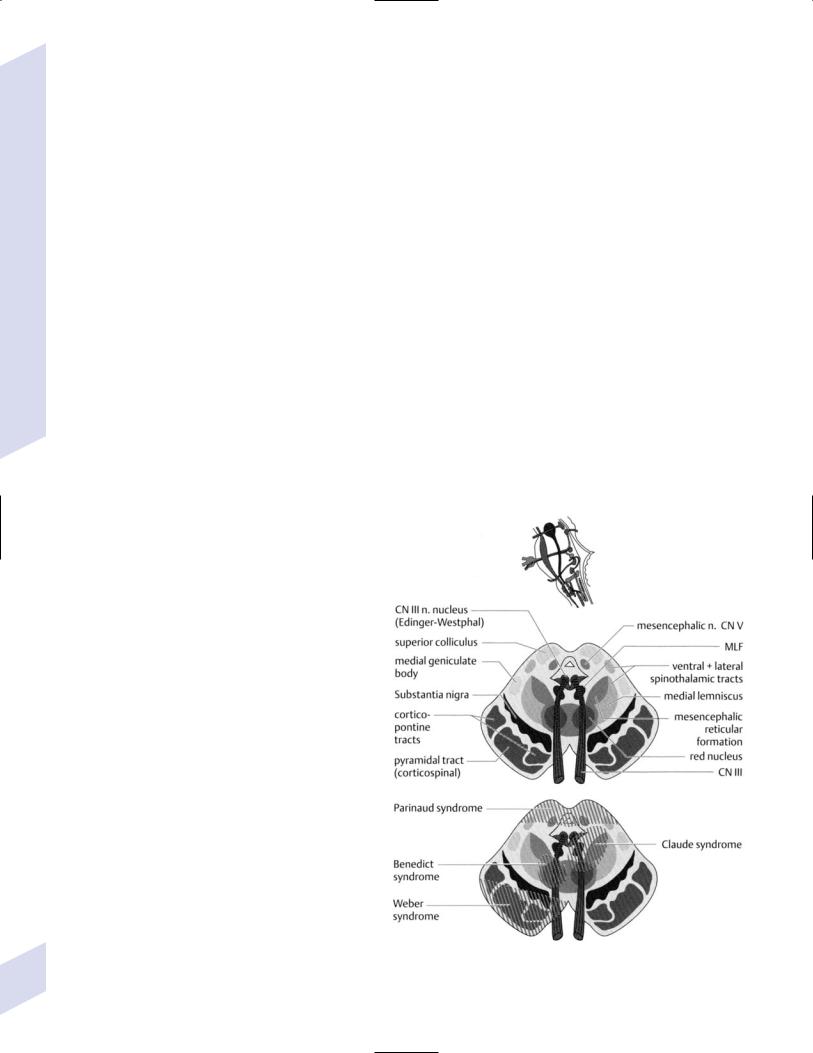
a.Weber’s syndrome—symptoms include ipsilateral CN III palsy (pupil-involving) with contralateral hemiplegia; caused by lesions of the cerebral peduncles
b.Claude’s syndrome—symptoms include ipsilateral CN III palsy (usually partial) with contralateral ataxia; lesion involves fibers of CN III and the dentato-rubro-thalamic tract as it decussates after leaving the superior cerebellar peduncle, but not always the red nucleus, which sits immediately superior to the dentato-rubro- thalamic tract
c.Benedikt’s syndrome—symptoms include ipsilateral CN III palsy with contralateral dyskinesias (parkinsonian tremor, chorea, athetosis); caused by lesions involving the red nucleus, medial substantia nigra, and fibers of CN III
d.von Monakow’s syndrome—symptoms include ipsilateral CN III palsy with contralateral hemisensory loss for vibratory and position sense; caused by lesion involving the fibers of CN III and the medial lemniscus
e.peduncular hallucinosis
i.symptoms
(1)formed visual hallucinations involving bizarre, vivid characters and objects that are often cartoonish in nature; hallucinations are recognized as such by the patient, and are purely visual in nature
Figure 1–19 Anterior mesencephalic syndromes. (From Tsementis SA, Differential Diagnosis in Neurology and Neurosurgery. Stuttgart, Germany: Georg Thieme; 2000:171, Fig. 15a. Reprinted by permission.)
(2)disrupted sleep–wake cycles
(3)occasionally CN III palsy
ii.specific lesions have been located bilaterally in the medial substantia nigra pars reticulata; symptoms may relate to dysfunction of the mesencephalic reticular formation with disinhibition of dream generation
f. top-o’-the-basilar syndrome
i.symptoms
(1)somnolence, coma
(2)amnesia
(3)visual and ophthalmologic dysfunction: hemianopia or complete vision loss; Anton’s syndrome, Balint’s syndrome, Parinaud’s syndrome (see pp. 43, 45)
(4)peduncular hallucinosis
ii.caused by occlusion of the basilar tip, leading to infarction of the tegmentum, medial thalamus, and parietal and occipital lobes; may be unilateral or bilateral
g.midbrain locked-in syndrome—symptoms are as per pontine locked-in syndrome (quadriparesis, anarthria) except that no voluntary eye movements are preserved; caused by bilateral lesions of the cerebral peduncles
h.hemiparkinsonism, contralateral to lesions of the substantia nigra pars compacta
i.hemiplegia
4.Dorsal mesencephalon syndromes
a.Nothnagel’s syndrome—symptoms include ipsilateral ataxia, vertical gaze palsy, and ipsilateral CN III palsy; caused by lesions in the pretectum involving the fibers of CN III and the superior cerebellar peduncle (Box 1.18)
b.ophthalmologic syndromes (see p. 44)—Parinaud’s syndrome, syndrome of the Sylvian aqueduct, retraction nystagmus, vertical one-and-a-half syndrome
Box 1.18
Different than Claude’s syndrome, which has contralateral ataxia
Brainstem
B. Pons (Fig. 1–20)
1.Motor output centers
a.motor trigeminal nucleus: innervates mostly the muscles of mastication and tensor tympani and tensor veli palatini muscles via CN V3
b.abducens nucleus (see p. 45)
c.facial nucleus: lower motoneurons for the upper face muscles (i.e., auricular, occipital, frontalis, and corrugator supercilii muscles) are positioned dorsal to those of the other facial muscles
i.the muscles of the upper face receive bilateral innervation from the corticobulbar tract, sparing the upper face from paralysis after unilateral upper motoneuron lesions
ii.involuntary facial movements involve projections from the basal ganglia, hypothalamus, and thalamus that do not travel in the internal capsule
2.Sensory input centers
a.principal trigeminal nucleus: receives ipsilateral ascending fibers of CN V that convey large-fiber sensory information from the face; most
efferent projections of the principal trigeminal nucleus decussate |
|
immediately and ascend along the side of the contralateral medial |
|
lemniscus to the ventral posteromedial thalamus, although some |
|
trigeminal fibers ascend to the thalamus ipsilaterally, which allows |
|
unilateral thalamic lesions to spare facial sensation (as in the chiro-oral |
23 |
pattern of sensory loss) |
1 Neuroanatomy
Figure 1–20 Anatomy of the pons. (A) Upper pass; (B) Lower pass. (From Tsementis SA, Differential Diagnosis in 24 Neurology and Neurosurgery. Stuttgart, Germany: Georg Thieme; 2000:172, Fig. 15b; 173, Fig. 15c. Reprinted by
permission.)

Table 1–5 Other Trigeminal Reflexes
Ciliospinal reflex |
Pinching the neck skin causes pupil dilation |
Corneal reflex |
Corneal touch causes blinking |
Corneomandibular reflex |
Corneal touch causes jaw movement and blinking, only becomes |
|
unmasked with frontal lobe lesions (i.e., a frontal release sign) |
Glabellar/blink reflex |
Tapping on the supraorbital ridge causes blinking |
|
|
b.mesencephalic trigeminal nucleus: contains the primary sensory neurons for the muscle spindles and Golgi tendon organs of the masticatory muscles; forms monosynaptic connection with the motor trigeminal nucleus to produce the jaw jerk reflex (Table 1–5)
3.Other centers
a.locus coeruleus (see p. 48)
b.auditory nuclei (see p. 48): nucleus of the lateral lemniscus, nucleus of the trapezoid body, superior olivary nucleus
4.Pathophysiology: the ventral pontine syndromes
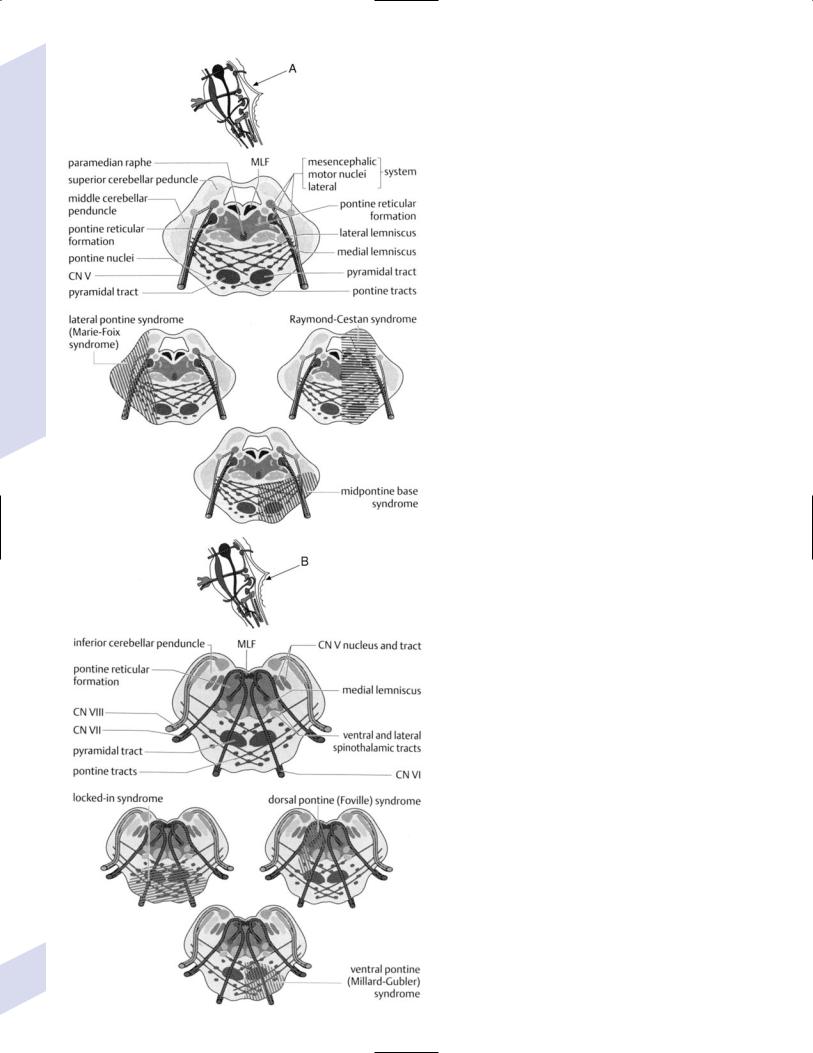
a.ataxic-hemiparesis/clumsy hand-dysarthria syndrome—symptoms include contralateral ataxia and weakness, dysarthria, and nystagmus
i.classically a pontine lesion, but can also occur with thalamocapsular or internal capsule/basal ganglia lesions
ii.may involve ipsilateral sensory loss, typically in the face, which is strongly indicative of a lesion in the thalamus
iii.usually the weakness is worse in the lower extremity, and the ataxia is worse in the upper
b.Millard-Gubler syndrome—symptoms include ipsilateral facial weakness, horizontal diplopia from CN VI palsy, and contralateral weakness of the upper and lower extremities; caused by lesions involving the facial nucleus, CN VI fibers, and the corticospinal tract
c.ventromedial pontine syndrome/Raymond’s syndrome—as per MillardGubler syndrome, but may also involve ipsilateral ataxia with middle cerebellar peduncle involvement and ipsilateral facial hemisensory loss with CN V root involvement
d.pseudobulbar palsy—dysarthria, dysphagia, unior bilateral facial weakness, extremity weakness, and emotional behaviors often without conscious perception of the emotion; caused by bilateral injury of the corticobulbar fibers that disconnects brainstem motor nuclei from the cortex
e.pontine locked-in syndrome—symptoms include weakness in all extremities, aphonia due to bilateral corticobulbar tract injury, and bilateral loss of horizontal eye movements with preservation of vertical eye movements; caused by bilateral ventral pontine lesions that involve the corticospinal and corticobulbar tracts, and bilateral CN VI fibers
f.hemiplegia—has a tendency to affect parts of the corticospinal tract (e.g., a single limb) because it is divided by the pontine nuclei
5.Pathophysiology: the dorsal pontine syndromes
a.Marie-Foix syndrome—symptoms include contralateral hemiparesis, ipsilateral ataxia, and a variable loss of small-fiber sensation; caused by lesions of the corticospinal tract, middle cerebellar peduncle, and spinothalamic tract
b.Foville’s syndrome—symptoms include ipsilateral facial weakness and lateral rectus weakness; caused by lesions involving the facial nucleus and abducens nucleus
c.Raymond-Gestan syndrome—symptoms include ipsilateral ataxia, contralateral pan-modal sensory loss, ipsilateral facial weakness, and horizontal diplopia from ipsilateral lateral rectus weakness; lesions involve the middle cerebellar peduncle, medial lemniscus and spinothalamic tract, facial nucleus or CN VII fibers, and abducens nucleus
d.internuclear ophthalmoplegia, one-and-a-half syndrome (see p. 45)
Brainstem
25
1 Neuroanatomy
26
C. Medulla (Fig. 1–21)
1.Motor output centers
a.hypoglossal nucleus: unilateral lesions cause ipsilateral tongue weakness and dysphagia dysarthria; bilateral tongue weakness may obstruct the airway
i.upper motoneuron lesions causing tongue weakness are rare
b.dorsal motor nucleus of the vagus: sends parasympathetic efferents to the heart, lungs, GI tract, and other abdominal viscera; viscerotopically organized
c.nucleus ambiguus: innervates muscles that are derived from the branchial arches, which includes masticatory, facial, pharynx, and larynx muscles
i.the cranial portion of the nucleus ambiguus innervates the soft palate (except tensor veli palatini [CN V-3]), oropharynx, and laryngeal muscles (via the recurrent laryngeal and pharyngeal branches of CN X)
ii.spinal portion of nucleus ambiguus (C1–5 levels) innervates the sternoclidomastoid and trapezius muscles
2.Sensory input centers
a.vestibular and cochlear nuclei
b.nucleus cuneatus and gracilis: receives the large fiber sensory input (from C2–T6 spinal cord levels via the fasciculus cuneatus, and from below T6 spinal cord level via the fasciculus gracilis); efferents decussate and ascend to the ventroposterolateral nucleus of the thalamus
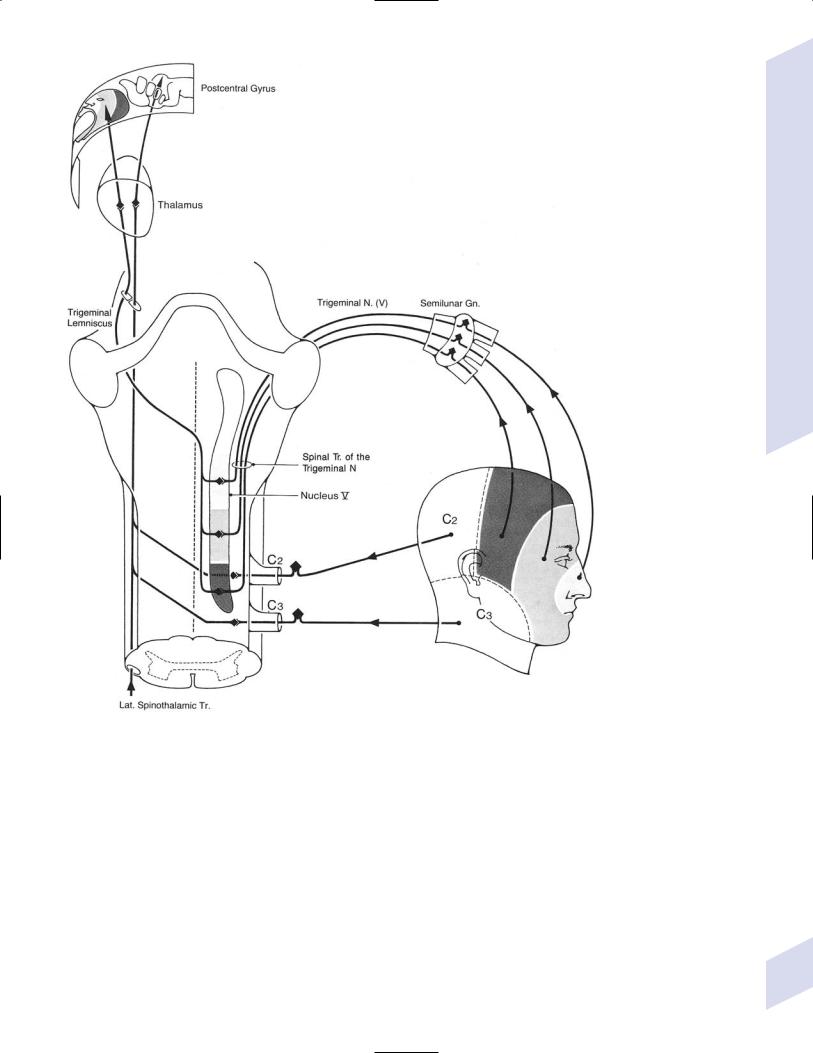
c.spinal trigeminal nucleus: receives the descending projections of CN V (for facial pain and temperature sensation) and general somatic afferents via CN V, VII, IX, X
i.facial sensory representation is divided according to CN V branch as well as into an onion-skin pattern (Fig. 1–22)
d.solitary tract nucleus
i.the rostral half of the solitary tract nucleus receives taste sensory fibers carried by CN VII, IX, X
ii.the caudal half of the solitary tract nucleus receives sensory inputs related to cardiovascular function, respiration, and GI function that are carried by CN IX and X
(1)blood pressure sense is from carotid body and sinus (via CN IX), and blood chemosensation from the aortic body and sinus (via CN X)
Figure 1–21 Anatomy of the medulla. (A) Midline medulla; (B) Inferior medulla. (From Tsementis SA, Differential Diagnosis in Neurology and Neurosurgery. Stuttgart, Germany: Georg Thieme; 2000:174, Fig. 15d; 175, Fig. 15e. Reprinted by permission.)
e.area postrema: chemosensitive for both intravascular blood and intraventricular cerebrospinal fluid; involved in triggering the vomiting reflex by either direct irritation or after irritation of thoracoabdominal CN X fibers
i. the only paired circumventricular organ
Brainstem
Figure 1–22 The onion-skin pattern of CN V sensory innervation. (From Mumenthaler M, Neurological Differential Diagnosis. 2nd ed. Stuttgart, Germany: Georg Thieme; 1992:144, Fig. 54. Reprinted by permission.)
3.Other centers
a.inferior olivary complex: composed of the principal and accessory olivary nuclei
i.afferents
(1)cerebral cortex via the corticospinal tract
(2)red nucleus via the central tegmental tract
(3)deep cerebellar nuclei, including some GABAergic projections
(4)spinoolivary tract that arises from all levels of the spinal cord
ii.efferents (to contralateral ipsilateral targets)
(1)cerebellar hemispheres and deep cerebellar nuclei (principal olivary nuclei)
(2) cerebellar vermis (accessory olivary nuclei) |
27 |

1 Neuroanatomy
28
b.arcuate nuclei: essentially are ectopic pontine nuclei (i.e., cerebellar relay nuclei) that send afferents to that cerebellum via the inferior cerebellar peduncle
c.perihypoglossal nuclei, which are accessory ocular motor nuclei (see
p.44)
4.Pathophysiology: The medullary syndromes
a.hemiplegia cruciata/cruciate paralysis—symptoms include ipsilateral lower extremity and contralateral upper extremity weakness, wherein the upper extremity weakness is worse than the lower extremity weakness; also may involve respiratory impairment, hypotension, sensory loss in the neck and posterior head, facial sensory loss in an onion-skin pattern (from spinal trigeminal nucleus injury; and/or lower cranial neuropathies (CN IX–XI)
i.caused by lesions of the lateral aspect of the corticospinal tract decussation where upper extremity fibers decussate rostral to the lower extremity fibers
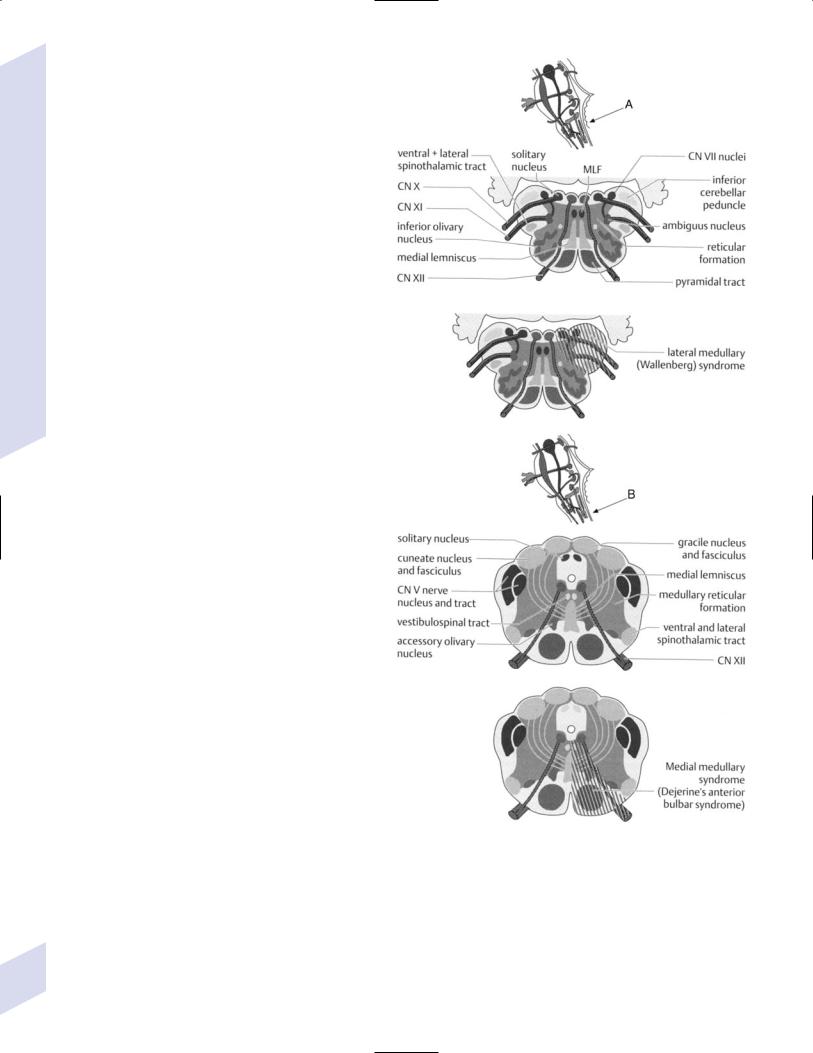
b.ventromedial medullary syndrome/Dejerine’s syndrome—symptoms include contralateral upper and lower extremity weakness sparing the face, ipsilateral weakness of the tongue, and ipsilateral loss of vibratory and position sense; caused by lesions involving the corticospinal tract, anterior CN XII fibers, and medial lemniscus
c.dorsolateral medullary syndrome/Wallenberg’s syndrome
i.symptoms
(1)ipsilateral: Horner’s syndrome; ataxia; loss of small-fiber sensation in the face; transient facial pain; weakness of the palate, larynx, and pharynx causing dysarthria and dysphonia
(2)contralateral: loss of small-fiber sensation in body
(3)hiccups
ii.caused by lesions of the lateral ponto-mesencephalic junction, usually vertebral artery infarction more so than posterior inferior cerebellar artery (PICA) infarction
d.hemimedullary syndrome/Babinski-Nageotte syndrome—the combination of ventromedial and dorsolateral medullary syndromes
D.Reticular Formation
1.Anatomy: a diffuse group of intrinsic brainstem and diencephalic neurons of varying size and shape that are separated by myelinated axons projecting in all directions {reticulum}; extends from the caudal medulla through the pons and mesencephalon into the diencephalon up to the posterior hypothalamus and thalamus (midline, intralaminar, and reticular nuclei)
2.Subdivisions and key nuclei
a.mesencephalic reticular formation
i.dorsal raphe nucleus: sends serotonergic projections to most of the forebrain and basal ganglia; many neurons colocalize serotonin with substance P
ii.pedunculopontine tegmental nucleus (acetylcholinergic): related to basal ganglia circuits
iii.ventral tegmental area (dopaminergic): related to the nucleus accumbens
b.pontine reticular formation
i.locus coeruleus: supplies norepinephrine to most of the brain and spinal cord except for the hypothalamus and the preganglionic sympathetic neurons of the spinal cord intermediolateral cell column, which receive adrenergic innervation from the medulla
(1)degenerates in conditions that affect other pigmented neurons (e.g., Parkinson’s disease) or in dementing disorders (e.g., Alzheimer’s disease)
ii.nucleus reticularis pontis: controls convergent and divergent eye movements and forms the ventral reticulospinal tract
iii.paramedian pontine reticular formation
c.medullary reticular formation (Box 1.19)
i.lateral reticular nucleus
ii.medullary raphe nuclei (pallidus, obscurus, magnus): forms serotonergic projections to the diencephalon, brainstem, and spinal cord, but not to the telencephalon; many neurons colocalize serotonin with substance P or galanin
iii.nucleus gigantocellularis
3.Afferents
a.spinoreticulothalamic tract, which carries poorly localizable pain sensation to the intralaminar thalamic nucleus and reticular formation
b.corticospinal and corticobulbar tracts
c.sensory and autonomic brainstem nuclei; fastigial nucleus; hypothalamus
4.Efferents
a.sensory and motor brainstem nuclei; cerebellum
b.ventral and lateral reticulospinal tracts
i.ventral reticulospinal tract: formed by projections from the nucleus reticularis pontis; has ipsilateral projections that are generally excitatory upon spinal motoneurons
ii.lateral reticulospinal tract: formed by projections from the nucleus gigantocellularis; has ipsilateral and contralateral projections that are inhibitory upon spinal motoneurons, causing atonia during REM sleep
c.spinal cord: nucleus raphe magnus reduces pain sensation via projections to the spinal trigeminal nucleus and posterior horn of spinal cord (endogenous analgesic systems)
i.the nucleus raphe magnus is regulated by the periaqueductal gray, which is itself regulated by the hypothalamus and amygdala
d.autonomic control centers
e.ascending reticular activating system (ARAS) and non-ARAS sleep centers
IX. Spinal Cord
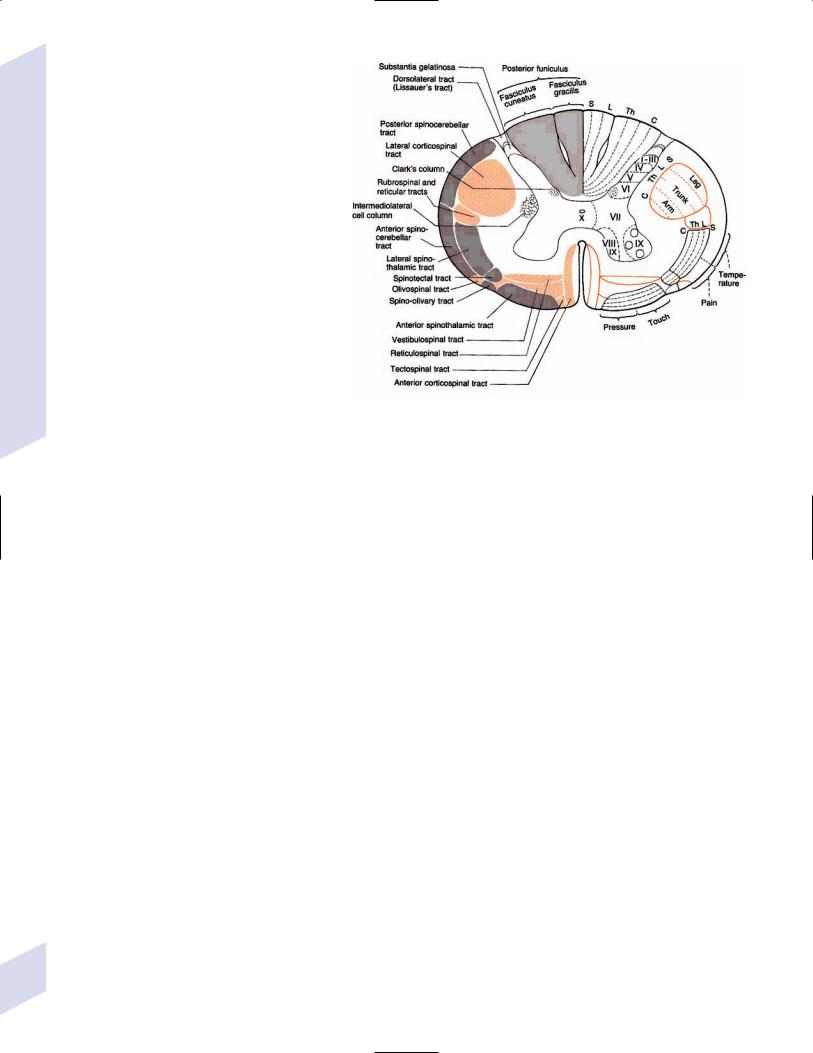
1.Long tracts (Fig. 1–23) a. anterior funiculus
i.spinothalamic tract/anterolateral system: formed by axons of secondorder neurons located in the contralateral Rexed laminae I, VI, and VII, which decussate as they ascend a few spinal cord levels
(1)fibers are arranged somatotopically in the spinal cord and also by modality (pain fibers are located anteriorly, temperature fibers are located posteriorly)
(2)carries localizable pain sensation; the spinoreticulothalamic tract carries poorly localizable pain sensation and terminates in the reticular formation and intralaminar nuclei of the thalamus
ii.anterior corticospinal tract
iii.medial longitudinal fasciculus: carries descending fibers to the cervical and upper thoracic spinal cord from the motor nuclei of the ocular muscles, nuclei of the auditory pathway, and vestibular nuclei (all of which control head positioning)
iv.vestibulospinal tracts (see p. 50): facilitates extensor antigravity muscle tone
v.ventral and lateral reticulospinal tracts
Box 1.19
The paramedian pontine reticular formation and the lateral reticular nuclei are also known as the precerebellar nuclei of the reticular formation, which form mossy fiber inputs to the cerebellum.
Spinal Cord
29
1 Neuroanatomy
b.lateral funiculus
i.lateral corticospinal tract (see p. 3): composed of projections from the
(1)primary motor cortex (Brodmann area 4) and supplementary motor area and premotor cortex (Brodmann area 6), which terminate in the anterior horn
(2)primary somatosensory cortex (Brodmann areas 3,1,2): terminates in Rexed lamina IV; modulates ascending sensory input
ii.rubrospinal tract: decussates immediately after leaving the red nucleus; projects to the anterior horn only in the upper cervical spinal cord levels
iii.spinocerebellar tracts: carry unconscious proprioception, exteroception, and somatosensory information
(1) dorsal spinocerebellar tract: |
Figure 1–23 Long tracts of the spinal cord. (From Duus P, Topical Diagnosis in Neurol- |
originates in the ipsilateral |
ogy. Stuttgart, Germany: Georg Thieme; 1998:20, Fig. 1.21. Reprinted by permission.) |
dorsal nucleus of Clarke that |
|
receives sensory inputs from |
|
spinal cord levels below T1; projects to the anterior cerebellar lobe via the inferior cerebellar peduncle
(2)ventral spinocerebellar tract: originates in contralateral Rexed laminae V–VII (lumbosacral levels), projects to the anterior cerebellar lobe via the superior cerebellar peduncle
2.Posterior funiculus: The dorsal columns, that is, the fasciculus gracilis (from lower thoracic, lumbar, and sacral spinal cord) and fasciculus cuneatus (from the spinal cord above T6)
a.both fasciculi are arranged somatotopically and according to modality (pressure and vibration are superficial, proprioception and touch are deep)
b.carry sensation for conscious proprioception, vibration, and discriminative touch (e.g., two-point discrimination, stereognosis, textures)
3.Gray matter
a.dorsal horn: important laminae include
i.substantia gelatinosa (Rexed lamina II): contains interneurons that release opioid agonists thereby limiting release of substance P from pain-sensitive dorsal root fibers
(1)supraspinal pain modulation is provided by descending norepinephrine fibers from the pontine A7 nucleus (not the locus coeruleus) and serotonergic fibers from the nucleus raphe magnus
ii.nucleus proprius (Rexed lamina III and IV): GABAergic interneurons
iii.lamina V projects into the spinothalamic tract (with laminae I and VII)
b.intermediate zone (Rexed lamina VII)
i.intermediolateral cell column: receives descending excitatory aminergic input from medullary C/A2 and A5 aminergic nuclei, and inhibitory serotonergic input from the medullary raphe nuclei
30
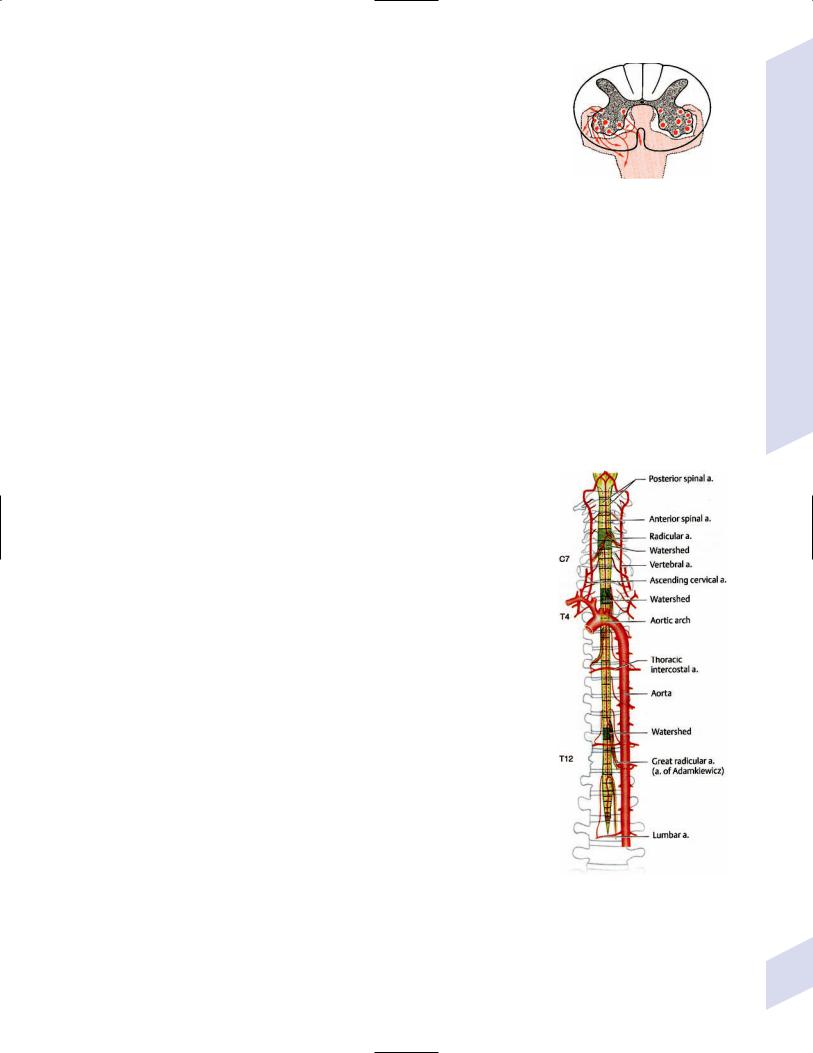
ii.Clarke’s column: located medial to the intermediolateral cell column between T1 and L3 spinal cord levels; relays proprioceptive and tactile sensory inputs from spinal cord levels below T1 (from the trunk and lower limbs) to the cerebellum through the dorsal spinocerebellar tract
c. anterior horn (Rexed lamina VIII, IX)
i.motoneurons
(1)subtypes
(a)-I motoneurons: have large cell bodies; innervate fatigable and fatigue-resistant fast-twitch muscle fibers
(b)-II motoneurons: have small cell bodies; innervate fatigueresistant slow-twitch muscle fibers
(c)motoneuron/fusimotor neurons: have very small cell bodies; innervate the intrafusal muscle fibers of the muscle spindles
(2)somatotopic arrangement of motoneurons (Fig. 1–24)
(3)modulated by excitatory fibers from locus coeruleus and inhibitory fibers from the medullary raphe nuclei
ii.interneurons: all are inhibitory, and use glycine GABA
(1)Ia “reciprocal” interneurons: receive direct excitatory input from sensory Ia (glutamate) muscle spindle afferents of the agonist muscle, and serve to inhibit the motoneurons of the antagonist muscle (e.g., during rapid contraction)
(2)Renshaw cells: innervated by excitatory acetylcholinergic collaterals of the agonist muscle motoneurons, and serve to inhibit those same motoneurons {recurrent inhibition} and the Ia interneurons of the antagonist muscles (i.e., increases antagonist tone)
(3)Ib interneurons: receive direct excitatory input from the sensory Ib afferents of the Golgi tendon organs of the agonist muscle and from cutaneous sensory receptors, and serves to inhibit the agonist muscle (e.g., to limit the force of exploratory touch)
4.Vascular supply (Fig. 1–25): Not continuous along the whole length of the spinal cord, allowing for watershed infarctions
5.Pathophysiology: incomplete spinal cord injuries
a.central cord syndrome—symptoms include bilateral weakness in the upper lower extremities because of involvement of the medial corticospinal tracts (which place fibers to the upper extremity medially) and the lower motoneurons of the cervical spinal cord
i.caused by neck hyperextension that compresses the spinal cord between an anterior osteophytic bone or herniated disk and the posterior ligamentum flavum; the pathological process is not necessarily infarction, but may involve traumatic injury and demyelination
ii.differentiate from cruciate paralysis by the presence of lower motoneuron-type weakness, the absence of facial sensory loss, and the absence of cranial nerve injury
b.anterior cord syndrome—symptoms include bilateral weakness, loss of pain and temperature sense below the level of lesion, and impaired bowel and bladder control; proprioception, vibration, and light touch are preserved
i.caused by lesions of the entire cord except the dorsal columns
ii.may develop painful dyesthesias below the level of lesion
Figure 1–24 Somatotopic organization of the spinal gray matter. (From Kahle W, Color Atlas/Text of Human Anatomy, Vol. 3: Nervous System and Sensory Organs. 4th ed. Stuttgart, Germany: Georg Thieme; 1993:47, Fig. C. Reprinted by permission.)
Figure 1–25 Vascular anatomy of the spinal cord. (From Rohkamm R, Color Atlas of Neurology. Stuttgart, Germany: Georg Thieme; 2004:23).
Spinal Cord
31
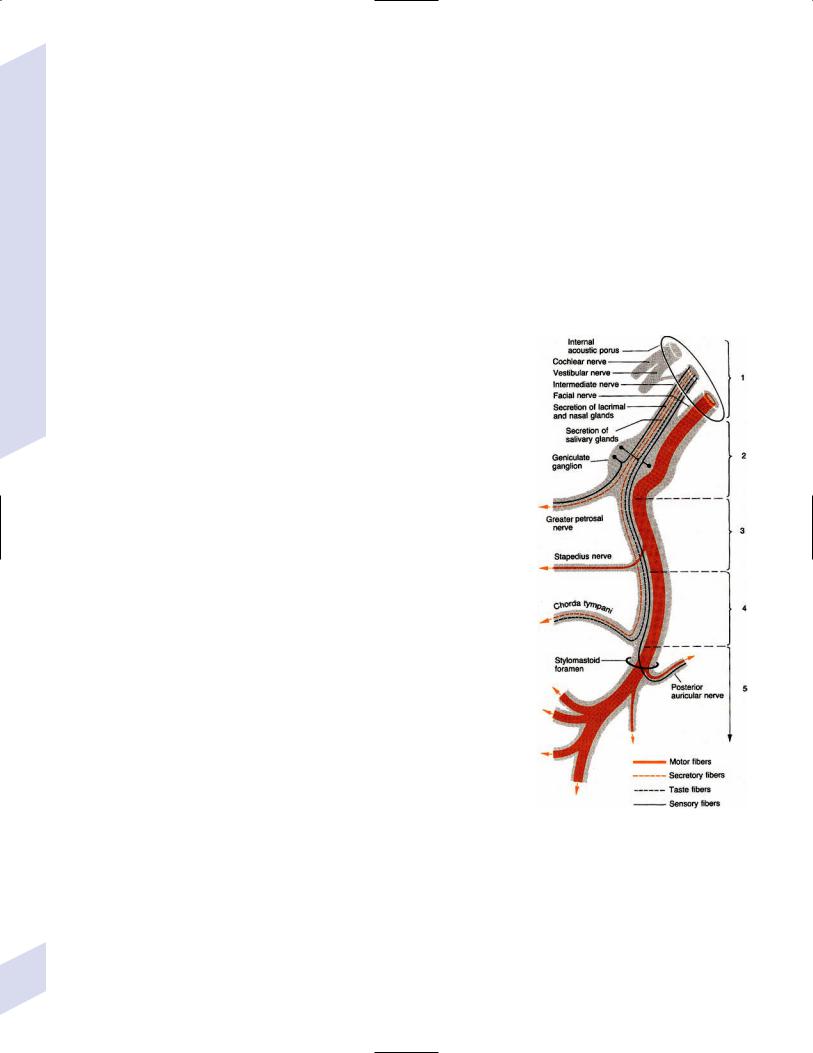
1 Neuroanatomy
32
Table 1–6 Conus Medullaris and Cauda Equina Syndromes
Conus medullaris syndrome |
Cauda equina syndrome |
|
|
Caused by lesions at L1-2 |
Caused by lesions at L4-5 or L5-s1 |
Symptoms include |
Symptoms include |
Symmetric weakness |
Asymmetric weakness |
Symmetric saddle anesthesia for small |
Asymmetric saddle anesthesia involving all |
fiber sensation |
modalities |
Normal knee reflex |
Decreased knee reflex |
Bowel and bladder incontinence, |
Bowel and bladder retention developing late |
developing early |
|
|
|
c.posterior cord syndrome—symptoms include loss of proprioception and vibratory sense below the level of lesion, and complete sensory loss in the dermatome at the level of the lesion (i.e., also involving small fiber sensation); caused by lesions of the dorsal columns
d.Brown-Sequard/hemisection syndrome—symptoms include ipsilateral sensory loss for vibration and proprioception, ipsilateral hemiplegia, and contralateral loss of pain and temperature sensation; touch is generally preserved due to representation in multiple bilateral ascending tracts
i.pain and temperature sensory loss tends to occur 1–2 levels below the level of vibration and proprioception loss because the projections of the second-order sensory neurons of the spinothalamic tracts ascend a few levels as they decussate
e.conus medullaris and cauda equina syndromes (Table 1–6)
X. Cranial Nerves
1.Anatomy
a.CN II (see p. 39); CN III, IV, VI (see p. 44)
b.CN VII: (Fig. 1–26)
c.CN XI: (Fig. 1–27)
2.Cranial nerve ganglia (Table 1–7)
3.Specific disorders of cranial nerves
a.optic neuritis ischemic optic neuropathy (see p. 38)
b.trigeminal neuralgia, glossopharyngeal neuralgia
c.hemifacial spasm—symptoms include irregular, repetitive contractions of the muscles of half of the face (including the platysma) that are induced by voluntary facial movements; often begins in the orbicularis oculi, and spreads to the other facial muscles over time
i.caused by compressive lesions of CN VII (e.g., tumor, basilar artery aneurysm) or as a complication of a resolving Bell’s palsy
ii.treat with antiepileptic drugs (carbamazepine) or surgical decompression
d.Bell’s palsy—there is generally no hyperacusis; loss of the stapedius reflex does not cause hyperacusis to normal sounds; principles of neurologic localization do not apply in Bell’s palsy; involvement of the stapedius or GSPN (dry eye) is an indication of greater severity of the lesion, not an indication of location of the lesion; the entire nerve is inflamed; the greatest degree of compression seems to be in the labyrinthine segment, where the fallopian canal is the narrowest
i.epidemiology: increased risk in diabetics but not during pregnancy
ii.symptoms: retroauricular pain that precedes facial weakness by 1–2 days; the facial weakness is relatively acute in onset but it increases over 2–5-day period
(1)associated symptoms (hyperacusis, loss of taste sensation) occur depending upon the segment of CN VII involved (Fig. 1–26)
Figure 1–26 Fiber components of CN VII and the nervus intermedius. Damage in different segments of the nerve (numbered 1–5) causes different neurological symptoms because of the involved nerve branches. The greater petrosal nerve carries parasympathetics to the lacrimal and nasal glands. The stapedius nerve acts to dampen the tympanic membrane oscillation. The chorda tympani carries taste sensation from the anterior tongue. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:113, Fig. 3.35. Reprinted by permission.)
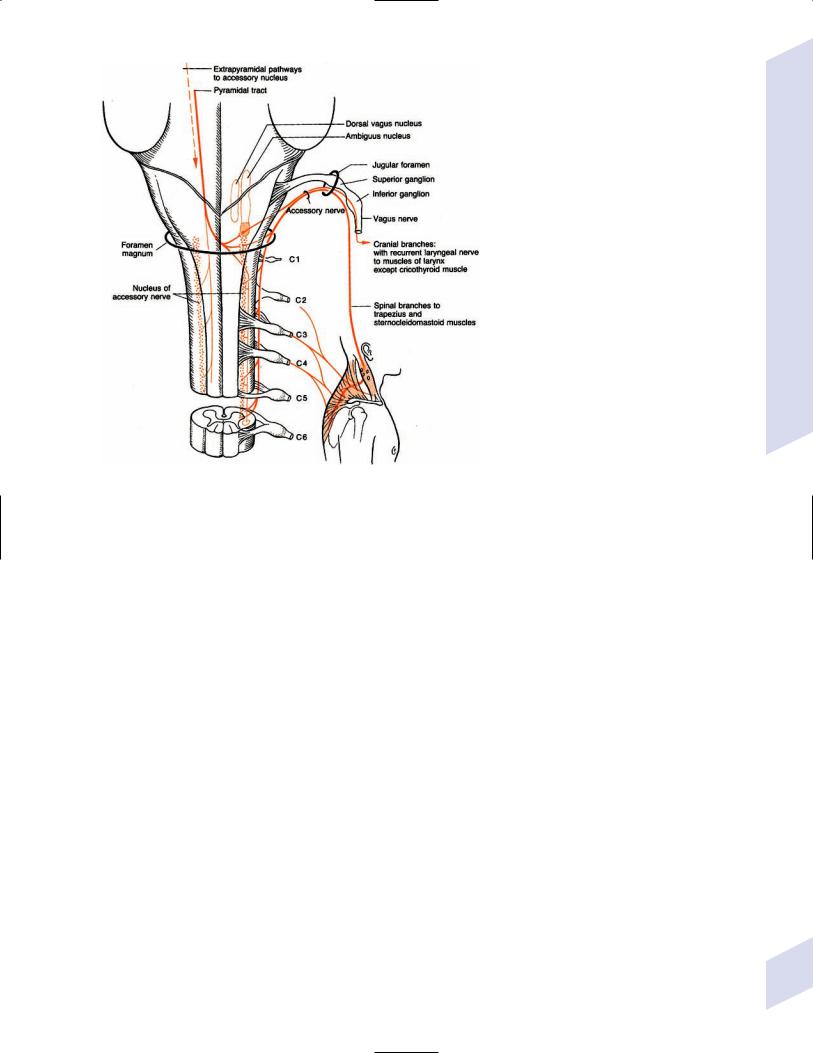
Figure 1–27 Course of CN XI. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany:
Georg Thieme; 1998:130, Fig. 3.43. Reprinted by permission.)
Table 1–7 Cranial Nerve Ganglia
Cranial nerve and ganglia |
Carries |
To |
|
|
|
|
|
CN III |
Parasympathetics from the Edinger–Westphal nucleus |
Pupil constrictor muscle, ciliary muscles |
|
Ciliary ganglion |
|||
CN V |
|
for lens accommodation |
|
General facial sensation |
Principle and spinal trigeminal nuclei |
||
Gasserian ganglion |
|||
CN VII |
Parasympathetics from the superior salivatory nucleus via |
Lacrimal and nasal glands |
|
Pterygopalatine ganglion |
|||
|
the greater petrosal nerve |
|
|
Submandibular ganglion |
Parasympathetics from the superior salivatory nucleus via |
Submandibular and sublingual glands |
|
|
the chorda tympani |
|
|
Geniculate ganglion |
General sensation from the external auditory meatus, |
Principle and spinal trigeminal nuclei |
|
|
pinna, and mastoid; taste sensation from anterior tongue |
Rostral solitary tract nucleus |
|
CN VIII |
Vestibular sensation from hair cells in ampula of the |
Vestibular nucleus and cerebellar vermis |
|
Vestibular/Scarpa’s ganglion |
|||
|
semicircular canals, and macula of utricle and saccule |
|
|
|
auditory sensation from the cochlear |
|
|
Organ of Corti |
Hair cells |
Cochlear nuclei |
|
CN IX |
Parasympathetics from the inferior salivatory nucleus |
Parotid gland |
|
Otic ganglion |
|||
Superior ganglion |
Taste sensation from the posterior tongue; general |
Rostral solitary tract nucleus; spinal |
|
|
sensation from the posterior tongue and oropharynx |
trigeminal nucleus |
|
Inferior ganglion |
As per superior ganglion |
As per superior ganglion; caudal solitary |
|
|
Chemoreception and baroreception from the carotid body |
tract nucleus |
|
|
and sinus |
|
|
CN X |
General sensation from the external auditory canal, pinna, |
Spinal trigeminal nucleus |
|
Superior/jugular ganglion |
|||
|
oropharynx, and larynx |
|
|
Inferior/nodose ganglion |
As per superior ganglion |
As per superior ganglion |
|
|
Nonpainful visceral sensation |
Solitary tract nucleus |
|
|
|
|
Cranial Nerves
33

1 Neuroanatomy
Table 1–8 Cranial Nerve Syndromes
Syndrome |
CN involved |
Location of lesion |
Typical cause |
|
|
|
|
Foix |
III, IV, V-1, VI |
Sphenoid fissure |
Mass lesions, aneurysm |
Tolosa-Hunt |
III, IV, V-1, VI; |
Cavernous sinus or |
Idiopathic granulomatous |
|
sympathetics |
superior orbital fissure |
disease, sinus thrombosis, or |
|
|
|
aneurysm |
Gradenigo |
V, VI |
Petrous apex |
Idiopathic inflammatory disease |
Vernet |
IX, X, XI |
Jugular foramen |
Mass lesions |
Collet-Sicard |
IX, X, XI, XII |
Around the occipital |
Mass lesions |
|
|
condyle |
|
Villaret |
IX, X, XI, XII; |
Around the occipital |
Mass lesions involving the |
|
sympathetics |
condyle |
internal carotid artery |
|
|
|
|
iii.treatment: protection and lubrication of the ipsilateral eye; 4–10-day course of prednisone antiherpetic drug (acyclovir, famcyclovir), started within 1 week of symptomatic onset
(1)no evidence in support of CN VII decompression surgery
iv.prognosis: 75% recover within 3 weeks; better outcomes occur in cases with partial weakness and a rapid recovery of taste
(1)increased tone of the facial muscles after CN VII regeneration may give the appearance that the facial palsy was on the opposite side
(2)facial synkinesis—aberrant regeneration of CN VII fibers that innervate the wrong target, often producing unintentional blinking, twitching, tearing, or snarling
4.Cranial nerve syndromes (Table 1–8)
XI. Autonomic Nervous System
1.Urination
a.motor innervation (Fig. 1–28)
b.sensory innervation: diffuse innervation by sympathetic and parasympathetic afferents, which are sensitive mostly to distension; somatic innervation of the external sphincter is provided by the pudendal nerve
2.Sexual function
a.erection: mediated by parasympathetic motor fibers from the intermediolateral cell column of the S2-4 spinal cord via the pelvic splanchnic nerves {nervi erigentes}
b.ejaculation: mediated by sympathetic motor fibers from the intermediolateral cell column of the L2–4 spinal cord via the hypogastric nerves
3.Pathophysiology
a.the syndrome of autonomic dysreflexia—caused by hyperactive visceral reflexes that are triggered by noxious stimuli applied to the skin or viscera below the level of the spinal cord lesion (e.g., intestinal or bladder distention, urinary tract infection, bladder catheterization, bed sores); occurs with spinal cord lesions above T6
i.symptoms: the simultaneous occurrence of
(1)hypertension (from splanchnic vasoconstriction) and reflex bradycardia
(2)profuse sweating, piloerection, and skin flushing above the spinal cord level
(3)pupillary dilatation
(4)erection and ejaculation; bowel and bladder incontinence
34 |
b. bladder dysfunction (Table 1–9) |
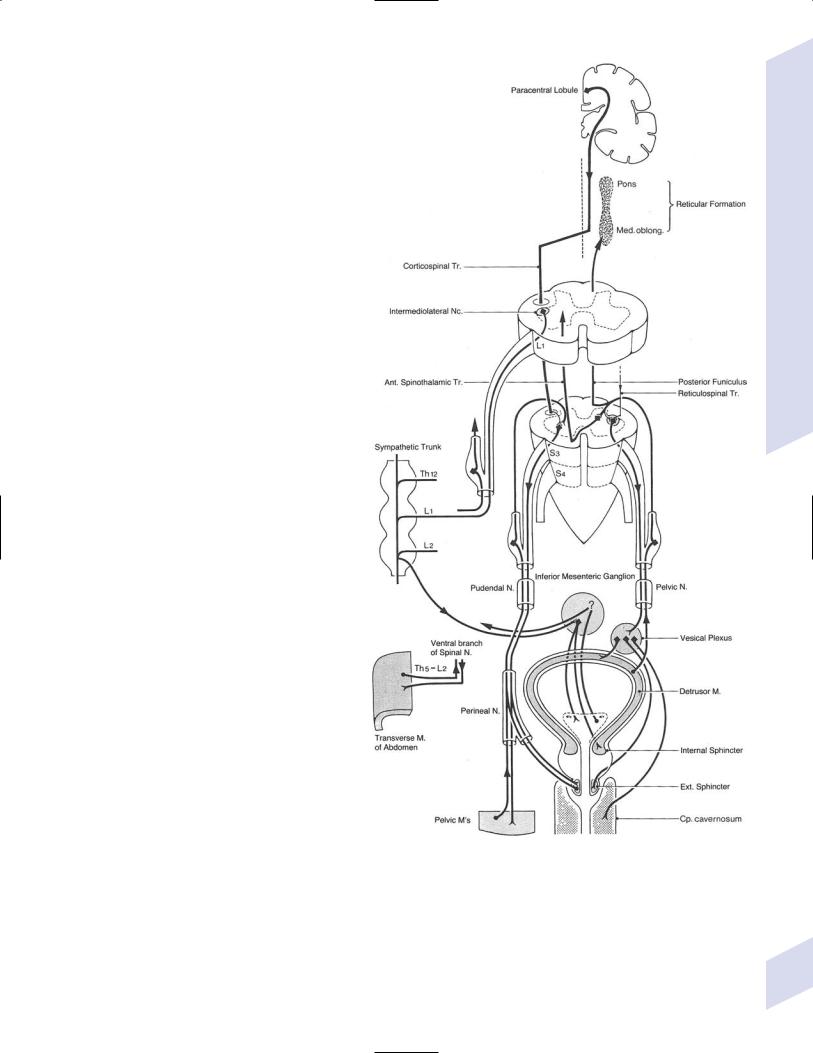
XII. Ventricle System and
Cerebrospinal Fluid (CSF)
1.Pathophysiology
a.traumatic lumbar puncture: differentiate from subarachnoid hemorrhage by declin-
ing red blood cell count in sequential tubes, a RBC–WBC (white blood cell) ratio 700 to 1, the absence of xanthochromia, the presence of clotting blood, and a protein level elevated no more than 1 mg/1000 RBCs
b.intracranial pressure/Lundberg waves: reflect arterial and venous pressure, and the respiratory cycle
i.Lundberg A waves: high amplitude increases in CSF pressure lasting 5 minutes, generally associated with increases in blood pressure
ii.Lundberg B waves: mid-amplitude in-
creases in CSF pressure lasting5 minutes, associated with cyclic breathing
iii.Lundberg C waves: low amplitude increases in CSF pressure lasting 30 seconds occurring on top of Lundberg A waves; unknown significance
c.pediatric hydrocephalus (see Ch. 12)
d.adult hydrocephalus: symptoms include dementia similar to that of bilateral frontal lobe dysfunction, usually without apraxia, agnosia, or aphasia
i.general diagnostic testing: neuroimaging
(1)requires temporal horns 2-mm wide, as well as one of the following
(a)compressed Sylvian fissures, interhemispheric fissures, and cerebral sulci
(b)frontal horn (FH)/internal diameter (ID) at the frontal horn level 0.5
(c)frontal horn/maximal intracranial diameter 0.3 {Evan’s ratio}
(2)nonspecific radiographic findings: frontal horn ballooning (“Mickey Mouse” ventricles); transependymal edema; upward bowing of the corpus callosum on sagittal sections
ii.pathophysiology: normal pressure hy- drocephalus/Hakim-Adams syndrome
Figure 1–28 Motor innervation of the bladder. (From Mumenthaler M, Neurological Differential Diagnosis. 2nd ed. Stuttgart, Germany: Georg Thieme; 1992:57, Fig. 157. Reprinted by permission.)
(1)a communicating hydrocephalus
that does not involve increased intracranial pressure; potential mechanisms include dilation caused by transiently increased intracranial pressure and/or altered elastic properties of the ventricle walls
(2)epidemiology: more common in males 60 years old
Ventricle System and Cerebrospinal Fluid (CSF)
35

1 Neuroanatomy
36
Table 1–9 Bladder Dysfunction
Type |
Lesion site |
Pattern of urination |
|
|
|
Upper motoneuron/ |
Between the frontal lobes |
Bladder contracts during filling |
spastic bladder |
and the pons |
instead of relaxing; poor emptying |
Bladder dyssynergy |
Below the pons but above |
Simultaneous detrussor and internal |
|
the conus |
sphincter contraction causes ureter |
|
|
reflux, poor emptying, and overflow |
|
|
incontinence |
Lower motoneuron/ |
Lower motoneurons |
Retention with poor emptying |
flaccid bladder |
|
|
Sensory paralytic |
Afferent sensory fibers, |
Retention with overflow incontinence; |
bladder |
dorsal columns, or |
preserved voluntary urination |
|
spinothalamic tracts |
|
Bladder areflexia |
Acute spinal shock; caudal |
Unrestricted bladder distention causes |
|
equina or conus medullaris |
overflow incontinence and retention |
|
injury |
|
|
|
|
(3)symptoms (in order of appearance)
(a)gait disturbance: Bruns’ frontal lobe ataxia (see p. 5)
(b)dementia (60%): predominant short-term memory impairment
(c)urinary incontinence from a spastic bladder; urinary urgency may precede incontinence
(4)specific diagnostic testing: no test exhibits high diagnostic accuracy
(a)lumbar puncture
(i)demonstrates normal pressure but continuous CSF pressure monitoring may exhibit Lundberg B waves or transient pressures 20 mm Hg
(ii)symptoms must improve with large-volume CSF drainage
(iii)measurement of resistance with fluid infusion into subarachnoid space is correlated with symptoms but is not predictive of shunting outcome
(b)radionuclide cisternography: retention of contrast in the ventricles 24 hours after administration is not useful for establishing the diagnosis or predicting the clinical response to shunting
(c)MRI CSF flow study: absence of CSF flow near the cerebral aqueduct does not predict the clinical response to shunting
(5)treatment: low-pressure ( 3-mm opening pressure) ventriculoperitoneal shunt
(a)patient should be sat upright slowly over several days after placement
(b)lumboperitoneal shunts generally results in overshunting and have high complication rates
(6)prognosis: incontinence and gait generally improve more than dementia
(a)responsiveness to shunting is prediced by
(i)the presence of the classic triad of symptoms
(ii)clinical improvement after lumbar puncture
(iii)opening pressure 7 mm Hg
(iv)the presence of high transient pressures on continuous CSF pressure monitoring
(v)the absence of ischemic changes on MRI
iii.hydrocephalus ex vacuo—dilation of the ventricular system due to brain atrophy that can be either diffuse or focal
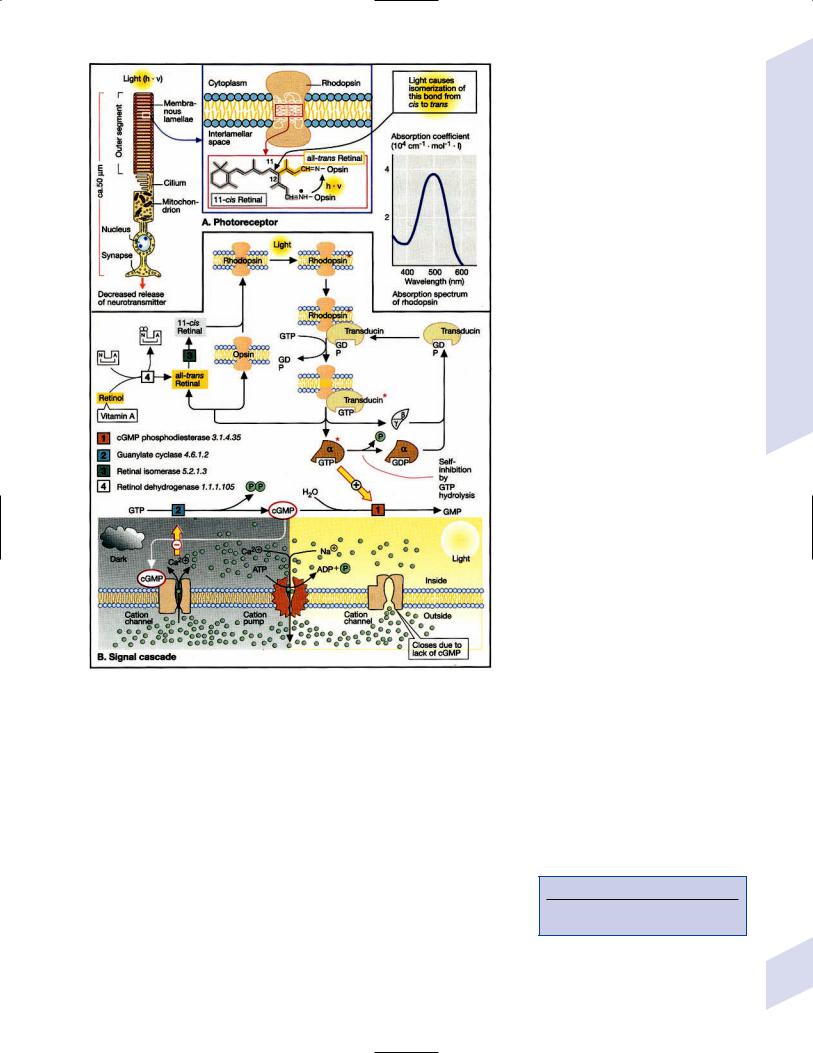
Neuroophthalmology
Figure 1–29 Photoreception in the retina. (From Koolman J, Rohm KH, Color Atlas of Biochemistry. Stuttgart, Germany: Georg Thieme; 1996:326. Reprinted by permission.)
XIII. Neuroophthalmology
A. The Sensory Visual System
1.Retina
a.vision reception (Fig. 1–29)
b.subtypes of retinal fiber bundles (Fig. 1–30)
c.pathophysiology: retinal fiber loss causes
i.decreased visual acuity with papillomacular bundle injury
ii.scotomata (Box 1.20)
(1)scotomata from papillomacular bundle injury (Fig. 1–31): central, centrocecal (a central scotoma that connects to the blind spot), or paracentral scotoma
(2)scotomata from arcuate bundle injury (Fig. 1–32): all connect with the physiological blind spot
Box 1.20
Failure of defect to connect to the blind spot suggests the lesion is retro-chiasmatic.
37

1 Neuroanatomy
38
- papillo macular bundle
Figure 1–30 Retinal fiber bundles. (From Duus P, Topical Diagnosis in Neurology. |
Figure 1–31 Papillomacular bundle injuries cause para- |
Stuttgart, Germany: Georg Thieme; 1998:83, Fig. 3.11. Reprinted by permission.) |
central (A), central (B), or centrocecal (C) scotomata. |
(a)Bjerrum/arcuate/scimitar scotoma
(b)Seidel scotoma: caused by lesions in the proximal arcuate bundle
(c)nasal step of Ronne: caused by lesions in the distal arcuate bundle
(d)isolated scotoma in Bjerrum’s area: caused by lesions in the intermediate part of the arcuate bundle
(3)scotomata from nasal bundle injury: wedge-shaped/ quadrantic temporal scotoma that connects to the blind spot but does not necessarily respect the horizontal meridian (Fig. 1–32)
d.diagnostic testing: the electroretinogram, which measures the electrical potential measured from the retina in response to a series of flashing lights; the electrical potential is generated by the ganglion cell layer, and is reduced by ganglion cell loss (e.g., optic neuropathies)
2.Optic disc: The collection of retinal ganglion cell projections (still unmyelinated); normally forms the blind spot located in the temporal visual field
a.blood supply: the circle of Zinn-Haller, which is formed by the short posterior ciliary arteries, choroid arteries, and pial arterial network
b.pathophysiology
i.scotoma and loss of visual acuity (as per retinal fiber bundle injuries)
ii.papilledema: caused by transmission of elevated intracranial pressure along the subarachnoid space of CN II, or else by elevated pressure central retinal vein; takes 24 hours to develop after elevation of intracranial pressure
Figure 1–32 Arcuate bundle injuries cause an isolated scotoma within the Bjerrum region (A), an altitudinal defect/fat Bjerrum scotoma (B), the Bjerrum scotoma (C), nasal step of Ronne (D), and the Seidel scotoma (E). Nasal bundle injury causes a temporal field defect (F).
(1)generally is asymptomatic unless long-standing and
severe, wherein it may cause visual blurring and/or loss of acuity
iii.anterior ischemic optic neuropathy (AION)—caused by ischemia of the optic disc and the most anterior portion of the optic nerve, wherein the ischemia is exacerbated by swelling of the ischemic neural tissue; usually from diseases of the short posterior ciliary arteries
(1)subtypes: both are common only in women 50 years old and in Whites

(a)arteritic AION: ischemia is the result of giant cell arteritis or other vasculitides
(b)non-arteritic AION: not related to giant cell arteritis, but rather to classic stroke risk factors; congenitally small discs may also predispose the patient to ischemia of the anterior optic nerve
(i)symptoms: painless (90%) vision loss that often develops during sleep; papilledema that can be limited to a segment of the optic disc {sectorial disc edema} and that is often associated with retinal flame hemorrhages
3.Optic nerve
a.subdivisions
i.intraocular segment (1-mm length): unmyelinated and therefore smaller diameter
ii.intraorbital segment (25-mm length): passes through the annulus of Zinn formed by the tendinous origins or the superior, inferior, and medial recti muscles
(1)normally exhibits a sinusoidal course in the orbit; a straight course suggests tension caused by proptosis, and it may cause tenting of the posterior globe
iii.intracanalicular segment (10-mm length): optic nerve turns medially and passes through optic canal; the dura of the optic nerve fuses with the periosteum, so the subarachnoid space of brain is continuous with the subarachnoid space of the optic nerve (all three layers of the dura are present in the orbit)
iv.intracranial segment (15-mm length)
b.blood supply: extracranial optic nerve is supplied by the ophthalmic artery; intracranial optic nerve is supplied by the middle cerebral artery (MCA) and the anterior communicating
artery (ACA) (Fig. 1–33)
c.pathophysiology
i.decreased visual acuity and scotomata (as for retinal fiber bundles)
ii.optic neuritis
4.Optic chiasm
a.fiber decussation
i.Wilbrand’s knee: an anterior deflection of the decussating fibers from the inferior nasal retina into the terminal part of the contralateral optic nerve; accounts for a superior temporal field cut contralateral to an optic nerve injury (Fig. 1–30)
ii.fibers from macula cross in posterior part of chiasm
Figure 1–33 The intracranial segment of CN II. (From Rohkamm R, Color Atlas of Neurology. Stuttgart, Germany: Georg Thieme; 2004:13. Reprinted by permission.)
b.blood supply: ACA, MCA, and posterior communicating artery
c.pathophysiology: almost invariably caused by mass lesions (e.g., pituitary tumors, suprasellar meningiomas, aneurysms)
i.lesions posterior to the chiasm do not decrease visual acuity (except for bilateral injury to the poles of the occipital cortex that
represent the macula)
ii.scotomata and field cuts
(1)anterior chiasmal lesions {junctional scotoma} (Fig. 1–34): ipsilateral vision loss (from optic nerve injury) and a contralateral superior temporal scotoma (from Wilbrand’s knee)
(2)lesions in the body of the chiasm: bitemporal hemi-
field scotomata |
Figure 1–34 The junctional scotoma. |
Neuroophthalmology
39
1 Neuroanatomy
40
(3)lateral chiasmal lesion: produces a nasal hemianopia; rarely occurs bilaterally
5.Optic tracts
a.contains the postchiasmal ipsilateral temporal and contralateral nasal retinal projections to the lateral geniculate nucleus
b.a small projection avoids lateral geniculate nucleus and terminates in the suprachiasmatic nucleus of the supraoptic hypothalamic region, superior colliculus, and pretectal nucleus
i.nongeniculate projections are responsible for
(1)setting circadian rhythms
(2)blind sight: the ability to respond to visual stimuli without the conscious perception of sight (i.e., cortical blindness)
(3)pupil reaction to light
c.blood supply: anterior choroidal branch of the MCA
d.pathophysiology: homonymous visual field deficits that are poorly matched in shape between eyes but that still respect the vertical meridian; congruity of the visual field deficits increases as the bilateral visual projections become more organized along the length of the optic tract
i.lesions do not decrease visual acuity, but there is a relative afferent pupillary defect in the eye ipsilateral to lesion (i.e., in the eye with the temporal field defect)
6.Lateral geniculate nucleus
a.subdivisions
i.subdivisions according to the type of vision
(1)magnocellular/M pathway: important for motion and stereopsis, but provides low spatial resolution; synapses in geniculate layers 1–2, projects to visual cortex layer 4C
(2)parvocellular/P pathway: important for fine spatial resolution and color vision; synapses in layers 3–6, projects to visual cortex layer 4C
ii.subdivisions according to eye of origin of the nerve fibers: fibers from the ipsilateral eye synapse in geniculate layers 2,3,5; fibers from the contralateral eye synapse in layers 1,4,6
iii.subdivisions according to the position of the nerve fibers in the retina: incoming nerve fibers of the optic tract are rotated 90° nasally (e.g., superior retina nerve fibers synapse medially in the lateral geniculate); outgoing nerve fibers from the lateral geniculate rotate an-
other 90° nasally (e.g., the medial lateral geniculate projections synapse inferiorly in the visual cortex)
b.blood supply
i.lateral portion: anterior choroidal branch of the MCA
ii.medial portion: posterior lateral choroidal branch of the posterior cerebral artery (PCA)
c.pathophysiology: sectoranopias, which still exhibits significant incongruity (Fig. 1–35)
7.Optic radiations
a.the projections of the lateral geniculate are divided into fibers carrying information from the superior and inferior quadrants of the retina
i.superior retinal quadrant projections from the lateral geniculate are carried straight posterior along the mesial parietal cortex before reaching the occipital lobe
ii.inferior retinal quadrant projections from the lateral geniculate must wrap anterior in front of the temporal horn before passing posterior toward the occipital cortex {Meyer’s loop}
Figure 1–35 Sectoranopias from anterior choroidal artery infarction (A) and lateral choroidal artery infarction (B).
b.pathophysiology: large lesions in Meyer’s loop cause superior homonymous quadrantic (“pie in the sky”) field defects, whereas small lesions cause scotoma-like defects; lesions in the mesial parietal lobe cause inferior homonymous quadrantic field defects (a defect that is more likely to be caused by occipital lobe lesions)
8.Primary visual cortex (Brodmann area 17/visual area I)
a.representation of visual fields on the primary visual cortex
i.visual field representation is completely inverted on the occipital cortex (e.g., the superior visual field is below calcarine fissure; the inner retina (macula) is represented on the outermost occipital cortex)
ii.macular retina has disproportionately large area of representation on the visual cortex
b.blood supply
i.outside of macular region: calcarine artery branch of the PCA
ii.macular region/pole of occipital cortex: in addition to the PCA, it may receive collaterals from the meninges that could account for the sparing of macular vision that can occur after bilateral occipital lobe infarcts
c.pathophysiology: highly congruous visual field defects; vision loss does not involve neglect, asymmetry of optokinetic nystagmus, or loss of blinking to threat
d.diagnostic testing
i.visual evoked potentials (VEP): the potential derived from occipital cortex caused by stimulation of the retina (e.g., with a strobe light or reversal of a checkerboard pattern) (Fig. 1–36)
A
Neuroophthalmology
B
Figure 1–36 The right (A) and left (B) eye VEP from a patient with optic neuritis. Delay in the P100 potential and a loss of amplitude are noted in |
41 |
the affected left eye. |
1 Neuroanatomy
42
(1)requires time-locked averaging, which averages-out the background EEG signal
(2)VEP predominantly tests macular vision because the macula has a disproportionately large cortical representation and is closer to the electrode
(3)VEP uses
(a)multiple sclerosis: prolonged latency of the positive-deflection at 100 ms (P100) can be considered as a subclinical demyelinating event, although it also occurs with other demyelinating diseases (e.g., vitamin B12 deficiency, spinocerebellar degeneration)
(b)optic neuropathy: reduced P100 amplitude is suggestive of ischemia, although it can also represent nerve compression or toxic neuropathies
(c)factitious disorders: normal VEP and electroretinogram make the subjective complaint of vision loss highly suspect
9.Association visual cortex
a.subdivisions
i.Brodmann area 18/visual area II
ii.Brodmann area 19/visual area III
iii.Brodmann area 36/visual area IV
iv.Brodmann area 37/visual area V: involved in the perception of motion {kinetopsia}, which may also involve visual area III
b.pathophysiology
i.achromatopsia (Box 1.21): caused by lesions in visual area IV (occipitotemporal junction of the fusiform and lingual gyri)
(1)hemiachromatopsia often occurs with a superior quadrantopsia and/or prosopagnosia
(2)color vision is also compromised early in disorders of the optic nerve and chiasm (particularly red perception) and may persist even if acuity returns
ii.akinetopsia: the inability to perceive moving objects in the contralateral visual hemifield, which suddenly appear when they become stationary; caused by lesions of visual area V
iii.visual neglect: the inability to perceive visual stimuli in the affected visual field when subjected to bilateral stimulation, but without outright vision loss; typified by patient complaints of running into objects on the neglected side
(1)associated with abnormal optokinetic nystagmus, and with abnormal drawing and copying abilities when the lesion is in the nondominant hemisphere
(2)caused by lesions in Brodmann areas 18 or 19/visual areas II or III
iv.prosopagnosia: the inability to recognize familiar faces; usually caused by bilateral lesions of the fusiform gyrus, although it may also occur with large injuries in the nondominant temporal lobe (e.g., as in progressive prosopagnosia, a subtype of frontotemporal lobar degeneration)
v.Anton’s syndrome—the denial of complete blindness (i.e., anosognosia for blindness) with confabulation
(1)caused by lesions that produce complete vision loss (typically bilateral medial occipital lobe lesions, although the lesions may be in the anterior visual system) in conjunction with
(a)bilateral lateral occipitoparietal cortex lesions (visual areas II and III)
(b)thalamic lesions, as in the top-o’-the-basilar syndrome
Box 1.21
Blue/yellow deficits occur early in glaucoma.
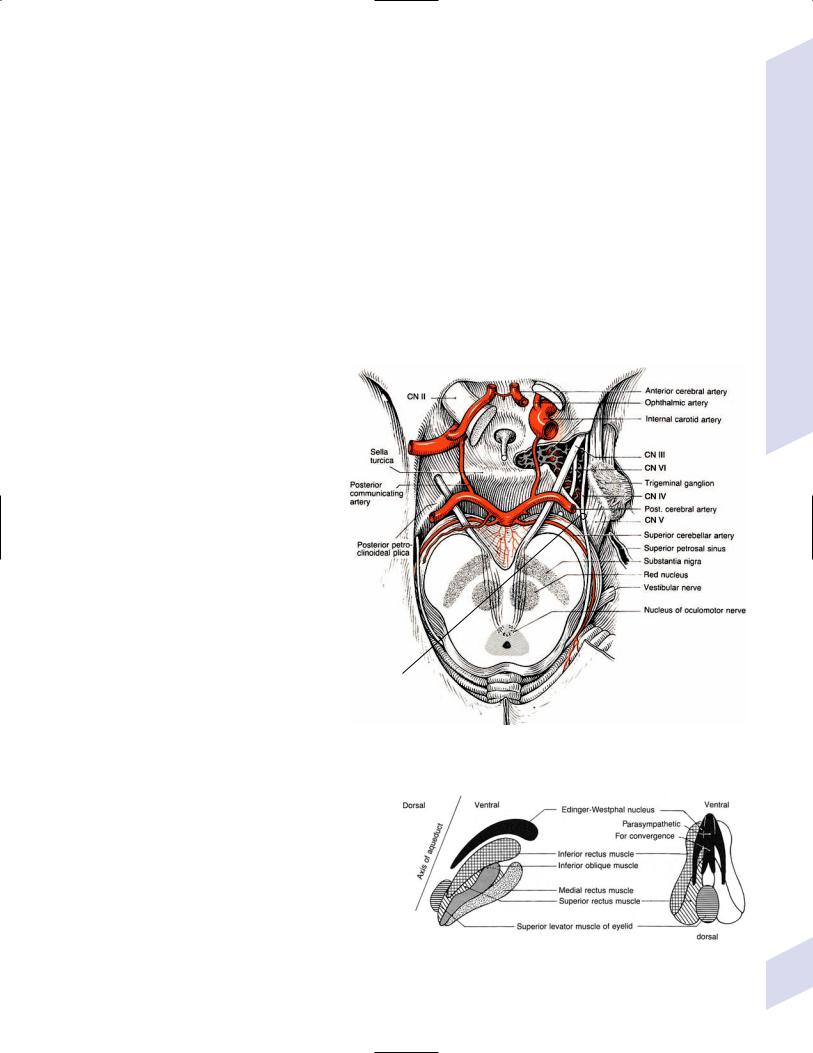
(c)any metabolic encephalopathy
(2)diagnostic testing: EEG exhibits loss of alpha rhythms that return with vision
vi.Balint’s syndrome—caused by bilateral lesions of the lateral occipitoparietal cortex, typically watershed infarction
(1)symptoms
(a)simultanagnosia: the inability to integrate an entire visual field
(b)ocular ataxia: clumsiness of voluntary movements that are guided by vision
(c)ocular apraxia: the inability to voluntarily direct gaze with saccadic eye movements
(d)neglect of the peripheral visual fields (causing apparent tunnel vision), or an altitudinal visual field neglect
B.The Ocular Motor System
1.Ocular motor cranial nerves (Fig. 1–37) a. pathophysiology
i.isolated CN III palsy
(1)risk of aneurysm (typically from the posterior communicating artery or distal basilar) is high when complete loss of parasympathetic function (e.g., ptosis and mydriasis) is accompanied by at least some ocular motor dysfunction
(a)high-risk patients should be evaluated immediately for aneurysm regardless of exam findings
(b)patients who are not at high risk should be reevaluated within a few days to determine if parasympathetic dysfunction has developed, which would make them high risk
(2)other causes
(a)pupil-sparing CN III palsy: diabetic neuropathy, atherosclerosis, vasculitis (e.g., giant cell arteritis)
Dorello’s canal
Figure 1–37 Entry of CN III, IV, V, and VI into the cavernous sinus. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:88, Fig. 3.16. Reprinted by permission.)
(b)pupil-involving CN III
palsy: tumors (chordoma, meningioma)
ii.isolated CN IV palsy: caused by trauma (40%), nerve infarction (20%), or mass lesion (10%)
iii.isolated CN VI palsy: in adults, usually caused by ischemia; in children, usually caused by viral infection
2.Oculomotor nucleus (Fig. 1–38)
a.Edinger-Westphal nucleus: contains the parasympathetic neurons that innervate the pupillary sphincter muscle and the ciliary bodies that perform lens accommodation
Figure 1–38 The oculomotor nucleus. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:87, Fig.3.15. Reprinted by permission.)
Neuroophthalmology
43
1 Neuroanatomy
44
b.convergence movements involving both medial rectus muscles are coordinated by interneurons located within the medial rectus subdivision of the oculomotor nucleus
c.pathophysiology: lesions of the oculomotor nucleus impair the ipsilateral ocular muscles innervated by CN III except for the superior rectus, which is contralaterally innervated; ptosis occurs only with lesions of the central caudal subdivision of the oculomotor nucleus, and is always bilateral
3.Trochlear nucleus: Efferent fibers decussate in the medullary velum and innervate the contralateral superior oblique muscle, which acts to move the eye downward when it is fully adducted
a.lesions cause extorsion of the affected eye and head tilting to the opposite shoulder
b.test for CN IV palsy in the presence of CN III palsy by having the patient attempt to look downward with the affected eye in abduction, which should produce a clockwise rotation of the eye if CN IV is functional
4.Abducens nucleus (Box 1.22)
a.types of neurons include
i.motoneurons projecting to the ipsilateral lateral rectus muscle
ii.neurons projecting to the contralateral oculomotor nucleus via the medial longitudinal fasciculus; these neurons are regulated by the paramedian pontine reticular formation (PPRF) to direct horizontal conjugate eye movements
(1)nucleus reticularis pontis of the pontine reticular formation coordinates the oculomotor and abducens nuclei during convergence and divergence
b.pathophysiology
i.internuclear ophthalmoplegia
(1)symptoms: lack of contralateral eye adduction during conjugate lateral gaze, but not during convergence; nystagmus in both eyes (greater in abducted eye) during conjugate lateral gaze
(2)caused by lesions of the medial longitudinal fasciculus in the pons, which is often bilateral due to the close proximity of the two fasciculi
ii.one-and-a-half syndrome—caused by lesions of both the medial longitudinal fasciculi and the abducens nucleus (an intranuclear ophthalmoplegia); symptoms include bilateral inability to adduct the eyes during conjugate lateral gaze and ipsilateral lateral rectus muscle weakness
5.Accessory ocular motor nuclei
a.mesencephalic accessory ocular motor nuclei
i.rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF): regulates vertical conjugate eye movements
(1)neurons controlling upward gaze (often specifically called the nucleus of the posterior commissure) project through the tectum (i.e., dorsal to the aqueduct) to the oculomotor and trochlear nuclei, therefore they are more sensitive to pineal masses causing Parinaud’s syndrome
(2)neurons controlling downward gaze project through the tegmentum (i.e., ventral to the aqueduct) to the oculomotor and trochlear nuclei
ii.interstitial nucleus of Cajal: involved in maintaining vertical gaze
iii.pretectal nucleus: regulates pupil light reaction; receives direct optic nerve input
(1)pathophysiology: Argyll-Robertson pupil, which is a functional elimination of the light reaction circuit without impairment of pupil constriction during accommodation
iv.nucleus of Darkschewitsch, which has an unknown function
b.pontine and medullary accessory ocular motor nuclei
Box 1.22
Near Triad Reflex
Convergence
Lens accommodation
Pupil constriction

i.paramedian pontine reticular formation (PPRF): irregulates horizontal conjugate eye movements acting via abducens nucleus
(1)excitatory burst neurons initiate saccode
(2)inhibitory burst neurons suppress contralateral abducens nucleus
(3)omnipause neurons block burst neurons and prevent saccodes
ii.perihypoglossal nuclei: have poorly defined roles in conjugate eye movements; not related to tongue movements
iii.nucleus prepositus hypoglossi: involved in maintenance of horizontal gaze along with the medial vestibular nucleus
iv.nucleus interpositus and the nucleus of Roller, which have unclear functions
c.pathophysiology involving the accessory ocular motor nuclei
i.Parinaud’s syndrome—caused by pineal-region masses or hydrocephalus that injures the pretectal region
(1)symptoms include
(a)supranuclear upgaze palsy, but preserved oculocephalic and oculovestibular-induced upgaze
(b)eyelid retraction {Collier’s sign} and lid lag with downgaze {setting sun sign}
(c)loss of convergence and accommodation
(d)Argyll-Robertson pupil
(e)convergence spasm and retraction nystagmus
(f)skew deviation
ii.syndrome of the Sylvian aqueduct—Parinaud’s syndrome also with a downgaze palsy
iii.vertical one-and-a-half syndrome—symptoms include bilateral upgaze palsy and ipsilateral monocular downgaze palsy; caused by poorly localized pretectal lesions
iv.skew deviation/vertical strabismus—symptoms include the perception of tilting of the visual fields, which is often compensated for by a head tilt
(1)caused by an imbalance in the vestibular input from lesions anywhere in the vestibular complex, vestibular division of CN VIII, or brain regions dealing with vestibular sensation including cortex; can also be seen with diffuse intracranial processes (e.g., elevated intracranial pressure, metabolic encephalopathy)
(a)variance of the skew deviation in different gaze positions suggests medullary lesion
(b)slowly alternating skew deviation suggests a mesencephalic tectal lesion
6.Superior colliculus: controls saccadic eye movements via projections to the PPRF and riMLF, and regulates gaze fixation on visual targets
7.Cerebellum: coordinates ballistic eye movement and braking during saccades
a.vermis and superior cerebellar peduncle lesions produce hypermetric contralateral saccades and hypometric ipsilateral saccades, which is the opposite of what fastigial nucleus lesions produce
b.flocculus lesions impair ipsilateral smooth pursuit movements and the ability to maintain gaze fixation after a saccade
8.Pulvinar (thalamus): involved in the maintenance and shifting of visual attention; promotes contralateral saccadic eye movements
9.Ocular motor circuit of the basal ganglia (see Table 1–2): acts selectively to gait all types of saccadic eye movements
10.Cortical gaze centers
a.frontal eye field (Brodmann area 8): directs intentional saccades and pursuit movements contralaterally; has separate neurons for selecting the new eye position and for actually moving the eyes
Neuroophthalmology
45
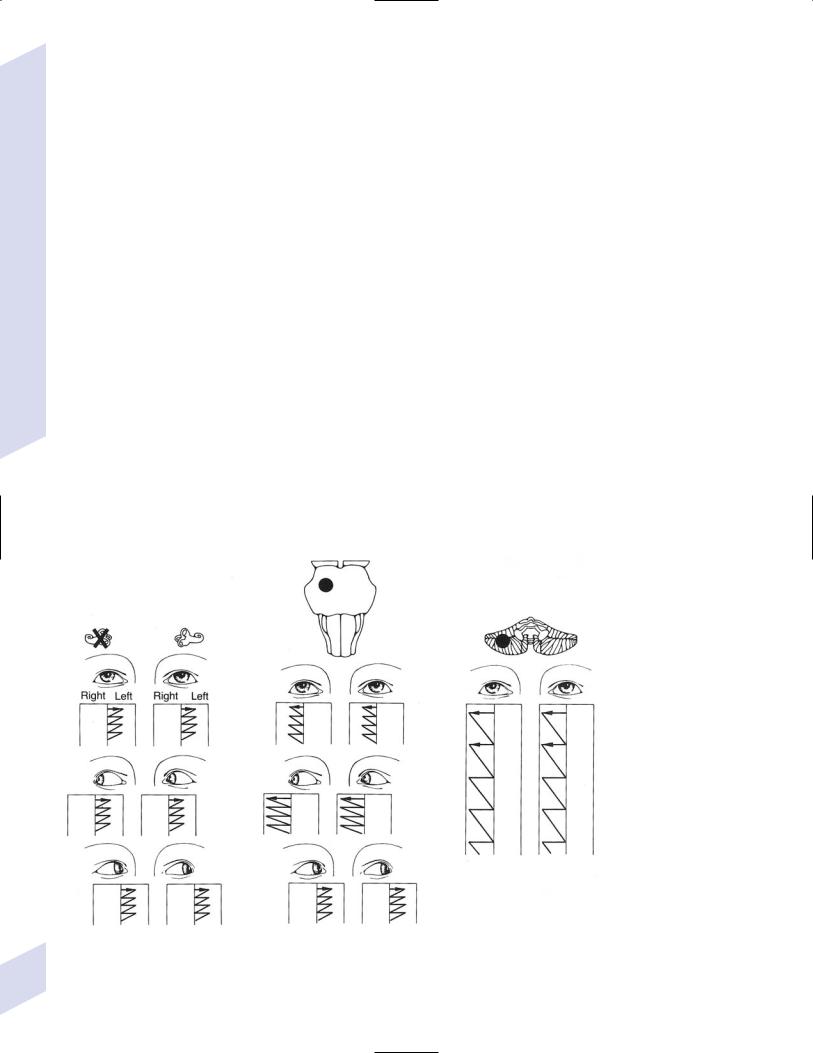
1 Neuroanatomy
i.lesions cause ipsilateral gaze preference acutely, which resolves within days
b.parietal eye field (Brodmann area 39 in the angular gyrus): directs unconscious saccades toward novel objects in the visual field, and mediates reflex pursuit of a visual target; visual area III of the occipital cortex may assist in reflex pursuit movements as well
i.the right parietal eye field is involved in bilaterally directed saccades whereas the left parietal eye field is involved only in contralaterally directed saccades, although lesions or either side cause an ipsilateral gaze preference
c.supplementary motor cortex (Brodmann area 6): involved in the generation of multi-step saccadic eye movements, and in suppressing unconscious saccades
d.pathophysiology
i.gaze deviation: oculocephalic/oculocaloric testing determines if the gaze limitation observed during voluntary eye movements can be overcome by involuntary brainstem mechanisms (i.e., the vestibular system); if a gaze palsy can be overcome by oculocephalic/oculocaloric testing, it is caused by a lesions above the brainstem (i.e., a supranuclear gaze palsy)
11.Localizable nystagmus syndromes (Fig. 1–39)
12.Inherited ocular motor disorders
a.Kearns-Sayre syndrome
i.pathophysiology: usually caused by a large deletion in the mitochondrial genome; sporadic inheritance, unlike other mitochondrial diseases
(1)number of mitochondria in a cell may be increased as a compensatory reaction to their poor function
(2)severity of symptoms is proportionate to the number of affected mitochondria
Vestibular |
Brun’s |
Cerebellar |
nystagmus |
nystagmus |
nystagmus |
|
Figure 1–39 Localizable nystagmus lesions. Other localizable nystag- |
malformation); periodic alternating nystagmus → cervicomedullary |
|
mus includes: Retraction nystagmus → mesencephalon tegmentum; |
junction cerebellum; ocular bobbing → pons. (From Mumenthaler M, |
46 |
seesaw nystagmus → diencephalon; upbeat nystagmus → medulla |
Neurological Differential Diagnosis. 2nd ed. Stuttgart, Germany: Georg |
lesion; downbeat nystagmus → cervicomedullary junction (i.e., Chiari |
Thieme; 1992:71–73, Fig. 24a–c,g,i,k,o. Reprinted by permission.) |
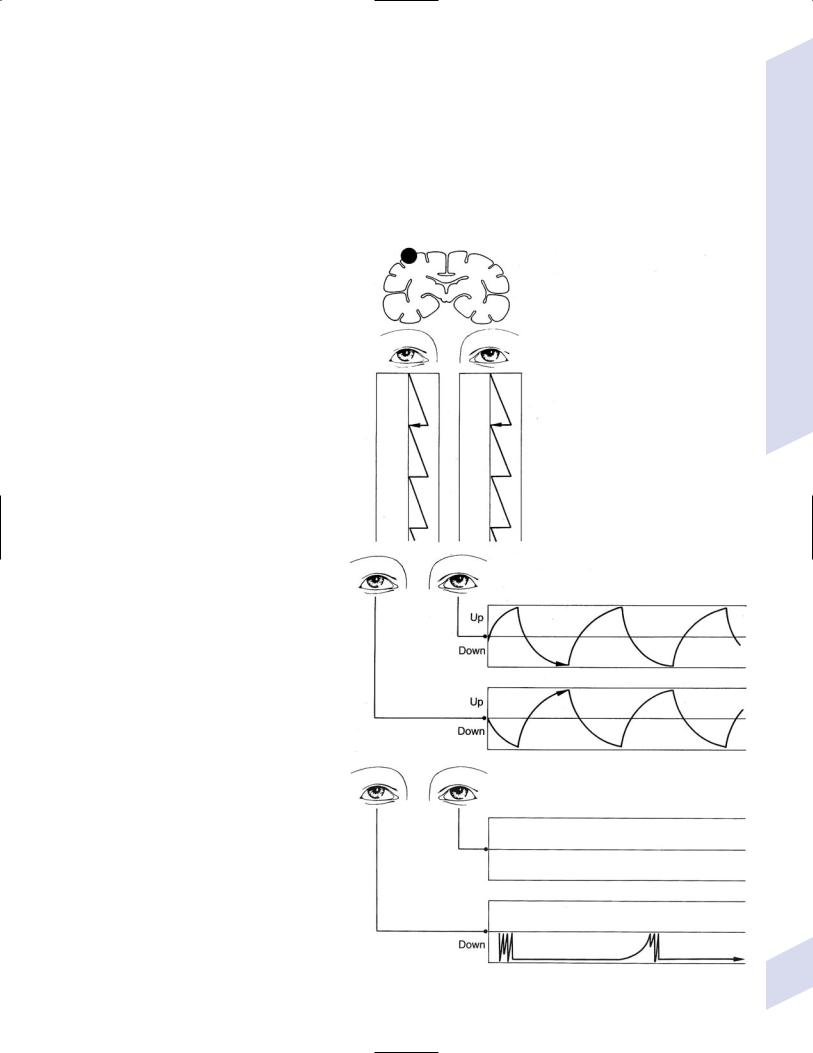
ii.symptoms: develops in childhood
(1)progressive ophthalmoplegia
(2)retinitis pigmentosa
(3)sensorineural hearing loss
(4)weakness, myalgias, and spasticity
(5)mental retardation
(6)cardiac arrhythmia; endocrinopathy from hypothalamic dysfunction; short stature
iii.diagnostic testing: muscle biopsy demonstrates ragged red fibers on light microscopy and distorted
mitochondria on electron microscopy; cerebrospinal fluid analysis demonstrates increased protein and decreased folate levels
iv.treatment: multivitamin supplementation
b.Leigh’s disease
i. subtypes: usually autosomal reces- |
ictal nystagmus |
|
|
sive inheritance, rarely maternal; |
|
due to |
|
(1)pyruvate dehydrogenase deficiency (25%): the enzyme normally converts pyruvate to citrate in the Kreb’s cycle, and is located in the mitochondrion
(2)cyclooxygenase deficiency (25%)
(3)mitochondrial respiratory chain complex I (25%) or V (15%) deficiencies
(4)point mutations in the mito-
chondrial genome (rare) |
seesaw nystagmus |
|
ii.histology: necrosis of the basal ganglia and periaqueductal gray
iii.symptoms: onset is usually in infancy; may have a progressive or episodic course
(1)apnea, particularly sleep apnea (one of the few causes of central sleep apnea)
(2)ophthalmoplegia (mostly with cyclooxygenase deficiency)
(3)ataxia; spasticity
(4)peripheral neuropathy
(5) seizures (only with complex V |
Heimann-Bielschowsky phenomenon |
deficiency) |
from monocular vision loss |
|
(6)retinitis pigmentosa (only with complex V deficiency)
iv.diagnostic testing
(1)lactate and pyruvate are elevated only after glucose challenge
(2)enyzme and genetic analysis
v.treatment: carnitine, coenzyme Q10, and B-complex vitamin sup-
plementation |
Figure 1–39 (Continued). |
Neuroophthalmology
47
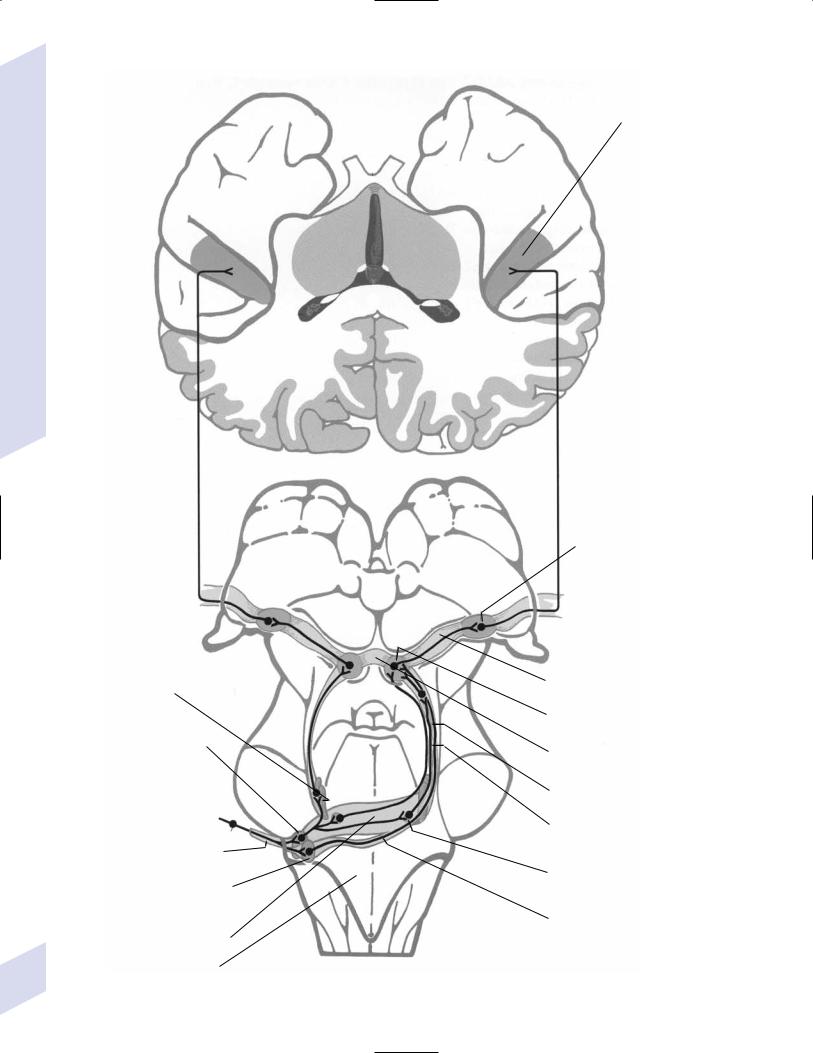
Transverse temporal/Heschl’s gyrus and monomodal auditory association area
(Brodmann area 42) on the planum temporale
1 Neuroanatomy
Thalamus
Superior olivary nucleus
Anterior cochlear nucleus
Pons
Cochlear portion of CN VIII
Posterior cochlear nucleus
Trapezoid body
Medulla
Medial geniculate body
Brachium of the inferior colliculus
Inferior Colliculus
Commissure of the inferior colliculus
Nucleus of the lateral lemniscus
Lateral lemniscus
Nucleus of the trapezoid body
Posterior acoustic stria
48Figure 1–40 The auditory system. (From Kretschmann HJ, Weinrich W, Neurofunctional Systems. Stuttgart, Germany: Georg Thieme; 1998:56, Fig. 28. Reprinted by permission.)
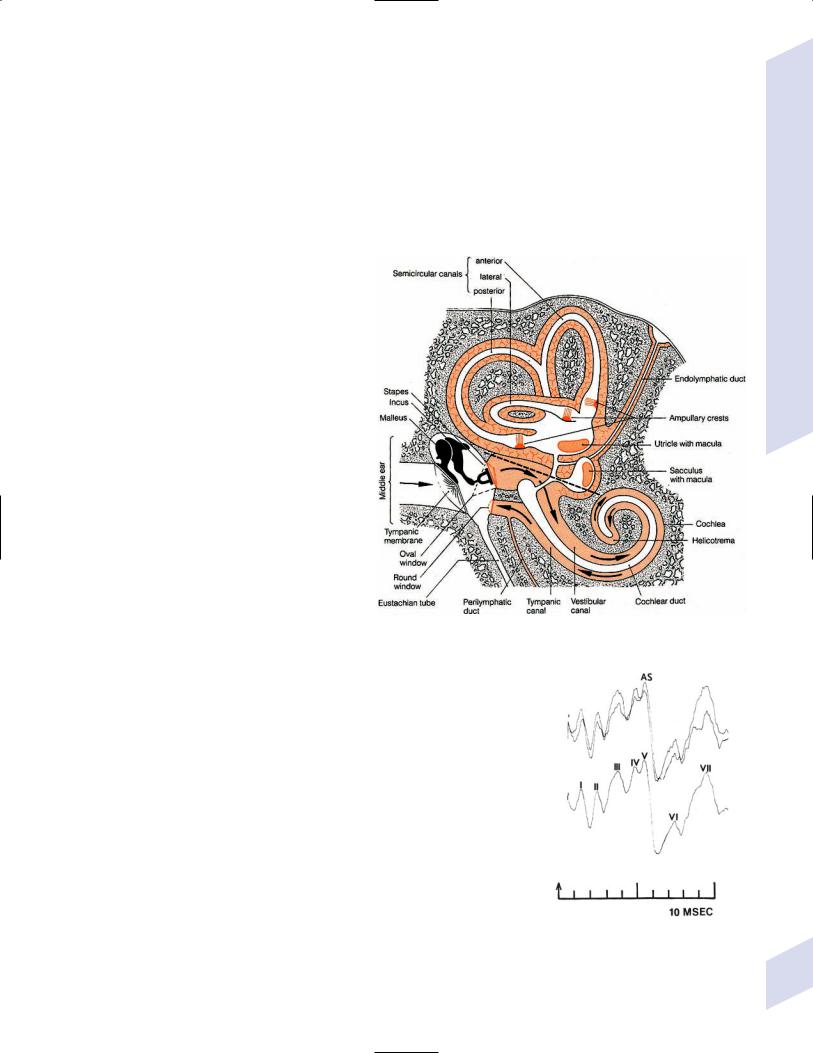
XIV. Neurotology
A. Auditory System (Fig. 1–40)
1.End organs: The cochlea and the organ of Corti, which are innervated by the acoustic part of CN VIII (Fig. 1–41)
2.Superior olivary nucleus: Regulates the stapedius and tensor tympani acoustic re-
flexes that are activated in response to intense acoustic stimulation; innervation |
|
Box 1.23 |
||
is bilateral, therefore hyperacusis does not occur with pontine lesions (Box 1.23) |
|
|||
|
CN VIII also sends cholinergic fibers to the |
|
||
a. |
stapedius acoustic reflex: acts to dampen the oscillation of the ossicles; |
|
||
|
fibers are carried by CN VII, which accounts for the hyperacusis of CN VII |
|
organ of Corti to modulate sensitivity. |
|
|
injury (e.g., Bell’s palsy) |
|
|
|
b. |
tensor tympani acoustic reflex: acts to increase |
|
|
|
|
the tension on the tympanic membrane; fibers |
|
|
|
|
are carried by the mandibular branch of CN |
|
|
|
|
V. Potentials are used for screening for retro- |
|
|
|
|
cochlear disease (vestibular schwannoma), |
|
|
|
|
for infant hearing screening, and for estima- |
|
|
|
|
tion of auditory thresholds in persons not |
|
|
|
|
able to cooperate for behavioral testing (e.g., |
|
|
|
|
infants, young children, malingering). The |
|
|
|
|
interpretation for retrocochlear diagnosis |
|
|
|
|
requires behavioral audiometry |
|
|
|
3. Diagnostic testing: brainstem auditory evoked |
|
|
|
|
potentials (BAER) |
|
|
|
|
a. |
unilateral ear stimulation (e.g., with a click) |
|
|
|
|
produces electrical potentials in the central |
|
|
|
|
nervous system that can be measured over |
|
|
|
|
the brainstem (with a contralateral mastoid |
|
|
|
|
or earlobe electrode) and the auditory |
|
|
|
|
cortex |
|
|
|
i.potentials are labeled sequentially, not according to latency (Fig. 1–42); potentials poorly relate to specific brainstem auditory nuclei
(1)potentials I and II correlate with CN VIII
Figure 1–41 The inner ear. (From Duus P, Topical Diagnosis in Neurology.
(2) potential III is certainly generated Stuttgart, Germany: Georg Thieme; 1998:115, Fig. 3.35A. Reprinted by permission.) within the brainstem
(3)potential VI is generated in the
upper mesencephalon, likely the medial geniculate nucleus
B.Vestibular System
1.End organs: innervated by the vestibular part of CN VIII
a.semicircular canals: sensitive to angular acceleration; only innervated at the distal enlargements (ampula)
b.utricle and saccule: sensitive to linear acceleration; only innervated at their maculae
c.Scarpa’s ganglion: bipolar neurons that form the vestibular part of CN VIII
2.Vestibular nuclei: located at the pontomedullary junction at the floor of the 4th ventricle
a.subdivisions (Table 1–10)
i.the medial vestibulospinal tract projects to the contralateral cervical and thoracic spinal cord, and functions in coordination of head and eye positioning
ii.the lateral vestibulospinal tract projects to the entire ipsilateral spinal cord, but particularly to the extensor musculature (i.e., for antigravity postures)
Figure 1–42 Brainstem auditory evoked potential and sequential potentials. (From Poser CM et al., The Diagnosis of Multiple Sclerosis. Stuttgart, Germany: Georg Thieme; 1984:121, Fig.1. Reprinted by permission.)
Neurotology
49
1 Neuroanatomy
50
Table 1–10 |
Vestibular Nuclei |
|
|
|
|
|
|
|
Afferents from |
Receives cerebellar |
|
Nucleus |
Scarpa’s ganglion |
projections from |
Efferents go to |
|
|
|
|
Superior |
Semicircular canals |
Flocculonodulus, uvula |
Ocular motor nuclei* |
Medial |
Semicircular canals |
Flocculonodulus, uvula |
Ocular motor nuclei,* cerebellar |
|
|
|
cortex, medial vestibulospinal |
|
|
|
tract |
Lateral |
Utricle |
Anterior vermis, |
Lateral vestibulospinal tract |
|
|
fastigial nucleus |
|
Inferior |
Saccule |
Fastigial nucleus |
Ocular motor nuclei,* cerebellar |
|
|
|
cortex, lateral vestibulospinal |
|
|
|
tract |
|
|
|
|
*Includes accessory ocular motor nuclei.
b.vestibular thalamus: there is no clear target for vestibular inputs, although a thalamic intermediary is very likely
c.vestibular cortex: vestibular sensation is likely represented in
i.Brodmann area 2v, located adjacent to the inferior edge of the lateral surface of Brodmann area 2 in the parietal lobe
ii.Brodmann areas 41 and 42, adjacent to the primary auditory cortex in the temporal lobe
C. Neurotology Pathophysiology
1.Hearing loss
a.conductive hearing loss—occurs with pathological process of the external acoustic meatus or middle ear structures; produces a uniform hearing loss across all frequencies that is usually associated with low-frequency tinnitus; conductive hearing losses are equal in all frequencies or may involve the low frequencies mostly; tinnitus is usually not present, but may be of any perceived frequency
i.the Rinne test produces a louder sound with bone conduction than with air conduction, and the Weber test lateralizes to the diseased ear
b.sensorineural hearing loss—caused by diseases of the cochlea more commonly than injury to CN VIII; produces hearing loss in the high-frequency ranges, and exhibits decreased sensitivity to low and mid-intensity sounds but increased sensitivity to high-intensity sounds; usually associated with high-frequency tinnitus; there are many patterns of SNHL; flat and high frequency patterns are more common; a low frequency SNHL is characteristic of cochlear hydrops
i.the Rinne test produces a louder sound with air conduction than with bone conduction, and the Weber test lateralizes to the normal ear
c.treatment
i.glucocorticoids (typically prednisone 1 mg/kg for 7 days) for rapid hearing loss; for sudden sensorineural hearing loss, after otolaryngic and audiologic evaluations
ii.hearing aids, for either type of hearing loss
iii.corrective surgical procedures for conductive hearing loss
2.Tinnitus—Associated with some degree of hearing loss in 95% of cases (Box 1.24)
a.generally tinnitus is only perceived by the affected individual; tinnitus that can be perceived by the examiner usually relates to muscular contractions of the nasopharynx (e.g., palatal myoclonus) or is transmitted from the carotid artery; tinnitus of vascular origin may be due to mild benign intracranial hypertension, primary or metastatic tumors, intracranial or extracranial vascular lesions
Box 1.24
Tinnitus is not the same as otoacoustic emissions, which are sounds that are normally produced by the outer hair cells of the cochlea in response to an auditory stimulus.
b.treatment
i.masking sounds (e.g., white noise generators) to interrupt the tinnitus
ii.hearing aids for hearing loss
iii.antidepressants, alprazolam
iv.biofeedback and stress management
3.Other abnormalities of hearing
a.pure word deafness/auditory agnosia—caused by injury to the dominantside monomodal auditory association cortex
i.reading and writing are intact, therefore it is not an aphasia
ii.comprehension of nonverbal sounds are intact
b.impaired ability to understand nonverbal sounds occurs with injury to nondominant monomodal auditory association area
c.cortical auditory hallucinosis—tend to be formed hallucinations with identifiable sounds, in contrast to pontine auditory hallucinosis
i.poorly localizes to the superior temporal gyrus in the dominant hemisphere; typically occur in the context of temporal lobe seizures in conjunction with other types of hallucinations (olfactory, gustatory) (Box 1.25)
d.paracusis—distortions in the perception of timbre, tone, or loudness in the presence of otherwise normal hearing; can occur with lesions anywhere in the temporal lobe
e.diplacusis—a difference in perception of sound between the two ears that causes a single sound to be heard as two separate sounds; most commonly a cochlear injury
f.hyperacusis—the perception of abnormal growth of loudness, which typically occurs with loss of outer hair cells, e.g., classic Meniere’s disease (Box 1.26)
4.Vertigo: Distinguishing central versus peripheral vertigo (Table 1–11)
a.benign paroxysmal positional vertigo (BPPV)
i.pathophysiology: caused either by movement of free otoliths in a semicircular canal (usually the posterior), or else by accumulations of basophilic material in the cupula of a semicircular canal that cause it to bend when the head is in certain positions; not caused by having the head in any particular position; caused by an angular acceleration in the plane of the semicircular canal that has the lesion
Table 1–11 Central versus Peripheral Vertigo
Feature* |
Central vertigo |
Peripheral vertigo |
|
|
|
Hearing loss |
Rare |
Common |
Ocular responses to caloric |
Symmetric |
Asymmetric, except for BPPV |
testing |
|
|
Nystagmus without vertigo |
Yes |
No |
Ataxia without vertigo |
Yes |
No |
Types of nystagmus |
Bidirectional; vertical; pure |
Unidirectional, torsional, |
|
horizontal is common; |
rarely pure horizontal, |
|
direction can be variable |
consistent direction |
Effect of visual fixation on |
None |
Reduces nystagmus |
nystagmus |
|
|
Habituation |
No |
Yes |
|
|
|
*The severity, exacerbation by movement, nausea, and tinnitus are not reliable distinguishing features. Vertigo may occur in isolation with brainstem injuries or in conjunction with only hearing loss due to infarction of the internal auditory artery (a branch of the anterior inferior cerebellar artery).
Abbreviation: BPPV, benign paroxysmal positional vertigo.
Box 1.25
Pontine auditory hallucinosis—Poorly formed sounds that are more complex than just tinnitus (e.g., ringing, buzzing); caused by poorly localized injuries that do not interfere with BAEPs
Box 1.26
Hyperacusis is most commonly caused by injury to the cochlea.
Neurotology
51

1 Neuroanatomy
52
(1)15% of cases develop after head trauma, and 15% develop after viral labyrinthitis (i.e., after a period of more intense vertigo)
(2)increased incidence in patients with Meniere’s disease
ii.symptoms: episodes of vertigo lasting 1 minute that are provoked by certain movements (lying down, turning over in bed, flexing or extending the head), although exacerbation by movement is nonspecific for all vertiginous disorders; vertigo typically develops several seconds after the provoking movement
(1)episodes tend to be more severe after awakening
(2)in the elderly, BPPV may last only a few seconds and be provoked only by head turning
iii.diagnostic testing
(1)Dix-Hallpike maneuver: produces 1-minute episodes (typically15 seconds) of nystagmus with a latency of a few seconds; the nystagmus is predominantly torsional but also has a vertical component
(a)sitting upright after the maneuver also causes symptoms and reverses the direction of the nystagmus
(b)repeating the maneuver causes acclimation that requires several minutes to fatigue
(c)unlike all other causes of vertigo, the nystagmus fast phase is toward the side of the bad ear; the nystagmus is torsional and geotropic, i.e., the upper part of the eye in the fast component beats toward the ground when the affected side of the head is moved into the Dix-Hallpike position
(2)caloric testing: normal
iv.treatment (in order of preference)
(1)repositioning maneuvers
(a)Epley maneuver: improves symptoms in 95% of cases within 1 week; decreases the 6-month recurrence rate to 5%
(b)Seamont maneuver: improves symptoms in 90% of cases within 1 month; decreases the 6-month recurrence rate to 5%
(2)antiemetics; vestibular suppressants (meclizine, benzodiazepines, amitriptyline)
(3)habituation exercises
(4)surgical treatments, for medically refractory cases
(a)occlusion of the posterior semicircular canal
(b)transection of vestibular part of CN VIII as it connects with the posterior semicircular canal
v.prognosis: episodes typically occur intermittently over a period of several weeks before spontaneously resolving, although BPPV may be a chronic condition in the elderly; without repositioning treatment, 25% resolve within 1 month
b.Meniere’s disease
i.pathophysiology: may be caused by endolymphatic distention {hydrops} that follows rupture of the membranous labyrinth, which ultimately causes cochlear hair cell death
(1)endolymphatic hydrops and cochlear hair cell loss are not always associated with the symptoms of Meniere’s disease, and may also occur after traumatic inner ear injury, labyrinthitis, or syphilis infection
(2)obvious cochlear hair cell loss occurs only in advanced Meniere’s disease; such cases also exhibit degeneration of other inner ear structures (e.g., atrophy of the cristae and the tectorial membrane)
(3)exhibits rare autosomal dominant and recessive inherited forms

ii.symptoms
(1)episodic symptoms
(a)tinnitus (generally low-pitched) and fullness in the ear, which sometimes precede the symptoms of vertigo and hearing loss by a few hours
(b)vertigo, lasting from 20 minutes to hours
(c)sensorineural hearing loss predominantly in the lowfrequency range that resolves over a period of a few hours
(2)chronic symptoms (present between episodes)
(a)sensorineural hearing loss (low high frequencies): may actually precede the onset of episodic symptoms
(b)mild ataxia late in the disease course; disequilibrium subjectively is more prominent than observable ataxia
iii.diagnostic testing
(1)audiometry demonstrates sensorineural hearing loss, characteristically in low frequencies
(2)caloric testing sometimes demonstrates a reduced sensitivity of the diseased ear to irrigation in cases of unilateral disease
iv.treatment
(1)low-salt diet
(2)medical therapy
(a)episodic treatment: meclizine (Antivert), antiemetics
(b)prophylaxis: diuretics
(3)surgical therapy for medically refractory cases
(a)intratympanic gentamicin
(b)selective vestibular nerve section
(c)endolymphatic sac procedures: usually decompression or endolymphatic fluid shunting into the mastoid
(d)labyrinthectomy, if all hearing is already lost
v.prognosis: 40% will develop some symptoms in the other ear within 5 years, although only 10% will develop the full Meniere’s syndrome in the second ear
(1)recurrence of episodes is highly variable and cases often involve periods of remission
(2)definitive spells usually end when the hearing loss reaches approximately 70 dB and 50% word recognition
c.vestibular neuronitis
i.pathophysiology: likely caused by viral infection of the vestibular part of CN VIII, because it occurs in epidemics; associated with mumps, measles, Epstein-Barr, and herpes zoster viruses
ii.symptoms: continuous vertigo, typically developing after an upper respiratory tract infection and a prodromal period involving the sensation of disequilibrium; does not involve hearing loss (unlike labyrinthitis)
iii.diagnostic testing: caloric testing demonstrates reduced sensitivity of the diseased ear to irrigation
iv.treatment: meclizine (Antivert) and antiemetics for 2–3 days to weeks, followed by vestibular rehabilitation
v.prognosis: spontaneous resolution over several days to weeks; rarely recurs, but inducible vertigo may persist for weeks
d.labyrinthitis
i.pathophysiology: caused by infection of the labyrinth (either bacterial or viral) or by erosion from chronic middle ear inflammation (e.g., from a cholesteatoma)
Neurotology
53

1 Neuroanatomy
Table 1–12 Other Diseases Associated with Vertigo
Disease |
Key features |
|
|
Vertigo associated with |
Vertigo lasts a few seconds. |
seizures |
Vertigo is part of an aura, usually with other types of sensations. |
|
Usually it is the sensation of linear movement, but may be |
|
rotational. |
|
Seizures are complex partial with a focus in the superior temporal |
|
lobe. |
Cervical vertigo |
Associated with pain in neck, usually due to trauma. |
|
May be caused by position-dependent flow reduction through the |
|
vertebral arteries. |
Migraine |
Adult migraine: 20% have vertigo associated with headaches; 10% |
|
report episodic vertigo between headaches. |
|
Recurrent episodes of transient vertigo in children may develop |
|
into classic migraine. |
|
Caloric testing is normal and symmetric. |
Head trauma/vestibular |
Heterogeneous group of ill-defined central and peripheral |
concussion |
disorders. |
|
Often related to for temporal bone fractures. |
|
|
ii.symptoms
(1)continuous vertigo, which is exacerbated by sneezing or the Valsalva maneuver (likely because of the development of a perilymph fistula)
(2)severe sensorineural hearing loss
(3)occasional meningitis-like symptoms
iii.diagnostic testing: temporal bone CT demonstrates bone erosion, mastoiditis, or abscesses; lumbar puncture to evaluate for meningitis
iv.treatment
(1)medical: antibiotics for an identifiable infection
(2)surgical: myringotomy or mastoidectomy for abscess drainage; removal of middle ear masses
e.medications causing vertigo: aminoglycosides, antiepileptics, antihypertensives, sedatives
f.other diseases associated with vertigo (Table 1–12)
54
2
Vascular Diseases of the
Nervous System
Note: Significant diseases are indicated in bold and syndromes in italics.
I. Ischemic Stroke
1.Subtypes: considering all ischemic strokes
a.30% are likely due to small artery occlusion
b.30% are likely due to large artery thromboembolism
c.20% are likely due to cardioembolism
d.20% are due to other mechanisms or are cryptogenic
2.Epidemiology: ischemic stroke accounts for 80% of all strokes
a.age: the most predictive factor for stroke
b.sex: male predominant disease until age 75
c.race: Blacks and Hispanics have higher stroke rates
3.Risk factors: identification of a risk factor that may have caused the stroke (e.g., atrial fibrillation) does not obviate the need to evaluate the patient for other possible risk factors
a.previous stroke, either clinical or radiographic
b.transient ischemic attack (TIA): 10% 90-day stroke risk, and 25% 90-day risk of TIA, stroke, or myocardial infarction
i.stroke risk is highest within 24–48 hours of TIA, which supports the recommendation of hospital admission and aggressive evaluation of TIA patients
c.intracranial atherosclerosis: 7%/year stroke risk; higher incidence in Blacks, Asians, and Hispanics
d.hypertension: systolic pressures 140 mmHg and diastolic pressures90 mm Hg are independent risk factors for stroke, and stroke risk is proportionate to the degree of hypertension
e.carotid stenosis: the degree of stenosis is proportionate to stroke risk in both symptomatic and asymptomatic patients
f.heart disease (Box 2.1)
i.atrial fibrillation
(1)overall stroke risk 6%/year; stroke risk is higher in chronic atrial fibrillation or atrial fibrillation due to thyroid disease
(a)1%/year stroke risk in patients 65 years old without other risk factors (can be treated with aspirin if need be)
(b)7–18%/year stroke risk in patients with at least one other stroke risk factor (must use Coumadin)
(2)stroke risk is highest at the time of onset of atrial fibrillation; conversion to sinus rhythm without anticoagulation is also high risk
(3)atrial enlargement without fibrillation is also a stroke risk factor
ii.congestive heart failure: stroke risk 4%/year
iii.myocardial infarction: wall motion abnormalities (particularly of the anterior wall) allow intracardiac thrombus formation that is a source of emboli
Ischemic Stroke
Box 2.1
Cardiovascular Procedure Stroke Risks
Angiography 1%
Coronary artery bypass graft 2%
Thoracic aorta operation 7% (higher incidence in emergent operations)
55

2 Vascular Diseases of the Nervous System
56
iv.valve abnormalities (stenosis, calcification, valve replacement) or the presence of coagulant material adherent to a valve (echogenic “strands”)
(1)mitral valve prolapse is unlikely to be a cause of stroke
(2)stroke risk for mechanical valves is greater than for bioprosthetic valves
v.patent foramen ovale: may allow a paradoxical embolism from the systemic venous system (e.g., a deep venous thrombosis) causing stroke
(1)patent foramen ovale is not conclusively a risk factor for stroke, although it is a risk factor when it is associated with an atrial septal aneurysm
(2)a patent foramen ovale is present in 20% of the general population
g.aortic arch calcifications and atheroma, particularly if 4-mm thick or mobile
h.cigarette smoking: stroke risk is proportionate to the amount of smoking; after cessation, the stroke risk reduces to nonsmoker’s level within 3 years
i.in addition to promoting atherosclerosis, cigarette smoking increases fibrinogen levels leading to a hypercoagulable state
i.dyslipidemia
i.low-density lipoprotein (LDL) 100 mg/dL, although the benefit of treating an LDL between 100–130 mg/dL in patients without other risk factors is unknown
ii.high-density lipoprotein (HDL) 40 mg/dL
iii.triglycerides 200 mg/dL
j.elevated homocysteine level: risk is proportionate to level, starting at10 mol/L
k.diabetes: a risk factor for all types of stroke, yet adequate glycemic control is known to reduce small vessel complications (e.g., retinopathy, nephropathy) but not large vessel complications
l.alcohol use: heavy use ( 4 drinks/day) has an inconsistent association with stroke that may be race dependent; low-to-moderate use (1–2 drinks/day) may reduce the risk for stroke, likely by improving the lipid profile
m.estrogen use
i.contraceptives: high-dose estrogen ( 50 g) contraceptives increase stroke risk likely because they increase blood coagulability and blood pressure; lower dose estrogen contraceptives may also increase stroke risk
(1)increased stroke risk is most marked in women 35 years old who smoke
(2)oral or injectable progesterone-only contraceptives have no apparent effect on stroke risk on their own, but may increase stroke risk in women with hypertension
ii.hormone replacement therapy: estrogen plus progesterone replacement therapy increases stroke risk in postmenopausal women
n.life-style factors: obesity, physical inactivity, diet, and psychological stress all are likely covariates with other risk factors
4.Symptoms: Focal neurological deficits, by definition 24 hours in duration for stroke and 24 hours in duration for TIA, although most TIAs last
15 minutes; speed of onset is generally considered to be acute, however rarely may develop over hours or days
a.headache: occurs in 40% of strokes; often stroke is preceded by a sentinel headache
i.headache is likely related to ischemia of the blood vessels or the meninges (Box 2.2)
Box 2.2
Only the proximal few centimeters of the named arteries are pain-sensitive.

b.seizure occurring concurrently with the stroke (6%)
i.risk of developing epilepsy is greater if the first seizure occurs 2 weeks after the stroke
5.Diagnostic testing
a.infarction (Fig. 2–1)
i.computerized tomography: acute infarction may be identified by hypodensities at the interface of the gray and white matter (e.g., the insula and extreme capsule, the basal ganglia, and internal capsule) or by sulcal effacement (Fig. 2–2); however, a CT scan within the first 3 hours is often normal
ii.magnetic resonance imaging (MRI): diffusion-weighted imaging (DWI)
and apparent diffusion coefficient (ADC) mapping identify 90% of acute infarctions (Fig. 2–3)
(1)DWI: a T2-like sequence that increases signal strength in areas where water molecules are not free to diffuse in the local environment
(a)during infarction, the failure of membrane ion transports may prevent transmembrane water movement and cause cellular swelling (e.g., cytotoxic edema) that reduces the extracellular space, thereby limiting the diffusion of water
(2)ADC: a calculated value that essentially accounts for any underlying increase in T2 signal (e.g., from vasogenic edema, as caused by tumors or inflammation) by subtracting the T2 signal from the DWI signal
Figure 2–1 Schematic of the arterial territories of the brain. (From Duus P. Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:309, Fig. 8.39. Reprinted by permission.)
(3)an increase in T2 signal and a decrease in T1 signal indicate tissue loss, and are not considered accurate for identifying infarction within 8 hours of onset
iii.hemorrhagic conversion of an ischemic infarction occurs in 20% by 3-weeks poststroke
(1)typically develops in a gyral pattern
(2)clinical deterioration tends to occur when the hemorrhage is30% of the infarcted area or when it exhibits mass effect
b.TIA: 40% exhibit neuroanatomically relevant DWI and ADC changes despite symptomatic resolution
c.carotid artery stenosis (Fig. 2–4)
i.for the purpose of identifying endarterectomy candidates, cerebral angiography should be used, although computed tomography angiography (CTA) provides essentially the same measures
(1)when not using angiography, carotid Doppler ultrasound and magnetic resonance angiography (MRA) are usually used together because either alone can overestimate the degree of stenosis in comparison with angiography
Ischemic Stroke
57
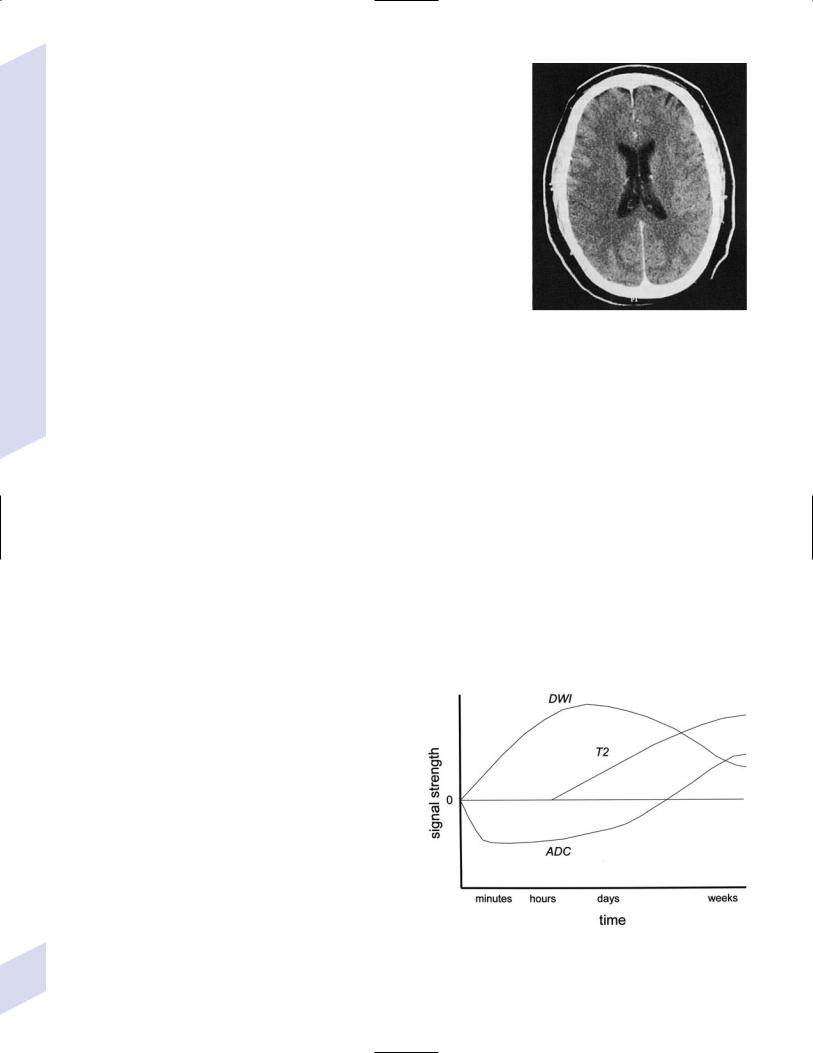
2 Vascular Diseases of the Nervous System
d.cardiac abnormalities: echocardiography may identify intracardiac thrombi and coagulated material (blood sludging, “spontaneous echo contrast,” “smoke”)
i.transesophageal echocardiography (TEE) is better than transthoracic echocardiography (TTE) specifically for left atrial appendage, septal abnormalities (e.g., patent foramen ovale), and aortic abnormalities; TEE also has a higher accuracy for overall detection of cardiac abnormalities when compared with TTE
ii.paradoxical embolism: evaluation for right-left shunt requires intravenous bubble contrast or Doppler echocardiography or transcranial Doppler ultrasound study
e.serologies
i.for patients 45 years old: evaluation of risk factors; when suspecting temporal arteritis in patients 50 years old include sedimentation rate and C-reactive protein serologies
ii.for patients 45 years old: evaluation of risk factors, as per older patients; consider evaluation for antiphospholipid antibodies, coagulation cascade disorders, and inherited stroke disorders
6.Treatment
a.acute setting
i.thrombolytic treatments
(1)tissue plasminogen activator (tPA): if given by IV within 3 hours from the time the patient was last known to be normal; relative risk reduction for disability at 3 months 30%
Figure 2–2 Early ischemic infarction demonstrating loss of gray-white differentiation in the right hemisphere, particularly around the basal ganglia. (From McKhann GM et al. Q&A Color Review of Clinical Neurology and Neurosurgery. Stuttgart, Germany: Georg Thieme; 2003:45, Fig. 36b. Reprinted by permission.)
(a)exclusion criteria
(i)absolute
1.occurrence of a seizure at the time of presentation
2.history of any intracranial hemorrhage or of a known vascular malformation, evidence of intracranial hemorrhage on CT, or clinical suspicion of intracranial hemorrhage irrespective of CT findings
3.active internal bleeding or arterial puncture at a noncompressible site within the preceding 7 days
4.gastrointestinal or urinary tract hemorrhage within 3 weeks
5.any neurosurgical procedure, major head trauma, or stroke within 3 months
6.blood pressure consistently 185/110 mmHg or that requires aggressive management to reduce to that level
7.blood glucose 50 or 400 mg/dL
8.abnormal prothrombin time or partial thromboplastin time; platelet count 100,000
(ii)relative: age 18; possibility of endocarditis or pericarditis; recent lumbar puncture; rapidly improving condition; obtundation or coma
(b)complications: 6% intracranial hemorrhage risk, proportionate to neurological impairment and infarct size but not to early CT findings of infarction
Figure 2–3 Time course of the change in diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping after cerebral infarction.
58
Ischemic Stroke
A B
Figure 2–4 (A) Measurement of carotid stenosis (arrow in the angiogram image) according to the NASCET criteria. The minimal lumen diameter is divided by the diameter of the distal ICA where the walls become parallel to avoid any poststenosis dilatation (B). (From McKhann GM et al. Q&A Color Review of Clinical Neurology and Neurosurgery. Stuttgart, Germany: Georg Thieme; 2003:67, Fig. 59 and Loftus CM and Kresowik TF. Carotid Artery Surgery. Stuttgart, Germany: Georg Thieme; 2000:28, Fig. 2-18. Reprinted by permission.)
(2)intraarterial prourokinase: relative risk reduction for disability of 35% at 3 months in patients with MCA occlusion when administered within 6 hours of symptomatic onset; t-PA has been substituted for prourokinase (which is not FDA-approved) and intraarterial thrombolytics have been used for occlusion of other major arteries, but without proof of efficacy
(a)10% risk of intracranial hemorrhage
ii.carotid endarterectomy: may be performed on an urgent basis in patients with TIAs that are referable to a severe stenosis
iii.anticoagulation and antiplatelet drugs (Fig. 2–5)
(1)aspirin: given acutely, relative risk reduction of recurrent stroke within 14 days 30%; also has small benefit in terms of mortality
(a)do not give aspirin (or other antiplatelet or anticoagulant agents) within 24 hours of thrombolytic administration
(2)heparin: reduces stroke recurrence but increases hemorrhage rate, therefore does not influence overall outcome when given in the acute setting
(a)sometimes given in cases with suspected cardiac sources of embolization until Coumadin can achieve clinical effectiveness
iv.blood pressure management: elevated blood pressures following a stroke are associated with better outcomes, thus hypertension is usually left untreated 2–3 days poststroke unless systolic blood pressure (SBP) 220, diastolic blood pressure (DBP) 130, or mean arterial pressure (MAP) 130 mmHg
(1)avoid using clonidine or diazoxide because they reduce cerebral blood flow
(2)blood pressure typically begins to fall on its own after 3–4 days
59
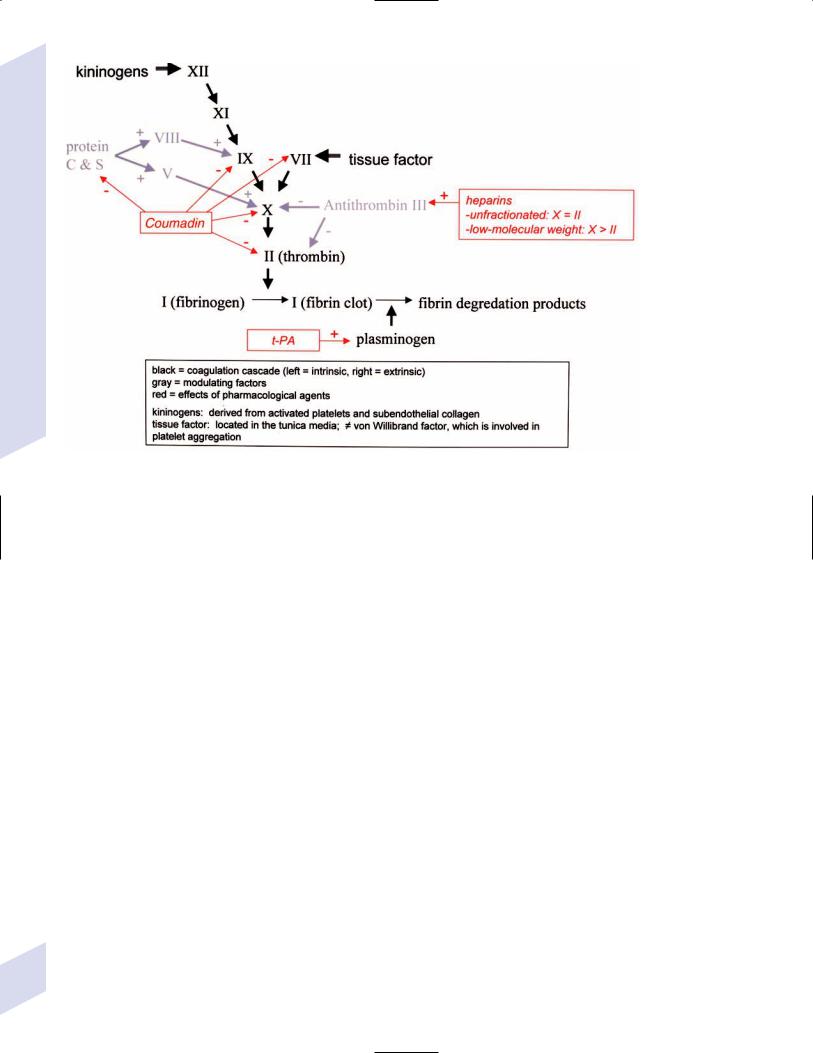
2 Vascular Diseases of the Nervous System
Figure 2–5 The coagulation cascade and sites of anticoagulant action.
v.hyperglycemia management: elevated blood glucose is associated with worsened outcomes in stroke patients irrespective of the patient’s diabetic status
vi.seizure prophylaxis: no indication in the absence of a seizure
(1)with a single seizure occurring less than 2 weeks from the time of stroke, treat with antiepileptics for 3–6 months before considering discontinuation (low risk of epilepsy)
(2)for a single seizure 2 weeks from the time of stroke or for multiple seizures, treat for 2–3 years before considering discontinuation (high risk of epilepsy)
b. subacute to chronic setting, and preventative measures
i.deep vein thrombosis (DVT) prophylaxis, as stroke patients are at particularly high risk for DVTs
ii.evaluation for cardiac ischemia, although large strokes cause transient ischemic-like EKG changes
iii.control of intracranial pressure: edema is maximal 3–5 days after large strokes, although it may be asymptomatic
iv.anticoagulation and antiplatelet drugs
(1)aspirin: relative risk reduction for recurrent stroke 25% with 75–325 mg qd (once daily)
(a)irreversibly inhibits cyclooxygenase production of thromboxane that causes platelet aggregation, but also inhibits vasodilatory prostacyclin production
(b)low-dose (e.g., 81 mg qd) or enteric-coated aspirin will inhibit platelet aggregation in only 40% of patients, in comparison with 70% of patients taking higher doses or uncoated aspirin
(2)dipyridamole aspirin (Aggrenox): modestly better than aspirin
60 |
alone (relative risk reduction for stroke 30%); high side-effect |
rate, particularly headaches |

Table 2–1 Uses of Coumadin in Stroke Prevention
|
Coumadin is better than aspirin |
Aspirin can be sufficient |
|
|
|
Established |
Intracardiac thrombus |
Atrial fibrillation without other stroke risk factors |
|
Artificial heart valve |
{lone atrial fibrillation} |
|
Atrial fibrillation with other stroke risk factors |
|
Limited evidence |
Significant aortic plaque |
Patients at risk for bleeding (e.g., fall risk) |
|
Large areas of cardiac hypokinesis |
|
|
|
|
(a)dipyridamole reduces platelet aggregation by inhibiting cAMP-phosphodiesterase and by blocking adenosine reuptake
(3)ticlopidine (Ticlid): relative risk reduction for stroke 25%; used in patients who cannot tolerate aspirin, but has a high side-effect rate (diarrhea, neutropenia [1%], thrombotic thrombocytopenic purpura)
(a)reduces platelet aggregation through a poorly understood mechanism
(4)clopidogrel (Plavix): equivalent efficacy against stroke in comparison with aspirin; benefit of clopidogrel over aspirin is its superior protection against myocardial infarction
(a)reduces platelet aggregation by blocking ADP binding to adenosine receptors
(b)same side-effect profile as ticlopidine; fewer hemorrhagic side-effects in comparison with aspirin
(5)Coumadin: 67% stroke risk reduction of stroke from atrial fibrillation when international normalized ratio (INR) is 2.0–3.0 (Table 2–1)
(a)for mechanical heart valves, target INR 3.0–4.0; for bioprosthetic heart valve, target INR 2.0–3.0
(b)side effects
(i)hemorrhage (usually gastrointestinal) 7%/year with INR 3.0; hemorrhage risk increases with age 80 years old
(ii)skin necrosis, usually occurring at the initiation of Coumadin therapy (rare)
(iii)blue toe syndrome: ischemia of the extremities caused by embolization of cardiac material
(6)heparin: no proven benefit in stroke management; used commonly as a bridge to Coumadin therapy and for temporary management of unstable major artery stenosis prior to surgical or endovascular intervention
(a)side effects: heparin-induced thrombocytopenia (HIT) syndromes
(i)type I HIT: develops 5 days after starting treatment and is clinically benign; heparin causes platelet aggregation, thereby increasing blood viscosity
(ii)type II HIT: develops 1–2 weeks after starting treatment and is associated with arterial and venous thromboses; heparin-induced autoantibodies cause thrombocytopenia but also a paradoxical hypercoagulable state
v.blood pressure management: reduction to a blood pressure 140/90 mmHg or as low as is tolerated
(1)dietary modification and salt restriction, exercise, and reduction in alcohol consumption should be attempted in conjunction with medication treatment
Ischemic Stroke
61
2 Vascular Diseases of the Nervous System
62
(2)unclear benefit of any particular antihypertensive agent, although first-line recommendations remain angiotensin converting enzyme (ACE) inhibitors or diuretic agents; effectiveness of specific drugs may be related primarily to the degree of blood pressure reduction they achieve
(3)treat isolated systolic or diastolic hypertension
vi.dyslipidemia management: as with hypertension treatment, the particular drug may not be as important as the degree of lipid lowering that it achieves
(1)HMG-CoA reductase inhibitors/statin agents: relative-risk reduction for recurrent stroke 25%
(a)have demonstrated ability to reduce arterial plaque size over time, and may have particular benefit in stroke prophylaxis in cases where aortic plaque is a suspected source of embolization
(2)other agents (clofibrate, cholestyramine, gemfibrozil, niacin) and dietary modification generally do not lower lipid levels as much as statin agents
vii.homocysteine reduction: reduction with vitamin therapy (i.e., vitamin B1 vitamin B12 folate) has an unclear effect on recurrent stroke prophylaxis, but reduction is certainly beneficial in inherited disorders of homocysteine metabolism (see p. 72)
viii.control of hyperglycemia: no proven benefit in stroke prevention; considerable evidence exists that small-vessel disease in other organs (e.g., retinopathy, nephropathy) is reduced with strict glycemic control
ix.carotid endarterectomy (Box 2.3)
(1)in some studies, asymptomatic stenosis of 60–99% has a 55% relative risk reduction for stroke if operative mortality 3% and if the patient has a 5-year life expectancy; untreated stroke risk of asymptomatic stenosis is 1%/year
(2)symptomatic stenosis of 70–99% has a 65% relative risk reduction for stroke if operative mortality 6% and if the patient has a 5-year life expectancy
(a)untreated stroke risk of symptomatic stenosis is 13%/year
(b)benefit of surgery persists for only less than 1 year after stroke or TIA, therefore surgery is best done as soon as possible after a stroke (usually within 4–6 weeks)
(c)50–70% symptomatic stenosis may also benefit from surgery in
(i)male patients
(ii)conjunction with plaque ulceration
(iii)cases involving nonlacunar hemispheric stroke or TIA
(d)endovascular stenting appears as effective as endarterectomy, with a greater restenosis rate but fewer procedural complications
II.Subarachnoid Hemorrhage
1.Causes
a.aneurysms: account for 80% of all nontraumatic subarachnoid hemorrhage (SAH)
b.other vascular malformations: arteriovenous malformations (AVM); moyamoya disease
c.head trauma: the most common cause of SAH
d.venous or capillary bleeding into the prepontine; and/or ambient cisterns {perimesencephalic hemorrhage}; not associated with aneurysms
e.venous thrombosis
Box 2.3
There is no proven treatment for completely occluded (i.e., 100%) carotid stenosis.
2.Risk factors: as per aneurysms (see p. 77), but also
a.hypertension
b.coagulopathy/anticoagulant use
c.smoking
d.drug use, particularly cocaine and amphetamines: suspecting drug abuse as the cause of SAH does not eliminate the need to search for a vascular malformation
e.estrogen use: high-dose preparations (e.g., birth control pills) may increased SAH risk; no effect of hormone replacement therapy
3.Epidemiology: accounts for 10% of all strokes
a.exhibits a familial tendency that is likely related to an inherited predisposition to aneurysm formation
4.Symptoms: pronounced headache, nausea, and stupor focal neurological deficits; severity of nonfocal symptoms in SAH is generally greater than in intracranial hemorrhage
a.seizure at the time of SAH (5%)
b.vision loss may be due to intracranial focal injury or to simultaneous retinal hemorrhage {Terson’s hemorrhage}
c.mild nonfocal symptoms often occur with small SAH {sentinel hemorrhage}
5.Diagnostic testing (Box 2.4)
a.neuroimaging: CT to determine Fisher grade of SAH and any hydrocephalus
i.thick blood layer or clot in subarachnoid space (Fisher grade III) is associated with vasospasm
ii.intraventricular blood (Fisher grade IV) is associated with noncommunicating hydrocephalus (Box 2.5)
iii.SAH can extend into the brain parenchyma (Fisher grade 4)
iv.CT identifies only 95% of SAH cases within the first 24 hours, therefore must evaluate for nonclearing hemorrhagic cerebrospinal fluid and xanthochromia if suspicion of SAH is high (see p. 35)
(1)cerebrospinal fluid xanthochromia is present in 70% of SAH cases within 6 hours and in 90% within 12 hours of SAH, but is rarely present within 2 hours of SAH
b.angiography, either conventional or CT angiography: MRA detects only70% of aneurysms and has high false-positive rate in the MCA and ACA territories
i.angiography is negative for aneurysms in 20% of SAH cases, probably because the aneurysm is hidden by the blood clot; therefore, if the first angiogram is negative, repeat it after 2–3 weeks
(1)50% of angiography-negative SAH is perimesencephalic hemorrhage
(a)if distribution of blood is typical of perimesencephalic hemorrhage and the first angiogram is negative, do not repeat the angiogram because there is low yield and good prognosis of the condition
ii.angiography demonstrates early venous drainage through AVMs, but associated aneurysms may be hidden in the AVM nidus
6.Treatment
a.acute treatment
i.blood pressure management: with an untreated aneurysm, reduce blood pressure to premorbid levels or to a MAP 130 mmHg while keeping cerebral perfusion pressure 70 mmHg
(1)“controlled” (e.g., clipped or coiled) aneurysms allow for aggressive use of hypervolemic–hypertensive–hemodilution (“triple H”) therapy for vasospasm
Box 2.4
Fisher Scale for SAH Using CT
1–No blood detected 2–Diffuse or thin blood 3–Clot or thick layer
4–Intraparenchymal or intraventricular blood subarachnoid hemorrhage
Box 2.5
Subarachnoid hemorrhage may also cause communicating hydrocephalus by direct obstruction of arachnoid granulations.
Subarachnoid Hemorrhage
63

2 Vascular Diseases of the Nervous System
64
ii.reverse coagulopathy
(1)for patients on Coumadin, use
(a)2–3 units fresh-frozen plasma; titrate to normalization of coagulation measures, which may require up to 6 units
(b)vitamin K 10 mg intramuscularly: takes 12 hours to have any effect
(2)for patients on heparin, use protamine 1 mg/100 units of heparin administered
iii.control of intracranial pressure and acute hydrocephalus: place
ventriculostomy to measure and control intracranial pressure so as to maintain cerebral perfusion pressure 70 mmHg; removal of bloody cerebrospinal fluid may also reduce the risk of vasospasm
iv.surgery
(1)timing of surgery: after aneurysmal SAH, the rebleeding rate is 20% in first week without controlling the aneurysm; the rebleeding rate for AVMs is only 6%/year
(a)early surgery (in 3 days) for aneurysmal SAH lowers the risk of rebleeding, but increases the risk of iatrogenic brain injury; generally reserved for patients exhibiting at most focal neurological deficits and lethargy
(b)late surgery (in 10 days) for aneurysmal SAH allows for stabilization of the patient’s clinical condition and reduction of tissue swelling for better surgical access; generally chosen for stuporous or comatose patients
b.preventative treatments
i.surgical treatment of unruptured aneurysms: the decision to surgically treat an unruptured aneurysm is made by comparing the cumulative
rate of aneurysm rupture against the expected surgical morbidity and mortality (typically 15%)
(1)rehemorrhage from 10-mm aneurysms: 0.05%/year unruptured; 0.5%/year ruptured
(2)rehemorrhage from 10–25-mm aneurysms: 1%/year unruptured or ruptured
(3)rehemorrhage from 25-mm aneurysms: 6%/year unruptured
ii.surgical treatment of unruptured AVMs: rupture risk is only 3%; risk is higher in AVMs that are small (due to concentrated exposure of the nidus to the high pressure of the feeding artery), that are associated with aneurysms, and that have limited venous drainage
(1)as with aneurysms, the risk of SAH must be balanced against the risk of surgical intervention, which is increased by the size of the AVM, its location in eloquent cortex, and its involvement of deep venous drainage
(2)may use endovascular treatment and/or stereotactic radiosurgery when craniotomy is not feasible, or in combination with partial surgical resection
(3)the treatment must completely destroy the AVM; residual AVM has a high risk of hemorrhage and can regrow
iii.reduction of hypertension, including isolated systolic or diastolic hypertension
iv.avoid use of anticoagulation but not necessarily antiplatelet agents
7.Complications of SAH
a.vasospasm: a focal arterial constriction possibly related to a loss of vasodilatory substances (e.g., nitric oxide, which is bound by hemoglobin), the release of vasoconstrictive compounds (e.g., endothelins, prostaglandins), and/or a proliferative vascular reaction
i.period of highest risk is 4–7 days post-SAH, but vasospasm may occur up to 3 weeks post-SAH
ii.symptomatic effects of vasospasm may be focal or diffuse (i.e., encephalopathy-like)
iii.diagnostic testing: screen patients for vasospasm with daily transcranial Doppler ultrasonography to detect increased flow velocities in major arteries
(1)ultrasound flow velocities are artificially increased by a low pCO2 or hematocrit
(2)confirm ultrasonographic findings as vasospasm with cerebral angiography
iv.treatment/prophylaxis
(1)nimodipine 60 mg PO q4h for 21 days beginning immediately after SAH
(a)may have direct protective effect on the brain because it does not have much effect on vascular caliber
(2)hypervolemic–hypertensive–hemodilution (“triple H”) therapy has no proven benefit, but is standard-of-care
(3)intraarterial papaverine or angioplasty of the vasospastic artery segments has no established benefit
b.hydrocephalus: treat with intraventricular shunt; intraventricular thrombolytics may be used with clipped aneurysms to clear the subarachnoid blood clot
c.hyponatremia: caused by natriuresis from excessive sympathetic activity (i.e., cerebral salt wasting (see p. 16)), rarely by syndrome of inappropriate antidiuretic hormone (SIADH)
d.cardiac arrhythmias and ischemic-like EKG changes due to increased sympathetic activity
e.seizures: prophylaxis is commonly used in the acute setting, but there is no evidence that it prevents the development of epilepsy
III. Intracranial Hemorrhage
1.Pathophysiology: hemorrhage within the brain parenchyma that separates tissue planes, forming a well-demarcated hematoma; intracranial hemorrhages (ICHs) are located more often in the putamen than in the cortex, thalamus, cerebellum, or pons
a.ICHs from large vessel disease are rare and generally are from rupture of an AVM or extension of SAH into the brain parenchyma (Fisher grade 4 SAH)
b.ICH from small vessel disease is usually related to
i.hypertensive angiopathy—breakdown of the blood–brain barrier in arterioles of chronic hypertensive patients leads to deposition of plasma proteins in the tunica media, inflammatory degeneration of the smooth muscle, and endothelial hypertrophy {lipohyalinosis}; affected arterioles usually also exhibit atherosclerotic changes
(1)many arteriolar microaneurysms {Charcot–Bouchard aneurysms} are likely coils of small arterioles, but aneurysmal dilation of severely hyalinized arterioles do occur; however, ICH does not necessarily occur by rupture of a microaneurysm
ii.amyloid angiopathy—develops when fibrillary proteins form a -pleated sheet conformation that deposits in the walls of arterioles as amyloid, causing wall thickening and lumen narrowing; amyloid deposits can extend into the surrounding perivascular tissue, resembling senile plaques of Alzheimer’s disease
(1)histology: similar to lipohyalinosis except that amyloid angiopathy is congophilic (i.e., exhibits apple green birefringence after Congo red staining) whereas lipohyalinosis is eosinophilic (i.e., stains with hematoxylin)
(2)ICH from amyloid angiopathy often extends into a SAH because of the cortical location of the ICH (Box 2.6)
Box 2.6
Other Symptoms of Amyloidosis
Dementia, either from multiple infarcts or from coincident Alzheimer’s disease
Peripheral neuropathy (e.g., carpel tunnel syndrome)
Renal failure, heart failure, purpura, macroglossia
Intracranial Hemorrhage
65
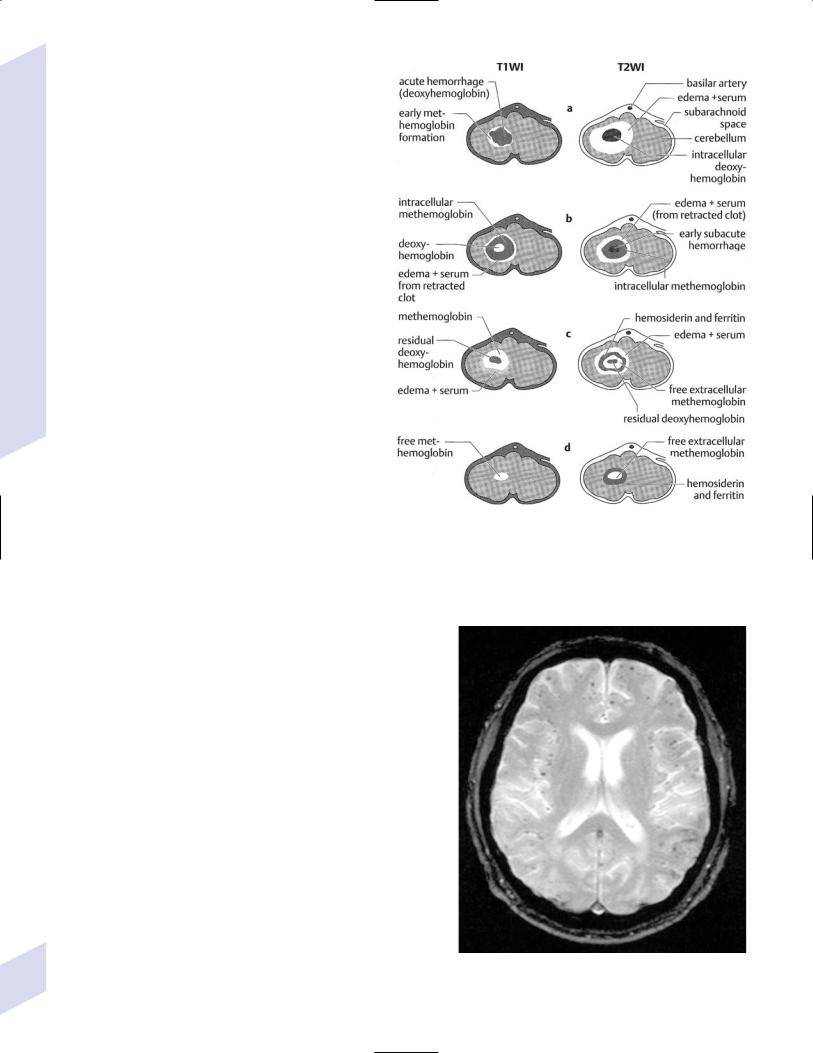
2 Vascular Diseases of the Nervous System
66
(3)genetics: most cases are sporadic and involve the amyloid precursor protein (APP; i.e., the A 1–42 protein) that is found in senile plaques of Alzheimer’s disease
(a)rare hereditary forms of amyloid angiopathy
(i)Icelandic-type amyloid angi- opathy—mutations of cystatin C, a protease inhibitor that accumulates as amyloid when mutated; causes fatal ICH by 20–30 years of age
(ii)Dutch-type amyloid angiopa- thy—mutations in APP that cause accumulation as amyloid; causes fatal ICH by 40 years of age
(iii)mutations of transthyretin, the thyroxine (T4) carrier protein
2.Other risk factors
a.coagulopathy/anticoagulant use: risk of ICH is particularly increased with INR 3
b.drug use (amphetamines, cocaine): tend to cause ICH in cortex; administration by IV is more prone to cause ICH than other routes
c.alcohol use, which is linearly related with the risk of ICH
d.hyperperfusion states (e.g., after carotid endarterectomy)
e.brain tumor, either primary (e.g., high-grade astrocytomas, pituitary adenoma) or metastatic (e.g., lymphoma, choriocarcinoma, melanoma)
f.apolipoprotein E 2 and 4 alleles
Figure 2–6 Stages of blood aging on magnetic resonance image scans. (A) Acute: 48 hours. (B) Early subacute: 3–7 days. (C) Late subacute: 7–10 days. (D) Chronic: 14 days. (From Tsementis SA. Differential Diagnosis in Neurology and Neurosurgery. Stuttgart, Germany: Georg Thieme; 2000:42, Fig. 4. Reprinted by permission.)
3.Symptoms: focal neurological deficits, often resem-
bling ischemic infarction; symptoms are always persistent, that is, never appearing as a TIA
a.headache (30%)
b.seizures: occur in 30% with lobar hemorrhage, but only 5% with subcortical hemorrhage
4.Diagnostic testing: neuroimaging may demonstrate some enlargement of the ICH within the first 12 hours; generally, the hemorrhage is a monophasic event
a.MRI signal characteristics of ICH (Fig. 2–6) as an incidental finding
i.small spots (1–2 mm) of decreased T2 signal are suggestive of microhemorrhages, which are typically observed in amyloid angiopathy (Fig. 2–7)
5.Treatment
a.acute treatment
i.medical: reduce blood pressure to premorbid levels or keep the MAP 130 mmHg with cerebral perfusion pressure 70 mmHg if premorbid blood pressure is unknown; reverse coagulopathy
ii.surgery: first-line treatment for cerebellar ICH 3 cm or in patients exhibiting clinical deterioration; otherwise surgery is only reasonable for expanding ICH in the nondominant hemisphere with simultaneous worsening of the patient’s clinical condition
Figure 2–7 Microhemorrhages demonstrated on T2 magnetic resonance image as multiple punctate areas of decreased signal.
(1)more favorable locations for surgery are in the putamen, frontal cortex, and temporal cortex
b.chronic and preventative treatment: control of hypertension; avoidance of anticoagulants
i.patients with a history of ICH are more likely to have another ICH than an ischemic infarction
IV. Inherited Stroke Disorders
1.CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy)
a.pathophysiology: caused by autosomal dominant mutations of the Notch 3 gene on chromosome 19, the protein of which acts as a cell–cell adhesion molecule with nuclear transcription regulatory properties
i.transcription-regulatory properties require cleavage of the protein by-secretase and transport to the nucleus (Box 2.7)
ii.Notch 3 protein binds the delta ligand on other cells
iii.Notch 3 gene is located very close to the CACNL1A4 gene, which is mutated in familial hemiplegic migraines
b.histology: fibrosis of the tunica adventitia and granular deposits in the tunica media that develop in arteries of 100–400 m in diameter (i.e., penetrating arteries)
i.deposits are of an unknown composition that is not amyloid
ii.deposits surround the smooth muscle cells, and also occur in muscle and skin arteries in some patients
c.symptoms: onset between 30–50 years of age
i.recurrent lacunar stroke and TIA, sparing the cortex
ii.dementia (30%): usually develops more than 10 years after the first stroke from a combination of multiple strokes (i.e., a vascular dementia) and progressive leukoencephalopathy
iii.migraine with aura (25%): does not develop in childhood like other migraines, and typically involves fever and/or stupor {confusional migraine}
iv.mood disorders: develop early in the disease course
d.diagnostic testing
i.gene testing
ii.MRI demonstrates
(1)patchy and diffuse T2 signal increases in the white matter that spares subcortical U fibers
(2)multiple small subcortical infarcts that are occasionally hemorrhagic
2.MELAS (Mitochondrial Encephalomyopathy with Lactic Acidosis and Strokes)
a.pathophysiology: caused by point mutations in a tRNA encoded by the mitochondrial genome; exhibits maternal inheritance
i.highly variable penetrance dependent on the number of affected mitochondria
b.symptoms: usually presents in childhood
i.stroke, particularly in the occipital region and frequently in a distribution that is not defined by a single artery
ii.migraine, often occurring at the time of a stroke
iii.vision loss from retinopathy; sensorineural hearing loss
iv.diffuse weakness from myopathy
v.seizures and dementia, which develop during adolescence
Box 2.7
-secretase also cleaves amyloid precursor protein (APP) into A 1–42 in Alzheimer’s disease.
Inherited Stroke Disorders
67

2 Vascular Diseases of the Nervous System
A
B
Figure 2–8 Ragged red muscle on biopsy. Some muscle fibers exhibit internal accumulations of dense material that represent mitochondria (A). Under EM, mitochondria in an area of proliferation exhibit paracrystalline inclusions (B). (From Sladky JT. Histopathological features of peripheral nerve and muscle in mitochondrial disease. Semin Neurol 2001:21, 296, Fig. 1; 298, Fig. 3. Reprinted by permission.)
68

c.diagnostic testing: lactic acidosis; ragged red fibers on muscle biopsy (Fig. 2–8)
d.treatment: B-complex vitamins niacin coenzyme Q10; dichloroacetate
V. Special Stroke Conditions
1.Endocarditis
a.marantic endocarditis—caused by the hypercoagulable state associated with chronic disseminated intravascular coagulation, AIDS, mucinsecreting tumors, or lupus (i.e., Libman–Sacks endocarditis)
i.diagnostic testing: transesophageal echocardiography demonstrates multiple vegetations 3-mm diameter on the aortic and mitral valves
ii.treatment: anticoagulation; treatment of the underlying causative condition
b.infectious endocarditis—causative organisms are Streptococcus viridans, Staphylococcus aureus, Enterococcus HACEK organisms (Haemophilus, Acinteobacillus, Cardiobacterium, Eikenella, Kingella), fungi
i.risk factors include IV drug abuse (usually causes right-sided endocarditis) and cardiac abnormalities (e.g., artificial heart valves)
ii.infectious endocarditis leads to an infectious arteritis that progresses to
(1)mycotic aneurysm formation on the distal superficial cerebral arteries
(2)microabscess formation
(3)meningoencephalitis
iii.symptoms
(1)stroke: occurs in 10% of infectious endocarditis cases; 75% of strokes are ischemic, 25% are hemorrhagic
(2)cerebral abscess and empyema formation
(3)bacteremia/fungemia
(4)peripheral emboli: painless lesions of the palms and soles {Janeway lesions}, septic pulmonary infarcts; conjunctival hemorrhages; multiple retinal hemorrhages {Roth spots}
(a)not a reliable finding in acute bacterial endocarditis
(5)immunological reactions: glomerulonephritis; painful nodules in the fingers and toes {Osler’s nodes}
(6)new cardiac murmur; valvular destruction that can progress to heart failure
iv.diagnostic testing
(1)echocardiography: demonstrates intracardiac mass, abscess, or new valvular regurgitation
(2)blood cultures: identification of the causative organism may require three sets of blood cultures prior to antibiotic administration
(3)angiography: may use MRA and CT angiography to detect mycotic aneurysms, and cerebral angiography to confirm their location
v.treatment: broad-spectrum antibiotics and antifungals initiated immediately after obtaining blood cultures, and adjusted to the sensitivities of isolated microorganisms as the blood cultures identify them
(1)avoid anticoagulation, which increases the risk of embolization of infected cardiac material and of hemorrhage from mycotic aneurysms
(2)surgical resection of enlarging or ruptured mycotic aneurysms
(3)cardiac valve replacement
Special Stroke Conditions
69

2 Vascular Diseases of the Nervous System
70
vi.prognosis: 50% of endocarditis patients who have had a stroke
(1)greater stroke risk and larger vegetation size in mitral valve disease; however, there is a lower 1-year survival rate in patients with aortic valve vegetations
2.Fibromuscular dysplasia
a.pathophysiology: smooth muscle hyperplasia and fibrosis of smalland medium-sized arteries (renal cervical visceral extremities); variants of the disease can involve hypertrophy of the tunica intima or adventitia
i.a noninflammatory condition that does not involve atherosclerosis or vascular calcification but that is associated with the development of cerebral aneurysms
ii.involvement of the extracranial carotid and vertebral arteries is much more common than involvement of the intracranial vasculature
b.epidemiology: usually occurs in middle-aged women
c.symptoms: ischemic stroke (30%); hypertension from renal artery stenosis; limb claudication and bowel ischemia
d.diagnostic testing: angiography demonstrates alternating segments of constriction and dilation {string of beads sign, tubular stenosis} (Fig. 2–9); arterial dissection rarely develops at sites of disease
e.treatment: antiplatelet agents or anticoagulation; surgical relief of obstruction in high-grade lesions
3.Global hypoxia and watershed infarction
a.pathophysiology: infarction occurs in areas between vascular territories during periods of diffusely reduced blood flow (e.g., after surgery that involves unexpected decreases in blood pressure, cardiac arrest with resuscitation, atrioventricular conduction block leading to syncope {Stokes-Adams attacks}) (Fig. 2–10)
b.treatment: hypothermic coma initiated acutely improves functional outcome in adults after cardiac arrest, and may improve outcome in neonates immediately after perinatal ischemia
4.Arterial dissection
a.pathophysiology: a tear in the tunic intima leads to blood collection underneath the tunica intima or inside the tunica media; blood collection in deeper layers, or absence of a connection between the dissection cavity and artery lumen (i.e., primary hemorrhage within the artery wall) are rare
i.caused by
(1)trauma (50%): carotid injury occurs with compression against transverse process of vertebrae during neck extension and rotation; vertebral injury occurs with stretching between C1 and C2 vertebrae during neck rotation
(2)connective tissue disorders: Ehlers-Danlos syndromes, Marfan’s syndrome, osteogenesis imperfecta
(3)fibromuscular dysplasia
(4)anomalous cerebral arteries (coils, kinks, etc.)
ii.idiopathic cerebral artery dissections
(1)careful evaluation of skin biopsies from such patients demonstrates a high frequency of abnormal elastin and collagen deposition
(2)patients with spontaneous dissection will rarely also demonstrate a bicuspid aortic valve, suggesting developmental abnormalities of neural crest structures
iii.dissection location
(1)carotid artery: usually within 2–3 cm of the bifurcation; rarely enters the petrous bone or extends intracranially
(2)vertebral artery: generally starts around C1–2 vertebrae levels and often extends intracranially
Figure 2–9 Fibromuscular dysplasia typified by an irregular extracranial carotid artery. (From Fischbein NJ et al. Teaching Atlas of Brain Imaging. Stuttgart, Germany: Georg Thieme; 2000:299, Fig. D. Reprinted by permission.)
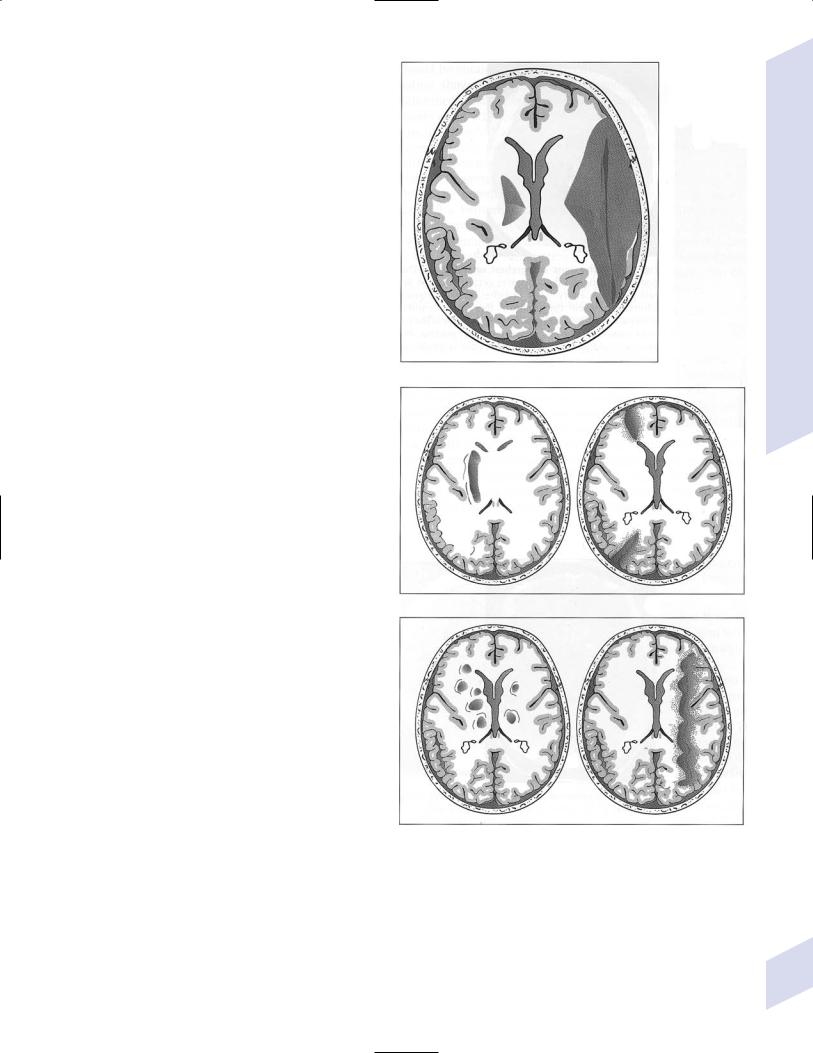
iv.epidemiology: increased risk in people with a family history of cerebral artery dissection
b.symptoms
i.unilateral head or facial pain
ii.stroke or TIA: 45% with carotid dissection, 75% with vertebral dissection
(1)intracranial vertebral dissection may often present with subarachnoid hemorrhage, which is very rare for carotid or extracranial vertebral dissection
iii.Horner’s syndrome, CN XII more than IX, X, XI palsies are caused by mass effect from an enlarged dissected carotid artery
c.diagnostic testing: 25% of cases exhibit multiple vessel dissections
i. angiography (Fig. 2–11) |
A |
ii.MRI: intramural hematoma is most visible on fat-saturation sequences (Fig. 2–12), which has a diagnostic accuracy comparable to cerebral angiography for carotid artery dissections
d.treatment
i.anticoagulation: immediate heparinization followed by conversion to Coumadin with a target INR 2–3; continue Coumadin for 3–6 months before reassessing the patency of dissected artery with neuroimaging
|
(1) anticoagulation has not been |
|
||
|
proven superior to |
antiplatelet |
B |
|
|
agents, and antiplatelet agents can |
|||
|
|
|||
|
be used if anticoagulation is con- |
|
||
|
traindicated (e.g., because the dis- |
|
||
|
section extends intracranially) |
|
||
ii. |
antiplatelet agents: used commonly for |
|
||
|
stroke prophylaxis after resolution of |
|
||
|
the dissection and completion of the |
|
||
|
anticoagulation treatment |
|
|
|
iii. |
endovascular stenting |
|
|
|
iv. |
surgery: carotid ligation with extracra- |
|
||
|
nial–intracranial |
bypass for patients |
|
|
|
with persistent |
ischemic |
symptoms |
|
|
while on anticoagulation |
|
|
|
e. prognosis: 1% annual risk of another dissec- |
|
|||
tion, but it rarely occurs in the same location |
C |
|||
i.1% stroke risk in the 30 months follow- Figure 2–10 Patterns of infarction. (A) Thromboembolic/large artery infarction.
ing initial dissection while on treatment |
(B) Watershed infarction. (C) Microangiopathic/small artery infarction. (From |
ii. majority of dissections will recanalize |
Hosten N, Liebig T. CT of the Head and Spine. Stuttgart, Germany: Georg Thieme; |
2002:28, Fig. 1.24. Reprinted by permission.) |
|
within 2–3 months |
|
5.Inherited coagulation cascade disorders: all exhibit autosomal dominant inheritance and develop symptoms at 30 years of age
a. subtypes
i.antithrombin III deficiency
ii.protein C or S deficiencies
Special Stroke Conditions
71
2 Vascular Diseases of the Nervous System
A B C
Figure 2–11 Carotid dissection. (A) Pouch sign. (B) Flame sign. (C) Double lumen sign. (From Martin JE, Armonda RA. Carotid dissection: a clinical review. Semin Neurosurg 2002:13, 267, Fig. 2A; and Stallmeyer
MJB et al. Emergency evaluation and treatment of injury to the extracranial carotid and vertebral arteries. Semin Intervent Radiol 2003:20, 267, Fig. 2A; 128, Fig. 1C;135, Fig. 8A. Reprinted by permission.)
iii.activated protein C resistance/factor V Leiden mutation: cause a decreased ability of protein C to cleave coagulation factor V due to a point mutation in the factor V gene
(1)activated protein C resistance is the biochemical measurement of factor V cleavage, whereas the factor V Leiden mutation is a genetic test
iv.prothrombin G20210A gene mutation
b.symptoms: usually limited to deep venous thrombosis; strokes are relatively rare and almost exclusively due to venous obstruction
c.treatment: acute treatment with heparin, chronic treatment with Coumadin for venous thrombosis prophylaxis
i.heparin must be given concurrently when initiating Coumadin therapy in protein C or S deficiencies because of the risk for skin necrosis
ii.standard antiplatelet agent therapy should be used in cases with ischemic stroke; anticoagulation is not proven to be superior
6.Homocystinuria/homocystinemia
a.general pathophysiology: accelerates atherosclerosis (in heterozygotes) and increases platelet aggregation (in homozygotes, which is often a lethal condition)
b.subtypes (Fig. 2–13)
i.cystathionine -synthase deficiency: exhibits autosomal recessive inheritance
(1)symptoms: onset 5 years of age in homozygotes; heterozygotes are asymptomatic
(a)ischemic stroke, myocardial infarction
(b)mental retardation
(c)glaucoma and downward lens dislocation; marfanoid habitus; osteoporosis
Figure 2–12 Intramural hematoma within a carotid dissection or fat-saturation MRI. (From Fischbein NJ et al. Teaching Atlas of Brain Imaging. Stuttgart, Germany: Georg Thieme; 2000:263, Fig. F. Reprinted by permission.)
72
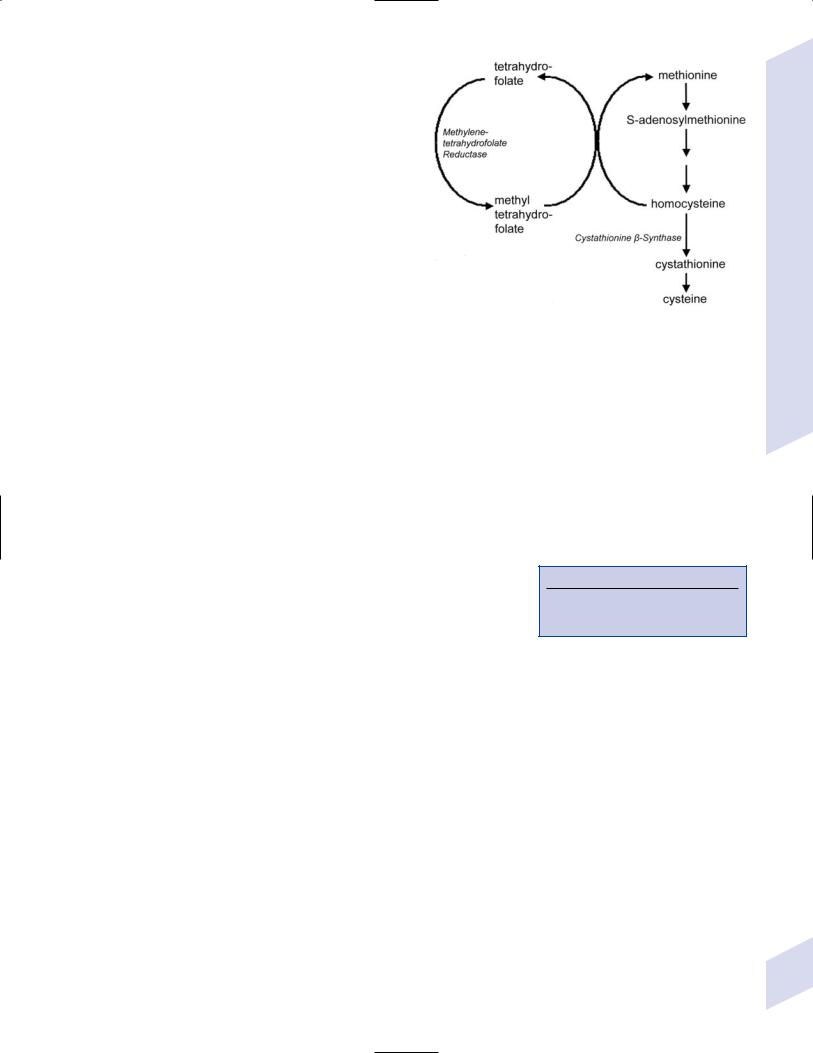
(2)diagnostic testing: elevated blood methionine and homocysteine levels
(a)routine neonatal testing is performed for increased methionine levels
(3)treatment: methionine-deficient, cysteinerich diet; vitamin B6 (pyridoxine)
ii.methylenetetrahydrofolate reductase (MHTFR) deficiency: exhibits autosomal recessive inheritance; homozygosity is very rare
(1)symptoms: similar to cystathionine -synthase deficiency, but more severe
(2)diagnostic testing: decreased blood methionine, increased blood homocysteine; decreased serum folate
(a)genetic testing for MHTFR mutations in
adults |
Figure 2–13 The homocysteine metabolic pathway. |
|
(3)treatment: folate vitamin B6 vitamin B12
iii.deficiencies of vitamin B12 coenzyme synthesis and transport
7.Antiphospholipid antibody syndrome
a.pathophysiology: includes anticardiolipin antibodies (immunoglobulin [Ig]G, IgM, IgA) or lupus anticoagulant (an antibody); antiphospholipid antibodies may increase stroke risk by
i.inducing platelet aggregation
ii.inhibiting endothelial prostacyclin release
iii.inhibiting protein C and/or antithrombin III activities
iv.inhibiting of endothelial release of plasminogen activator b. epidemiology: occurs mostly in young women
i.frequently associated with lupus, other autoimmune diseases, cancer, or infection (including HIV infection)
c. symptoms (Box 2.8)
i.spontaneous abortions, intrauterine growth retardation, preeclampsia
ii.venous thrombosis in extremities and abdomen (renal vein, hepatic vein) {Budd-Chiari syndrome}; rarely acute and disseminated
iii.cardiac vegetations may be an embolic source for strokes
iv.ischemic encephalopathy
d.diagnostic testing: RIA or ELISA of IgG, IgA, or IgM antibodies
i.antiphospholipid antibodies may cause a false-positive VDRL, prolonged PTT, and/or thrombocytopenia
e.treatment: Coumadin with a target INR of 3–4, although this has not proven to be superior to antiplatelet therapy
i.acute disseminated thrombosis may also require glucocorticoids and/or immunosuppressants
f.prognosis: risk of recurrent stroke within 2 years is increased only in patients who have both the lupus anticoagulant and anticardiolipin antibodies
8.Sickle cell disease
Box 2.8
Sneddon’s syndrome—lupus anticoagulant, livedo reticularis, and strokes (arterial or venous)
a.pathophysiology: caused by autosomal dominant mutations in the adultsubunit of hemoglobin that causes irreversible polymerization of hemoglobin when it is deoxygenated (i.e., during relatively hypoxic states)
i.distortion of the erythrocytes’ shape leads to cell–cell and cell– endothelium adhesion, causing vascular occlusion
Special Stroke Conditions
73

2 Vascular Diseases of the Nervous System
(1)erythrocyte adhesion damages the endothelium, promoting thrombus formation and luminal narrowing or aneurysm formation
b.epidemiology: 8% of Blacks are sickle cell carriers, but only 0.2% are homozygotes with symptomatic disease
c.symptoms
i.painful vaso-occlusive crises {sickle cell crisis}
ii.frequent infections that can involve the brain
iii.stroke: 75% are ischemic, 25% are hemorrhagic; risk for hemorrhagic stroke increases with age
(1)greater than 60% recurrence rate after first stroke if untreated
(2)average age of initial stroke 8 years old; 10% of sickle cell patients have had a stroke by 14 years of age, and 22% have a recurrent stroke within 10 years
iv.hearing loss
d.diagnostic testing
i.hemoglobin electrophoresis
ii.transcranial Doppler ultrasonography should be used for regular screening to detect elevated flow velocities in the MCA ( 200 cm/s), which is associated with an increased risk of stroke and is an indication for transfusion
e.treatment
i.ischemic stroke
(1)acute management: oxygen supplementation; transfusion and blood exchange; hydration; pain management
(2)prophylactic management
(a)transfusion and blood exchange to reduce sickle hemoglobin to 30% of total hemoglobin, keeping the total hematocrit35 mg/dL to prevent hyperviscosity
(i)reduces risk of recurrent stroke by 80%
(b)hydroxyurea: increases the amount of fetal hemoglobin, which is resistant to aggregation
(c)bone marrow transplantation: reduces risk of recurrent stroke by 80%
ii.hemorrhagic strokes: sickle cell patients with hemorrhagic strokes must be evaluated for vascular malformations; transfusion and blood exchange do not have an established effect against hemorrhagic strokes
9.Venous infarction
a.pathophysiology: can involve either cortical draining veins or venous sinuses
i.conditions directly affecting venous system that promote venous infarctions
(1)sarcoid; malignancy
(2)infections: meningitis; mastoiditis; periorbital or sinus infection (typically causing cavernous sinus thrombosis)
ii.conditions indirectly affecting the venous system that promote venous infarctions
(1)inherited coagulation cascade disorders
(2)oral contraceptive use, usually in conjunction with smoking
(3)pregnancy: 90% of infarctions during pregnancy are arterial, but 80% of strokes immediately after birth are venous
(a)pregnancy is a hypercoagulable state due to
(i)increased fibrinogen levels
(ii)decreased antithrombin III, protein S, and protein C levels
74 |
(iii) increased platelet adhesiveness |
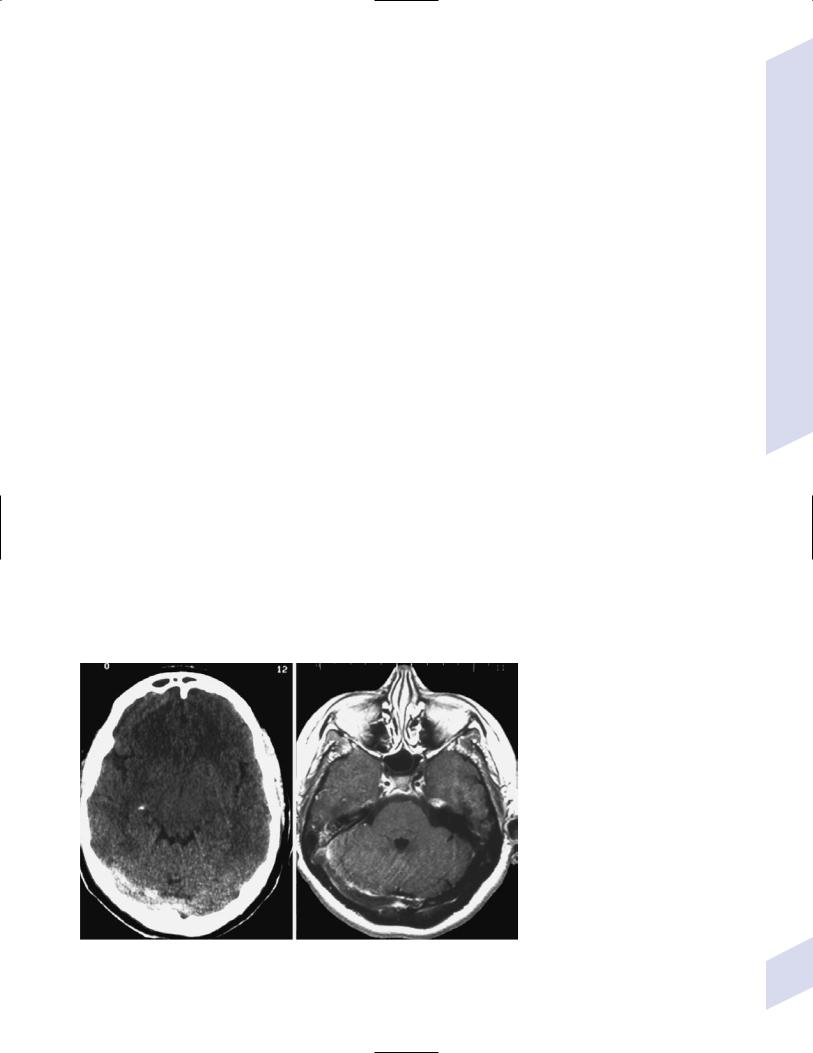
(4)polycythemia; paroxysmal nocturnal hemoglobinuria
(5)cancer, causing a hypercoagulable state or leukemic leukostasis
(6)drugs: asparaginase-induced antithrombin III deficiency
b.symptoms: focal neurological injury from ischemic or hemorrhagic infarction, and from increased intracranial pressure (e.g., headache, papilledema); frequently exhibits a step-wise symptomatic course, but often has an acute onset
i.cavernous sinus thrombosis also can cause proptosis from orbital vasocongestion, ophthalmoplegia, and vision loss from retinal hemorrhages
c.diagnostic testing: only 50% of cases have an identifiable cause for venous thrombosis
i.neuroimaging may reveal infarction with hemorrhagic conversion in a nonarterial pattern associated with a disproportionate amount of edema, or even the venous thrombus (Fig. 2–14)
(1)venography (MRV, magnetic resonance venography; venousphase angiography) demonstrates a restricted venous outflow pattern in 30% of normal subjects
ii.elevated intracranial pressure as measured by lumbar puncture
d.treatment
i.anticoagulation for acute treatment and for prophylaxis, although it has not been proven superior to antiplatelet therapy
ii.local intravascular thrombolytics or endovascular clot removal; surgical thrombectomy
10.Vasculitis
a.general symptoms include flu-like illness, headache, weight loss, and skin rash
b.subtypes
i.temporal arteritis/giant cell arteritis
(1)pathophysiology: granulomatous inflammation with monocyte infiltration and giant cell formation in segments of large arteries that may be initiated by antibody deposition in the internal elastic lamina
A B
Special Stroke Conditions
Figure 2–14 Thrombosis of the right transverse sinus on noncontrast CT (A) and T1 MRI (B). (From |
|
Citow JS et al. Neuropathology and Neuroradiology. Stuttgart, Germany: Georg Thieme; 2001:151, Fig. |
75 |
196. Reprinted by permission.) |

2 Vascular Diseases of the Nervous System
(2)epidemiology: incidence increases with age 50 years old ( 0.2% of cases are 50 years of age); threefold more common in women
(a)associated with polymyalgia rheumatica in 60% (Box 2.9)
(3)specific symptoms: most diagnostic are palpable cranial artery abnormalities, jaw claudication, diplopia, and cranial tenderness
(a)headache (70%): generally is bilateral, throbbing, and slowly increasing in severity over many days
(i)associated with scalp and cranial artery tenderness
(b)stroke: monocular vision loss (10%); nonocular strokes (7%), of which 50% are in posterior circulation
(c)ischemia of soft tissues of the head, leading to use-dependent symptoms (e.g., jaw claudication [40%], tongue claudication [5%], sore throat [10%])
(d)ischemia of extremities, leading to claudication (10%)
(4)diagnostic testing: useful in clinically suspected patients only
(a)erythrocyte sedimentation rate (ESR): elevated in only 80%, and low sensitivity of high ESR thresholds makes it a poor screening test; typically is 100 in biopsy-confirmed cases
(b)C-reactive protein (CRP): typically is 4 mg/dL; more sensitive than ESR, and is not affected by patient age or gender as is the ESR
(c)temporal artery biopsy: 90% diagnostic accuracy with large, bilateral samples
(i)utility of biopsy is not reduced by glucocorticoid treatment even after several days of treatment
(5)treatment: high-dose IV or oral glucocorticoids for 3–5 days, tapered over a period of several months such that symptoms do not recur and the ESR and CRP levels stay within the normal ranges
(6)prognosis: treatment protects the remaining vision, but does not restore vision
(a)1% 5-year risk of vision loss when treatment is begun before vision loss
(b)13% 5-year risk of further vision loss when treatment is begun after vision loss
ii.Takayasu’s arteritis—inflammation followed by progressive lumen occlusion in the aorta and large branches thereof including the carotid and vertebral arteries
(1)specific symptoms: predominantly position dependent symptoms that may have a focal distribution
iii.polyarteritis nodosa—a necrotizing vasculitis of smalland mediumsized arteries associated with hepatitis B and C infection, rheumatoid arthritis, and collagen-vascular diseases
(1)specific symptoms: mononeuritis multiplex, new-onset hypertension, renal failure
(2)specific diagnostic testing: 30% exhibit a positive antinuclear antibodies (ANA) test; visceral angiography demonstrates microaneurysms
iv.isolated CNS vasculitis—involves small to large intracranial or spinal cord vessels (most often small leptomeningeal vessels); associated with Hodgkin’s lymphoma (40%) and HIV infection
Box 2.9
Symptoms of Polymyalgia Rheumatica
Muscle stiffness and pain, particularly in the morning
Fatigue, malaise
Fevers
Night sweats
Unexplained weight loss
76

Table 2–2 Infectious Vasculitis
Type |
Vessels |
Notes |
|
|
|
Bacterial or fungal meningitis |
Arteries near the infection |
Infarction in 10% of adults, 25% of children |
Tuberculosis |
Basal arteries |
Infarction in 40%, develops during chronic meningitis |
|
|
Can produce aneurysms in small arteries |
Tertiary syphilis |
MCA branches |
Strokes usually present after a subacute course of encephalitis and insomnia |
|
|
Focal neurological injury develops progressively |
Lyme disease |
Small vessels |
Can progress to subarachnoid hemorrhage |
Herpes virus |
ACA and MCA |
Usually follows 2–3 weeks after ophthalmic infection |
Varicella zoster virus |
Unilateral ACA and MCA |
Large vessel disease usually follows 2–3 weeks after ophthalmic infection |
HIV |
Large and/or small arteries |
Vasculitis develops in 30% of HIV patients |
Whipple’s disease |
Small arteries |
Causes a pendular convergence–divergence eye movement and |
|
|
contraction of the mastication muscles {oculomasticatory myorhythmia} |
|
|
that persists in sleep |
|
|
Also causes dementia, myoclonus, panhypopituitarism, supranuclear |
|
|
ophthalmoplegia |
|
|
Isolated brain involvement in 30% of patients |
|
|
|
Abbreviations: ACA, anterior cerebral artery; HIV, human immunodeficiency virus; MCA, middle cerebral artery.
(1)specific symptoms: encephalopathy (from small vessel disease) and/or focal neurological injury (from large vessel disease)
(a)cases with focal neurological injury will eventually progress to diffuse small vessel involvement and encephalopathy
v.drug-induced vasculitis: most strongly associated with use of heroin or amphetamines; cocaine appears to cause a vasospasm-like reaction, not vasculitis
vi.infectious vasculitis (Table 2–2)
VI. Cerebrovascular Malformations
1.Aneurysms
a.pathophysiology: sporadic aneurysms do not exhibit tunica media or internal elastic lamina {a true aneurysm}, and often endothelial surface is irregular, inflamed, and atherosclerotic
i.development of aneurysms is associated with polycystic kidney disease, aortic coarctation, collagen-vascular diseases (Marfan’s disease, Ehlers-Danlos, pseudoxanthoma elasticum), fibromuscular dysplasia, and vasculitis (including lupus)
ii.aneurysms are usually located at arterial bifurcations, probably because of a relative weakness of the tunica media at those sites
(1)anterior circulation (90% of all aneurysms): anterior communicating artery posterior communicating artery MCA
(2)posterior circulation (10% of all aneurysms): basilar tip vertebral-PICA junction
iii.25% of SAH patients have multiple aneurysms
b.size: 25-mm diameter is considered giant, and giant aneurysms have a greater risk of hemorrhage and generally are located in the carotidophthalmic artery region
Cerebrovascular Malformations
77
2 Vascular Diseases of the Nervous System
A
Figure 2–15 AVM on T1 MRI demonstrating multiple flow voids on T1 MRI (A) and enlarged arterial channels supplying the nidus as well as early venous drainage on anteroposterior angiogram (B). (From
c.shape
B
Fischbein NJ et al. Teaching Atlas of Brain Imaging. Stuttgart, Germany: Georg Thieme; 2000:277, Fig. C; 278, Fig. F. Reprinted by permission.)
i.saccular/berry: exhibit a well-formed neck; develop more commonly in the carotid circulation
ii.fusiform: longitudinal dilatation of an artery that has no neck; develop at sites of atherosclerotic vessel injury or a winding arterial course {dolichoectasia}
(1)more commonly in the vertebrobasilar circulation
iii.serpentine: a fusiform aneurysm with an irregular lumen that develops after thrombosis and recanalization
2.Arteriovenous malformations (Fig. 2–15)
a.pathophysiology: formed by anastomoses between superficial feeding arteries and draining veins that form a nidus of dysplastic vessels; 90% are supratentorial
i.vein of Galen malformations are direct arterial–venous connections
78 |
that may present in adulthood with SAH and/or ICH |
b.sizes: large AVMs are usually wedge-shaped with a broad base on cortex surface; small AVMs are usually spherical
c.histology: AVMs are developmental malformations; therefore, no brain parenchyma exists within the AVM
i.the surrounding brain parenchyma is often stained with hemosiderin suggesting previous small hemorrhages, and exhibits gliosis with Rosenthal fibers
3.Cavernous angioma/cavernoma/cavernous hemangioma—a cluster of vascular channels with thick, hyalinized walls with thrombosis of large vascular lumens and the absence of intervening brain parenchyma (Fig. 2–16)
a.entirely venous structures, unlike AVMs
b.genetics: most are sporadic, but some are related to autosomal dominant mutations of the Krit1 gene (a tumor suppressor gene)
4.Venous angioma—a cluster of normal venous channels of varying sizes separated by normal brain parenchyma
5.Moyamoya disease—idiopathic development of abnormal small arteries from the circle of Willis associated with a narrowing of an intracranial basal artery (typically the MCA, and often bilateral); abnormal small arteries have thickening of all layers and are prone to occlusion or rupture
a.epidemiology: peak incidences in children 10 years old and in adults 20–30 years old; more common in Asians
b.specific diagnostic testing: angiography demonstrates the tangle of small arteries {puff of smoke} (Fig. 2–17)
Figure 2–16 Cavernoma of the pons (arrow). Cavernomas generally exhibit high T2 MRI signal but often involve areas of low signal from calcifications or hemorrhage (as on the rim of this cavernoma). (From Antunovic V et al. Magnetic Resonance in the Diagnosis of C.N.S. Disorders. Stuttgart, Germany: Georg Thieme; 2001:61, Fig. 60. Reprinted by permission.)
Cerebrovascular Malformations
Figure 2–17 Lateral carotid view of moyamoya disease in the lenticulostriate region (arrowheads) |
|
with a prominent posterior communicating artery (double arrow) and ophthalmic artery (single |
|
arrow). Pruning of MCA and ACA branches helps differentiate this condition from an AVM. (From |
|
Fischbein NJ et al. Teaching Atlas of Brain Imaging. Stuttgart, Germany: Georg Thieme; 2000:316, |
79 |
Fig. E. Reprinted by permission.) |
