
- •Contents
- •Preface
- •Contributors
- •01 Neuroanatomy
- •03 Seizures and Epilepsy
- •04 Disorders of Myelination
- •05 Tumors of the Nervous System
- •06 Headache and Pain Disorders
- •I. Episodic Headache
- •A. Episodic Headaches Lasting More than Four Hours
- •07 Behavioral Neurology
- •08 Movement Disorders
- •09 Diseases of the Nerves
- •10 Diseases of the Muscles
- •I. Bacteria
- •I. Diabetes Mellitus (DM)
- •Index
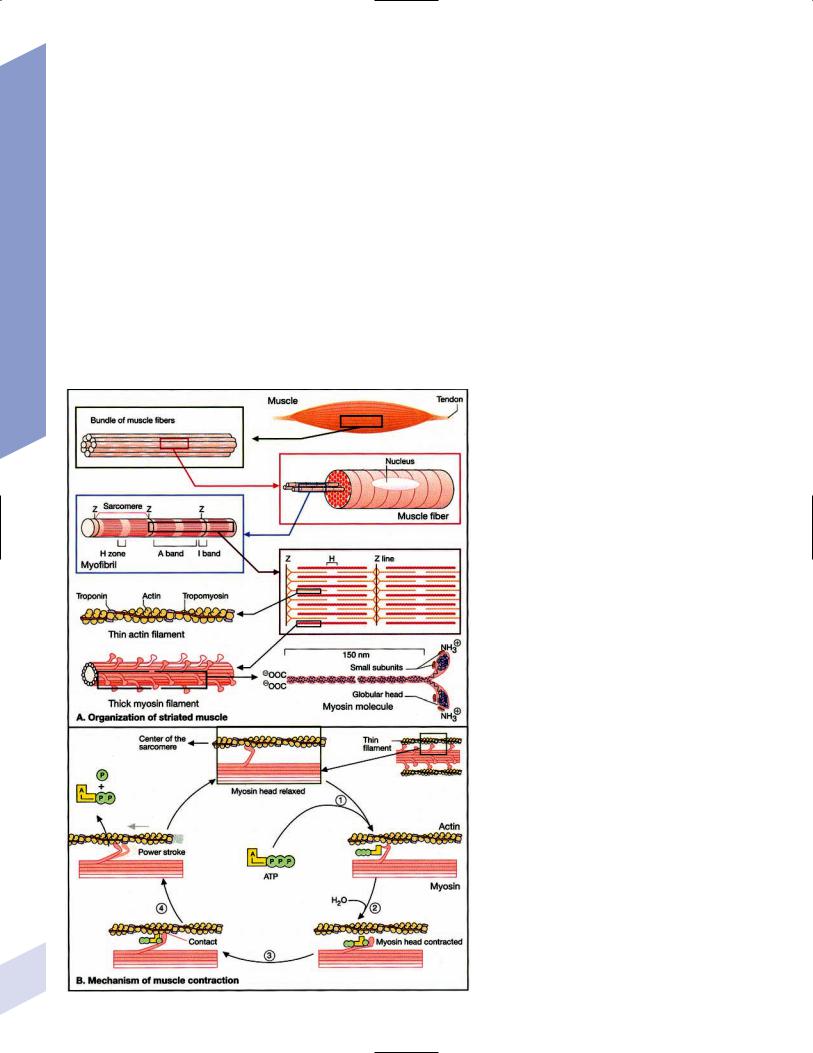
10 Diseases of the Muscles
10
Diseases of the Muscles
Note: Significant diseases are indicated in bold and syndromes in italics.
I. Muscle Physiology
1.Muscle contraction (Fig. 10–1) and neuromuscular transmission (Fig. 10–2)
2.Muscle fibers: distinguished biochemical by the expression of different isozymes of myosin
Figure 10–1 Muscle contraction. (From Koolman J, Rohm 230 KH. Color Atlas of Biochemistry. Stuttgart, Germany:
Georg Thieme; 1996:307. Reprinted by permission.)

Muscle Physiology
Figure 10–2 Neuromuscular transmission. SR smooth endoplasmic reticulum. (From Koolman J, Rohm KH. Color Atlas of Biochemistry. Stuttgart, Germany: Georg Thieme; 1996:309. Reprinted by permission.)
a.slow twitch/type I muscle fibers: characterized by a small diameter, a high myoglobin content, and numerous mitochondria; relies on aerobic metabolism, which is better for tonic contraction
b.fast twitch/type II muscle fibers: characterized by a large diameter, a low myoglobin content, and few mitochondria; relies on anaerobic metabolism, which is better for phasic contraction (Fig. 10–3)
c.super-fast muscle fibers: found in extraocular muscles
d.tonic muscle fibers: found in extraocular muscles, tensor tympani
3.The motor unit: composed of the motoneuron and the muscle fibers it innervates; the muscle fibers for a particular motoneuron are always of the same type, but are not necessarily located near to each other in the muscle
a.motoneurons innervate a greater number of muscle fibers in larger muscles; conversely, motoneurons for the small muscles of face and hands may innervate less than 10 muscle fibers, allowing for fine motor control
Figure 10–3 Normal gastrocnemius muscle. ATPase stain demonstrates fast-twitch (f) and slow-twitch (s) muscle fibers. (From Beltri K et al. Contribution of the distal nerve sheath to nerve and muscle preservation following denervation and sensory protection. J Recon-
struct Microsurg 2005, 21:67, Fig. 8e. Reprinted 231 by permission.)

10 Diseases of the Muscles
b.types of motor units listed in order of recruitment
i.fatigue-resistant slow-twitch motor units: respond to low levels of activation of the motoneuron pool
ii.fatigue-resistant fast-twitch motor units: respond to intermediate levels of activation of the motoneuron pool; produce only half the contraction force of fatigue-resistant slow-twitch motor units
iii.fatigable fast-twitch motor units: respond to the highest levels of activation of the motoneuron pool; generates the largest force during twitch or tetanic contraction
4.Recruitment principle: the force of a muscle contraction is produced by activating an increasingly larger number of motor units in a fixed order beginning with the weakest and progressing to the strongest; this simplifies the modulation of force, because only the magnitude of the response has to be determined, not the identity of the responding motor units
a.the recruitment principle also keeps fatigable fast-twitch motor units quiescent until they are absolutely needed, which is metabolically advantageous
b.the order of activation can be reversed under certain physiological conditions, for example, activation of cutaneous pain receptors that inhibit fatigueresistant slow-twitch motor units while promoting the fast-twitch motor units, thereby allowing rapid force modulation
II. Diagnostic Testing
1.EMG: normal motor unit potentials (MUPs) (Fig. 10–4) a. pathological MUPs
i.myopathic changes on EMG
(1)MUPs tend to exhibit a decreased, not increased, amplitude after myopathic changes, because the muscle fibers are atrophying
(2)contractions of a diseased muscle results in an increased number of MUPs that are firing at normal or slower-than-normal rates
ii.neuropathic changes on EMG: involve a lesser degree of denervation and reinnervation than do myopathic changes
(1)denervation: acutely denervated muscle fibers are electrically
inactive unless they are irritated (e.g., at the time of needle insertion), until the development of fibrillation potentials 3 weeks after injury
(a)fibrillation potentials: spontaneous, regular potentials of chronically denervated muscle fibers
(i)the most accurate indicator of axonal loss in a neuropathic disorder, but fibrillation potentials last only 2 years after denervation before they spontaneously resolve
(ii)presence of fibrillation potentials in the proximal musculature (e.g., paraspinal muscles) indicates a motor root injury (versus peripheral nerve disease), but it is not always present
(2)reinnervation: can be accomplished by regeneration of an injured axon or by collateral sprouting of nearby intact motoneuron terminals
(a)MUPs from collateral sprouts are different from those of normally innervated motor units in that they
(i)have an increased duration
(ii)have an increased amplitude
(iii)have a polyphasic waveform
232
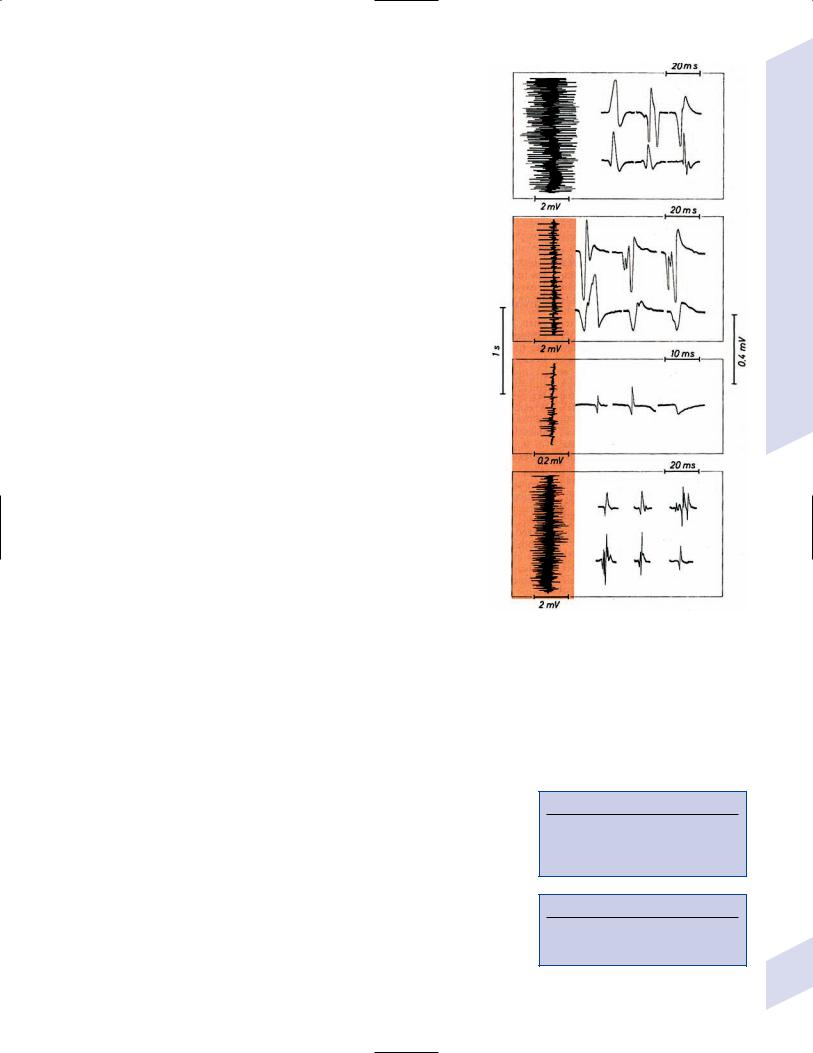
(iv)are of reduced number and are firing at faster than normal rates ( 15 Hz) during muscle contraction (i.e., abnormal recruitment pattern)
iii.abnormalities of insertional activity and irritability on EMG (Fig. 10–4)
(1)reduced insertional activity and irritability are caused by replacement of the muscle by other soft tissues or by physiological contracture (e.g., as in McArdle’s dis-
ease, phosphofructokinase deficiency, paramyotonia |
A |
congenita, or during episodes of the periodic paralysis |
|
disorders) |
|
(2)increased insertional activity and irritability exists in several forms including
(a) |
fibrillation potentials: bior triphasic waveforms |
|
|
|
with an initial negative potential originating |
|
|
|
in the muscle; occurs at 1–30 Hz at a regular |
|
|
|
rhythm |
|
B |
|
(i) caused by lower motoneuron disease, muscu- |
||
|
|
||
|
lar dystrophies, and inflammatory myopathies |
|
|
|
(ii) insertional sharp waves: essentially a fibrilla- |
|
|
|
tion potential caused by the initial irritation |
|
|
|
of a muscle fiber during insertion of the EMG |
|
|
|
needle |
|
|
(b) |
fasciculation potentials: less than 6 Hz poten- |
C |
|
|
tials with an irregular rhythm and variably |
||
|
|
||
|
shaped waveforms caused by pathological |
|
|
|
processes that irritate the lower motoneuron |
|
|
|
anywhere along its course (even within the spinal |
|
|
|
cord, as with amyotrophic lateral |
sclerosis) |
|
|
(Box 10.1) |
|
|
(c) |
myokymic discharges: short bursts of sharp |
|
|
|
waves occurring at regular intervals and that pro- |
|
|
|
duce a rhythmic, undulating muscle contraction; |
|
|
|
occurs at 5–150 Hz |
|
D |
(i)can be caused by most diseases of the muscle Figure 10–4 Normal (A) and abnormal EMG activity. Rein-
(d) myotonic discharges: sustained runs of positiveor |
nervation (B) demonstrates broad, high-amplitude wave- |
|
forms. Complete denervation (C) demonstrates fibrillation |
||
negative-sharp waves (depending upon the site of |
||
potentials (left and middle potentials) and positive sharp |
||
electrode placement) with fluctuant amplitudes, |
waves (right potential). Myopathy (D) exhibits low-amplitude, |
|
producing a tonic muscle contraction; occurs at |
polyphasic waves that are often split. (From Mumenthaler |
|
50–100 Hz (Box 10.2) |
M, Neurology. 3rd ed. Stuttgart, Germany: Georg Thieme; |
|
1990:389, Fig. 10. 2a–d. Reprinted by permission.) |
||
|
(i)neuromyotonic discharges: similar to myotonic discharges, except they occur at 150–300 Hz,
are continuous even during sleep, and are induced by physical stimulation; caused by diseases of the nerve that involve aberrant activity of the most distal portion of the nerve branches (e.g., Isaac’s disease)
(e)complex repetitive discharges: a coordinated burst involving several MUPs induced by a “pacemaker” muscle fiber that drives nearby muscle fibers, producing a stable but abnormal waveform; occurs at 5–100 Hz
b.technical considerations for EMG
i.limb temperature variations: cold prolongs the MUP and increases its amplitude
ii.patient age
(1)neonates: MUPs amplitudes are proportionately small due to immature size of the muscle fibers
Box 10.1
Differential diagnosis of fasciculation potentials—Motoneuron diseases; chronic radiculopathy or neuropathies; cholinesterase use
Box 10.2
Differential diagnosis of acquired myotonia—
Chronic radiculopathy or neuropathy; severe myopathies; drug exposure
Diagnostic Testing
233

10 Diseases of the Muscles
234
(2)elderly: MUP amplitudes are larger than in young adulthood due to compensatory reinnervation caused by age-appropriate loss of motoneurons
2.Muscle biopsy
a.open biopsy allows for multiple sampling sites that may be necessary for muscle diseases that do not involve the whole muscle body (i.e., are patchy, as in the inflammatory myopathies)
b.evaluate both frozen and glutaraldehyde-fixed sections; fixed sections should be prepared by embedding in plastic (for electron microscopy, if necessary) and paraffin
c.technical limitations
i.in cases of chronic muscle diseases, avoid biopsies of severely affected muscles; in cases of relative acute muscle diseases, select severely affected muscles
ii.do not perform within 1 month of an episode of rhabdomyolysis, and avoid muscles that have suffered recent trauma (e.g., EMG testing)
III. Myasthenic Syndromes
1.Myasthenia gravis
a.pathophysiology: caused by antibodies that disrupt the function of postsynaptic nicotinic acetylcholine receptors on striated muscle; these antibodies may be derived from
i.a paraneoplastic syndrome: anti-acetylcholine receptor antibodies block acetylcholine receptor binding and crosslink the receptor proteins causing their internalization and destruction; antibodies target the gamma subunit of the receptors, which are most concentrated in the extraocular muscles
(1)antibodies on the myocytes induce (Box 10.3)
(a)complement membrane attack complexes: damage to the postsynaptic membrane initially causes loss of infoldings of the muscle fiber membranes {T tubules} and disorganization of the muscle end plate with widening of the synaptic cleft, and may ultimately cause muscle fiber death
(b)mild lymphocyte invasion of the muscle, in severe cases
(2)associated with thymoma (15% of cases) or thymic hyperplasia (80% of cases)
(a)B lymphocytes produce the anti-acetylcholine receptor antibodies but require T lymphocytes to make them in the form of IgG, therefore thymectomy changes the antibody expression from IgG to the less damaging IgM form
ii.an autoimmune reaction: antibodies against the muscle specific tyrosine kinase (MuSK) may disrupt acetylcholine receptor aggregation in the muscle end plate by deregulating the function of the intracellular protein agrin
(1)this form of myasthenia gravis is associated with HLA-B8 and –DR3 in young patients, and with other autoimmune disorders (rheumatoid arthritis, lupus, polymyositis, pernicious anemia) or immunosuppression
b.epidemiology: a bimodal prevalence with peaks at 20–30 years of age (female predominance) and 60–80 years of age (male predominance)
(1)myasthenia gravis patients greater than 40 years of age commonly have a thymoma; patients less than 40 years of age are more likely to have thymic hyperplasia
c.symptoms
Box 10.3
Thymoma paraneoplastic syndromes—
Myasthenia gravis; Isaac’s syndrome; cerebellar degeneration (CV-2 paraneoplastic autoantibody); pure red cell aplasia

i.weakness of the extraocular and facial muscles neck (particularly flexion), bulbar, and oropharyngeal muscles proximal upper extremity muscles lower extremity muscles
(1)weakness is typically asymmetric and becomes evident after prolonged use of a muscle (i.e., it is not present with immediate movements)
(a)weakness is often evident as a loss of postural tone, or as tremulousness during prolonged muscle use
(2)the weakness may be specifically limited to the extraocular muscles; the pupils are spared in myasthenia gravis, unlike botulism
ii.muscle soreness
iii.muscle atrophy occurs in only 15% of chronic cases and reflexes are always normal
(1)the tongue often exhibits longitudinal furrows in patients with MuSK antibodies, but this is a poorly understood abnormality that is not true atrophy
d.diagnostic testing
i.edrophonium (Tensilon) test: double-blinded injection of edrophonium (10 mg total in divided doses) produces improvement in a preexisting weakness within 1 minute of injection
(1)best to measure the degree of ptosis, which can be easily quantified
(2)requires a 2-mg test dose to assess tolerability; monitoring for bradycardia
(3)not as accurate as serological tests and EMG, because the response to edrophonium is not specific for myasthenia gravis
ii.serum antibodies
(1)acetylcholine receptor: present in 95% of myasthenia gravis cases
(2)striated muscle: present in 30% of myasthenia gravis cases; more common in older patients
(3)MuSK: present in 65% of acetylcholine receptor-negative patients; patients usually exhibit mild symptoms limited to the extraocular muscles
(4)titan: more common in patients with normal thymus glands
(5)ryanodine-sensitive voltage-gated calcium channels (Box 10.4)
iii.EMG demonstrates increased jitter, at least in facial muscles if not diffusely
iv.nerve conduction studies: repetitive nerve stimulation produces decremental responses
v.muscle biopsy can be used to demonstrate immune deposits in seronegative cases
vi.thymoma screening with CT chest, which always should be performed irrespective of the likelihood of its occurrence
e.treatment (Fig. 10–5)
i.pyridostigmine (Mestinon): better for paraneoplastic myasthenia (i.e., anti-acetylcholine receptor antibody), and is minimally effective in autoimmune myasthenia (i.e., anti-MuSK antibody)
(1)the results of edrophonium testing should not be used to adjust medication doses
ii.immunosuppressants: better for autoimmune myasthenia (Box 10.5)
(1)chronic prednisone, which may cause transient worsening of the weakness at the time of initiation of the therapy
(2)chronic cyclosporine, azathioprine, or mycophenolate in patients who are poorly responsive to glucocorticoids
(3)plasma exchange or intravenous immunoglobulin (IVIg) for severe bulbar and oropharyngeal dysfunction
Figure 10–5 Myasthenia gravis. Significant decrement at rest and postexercise potentiation followed by exhaustion. (From McKhann GM et al. Q&A Color Review of Clinical Neurology and Neurosurgery. Stuttgart, Germany: Georg Thieme; 2003: 131, Fig. 130. Reprinted by permission.)
Box 10.4
The ryanodine-sensitive voltage-gated calcium channel is also mutated in central core myopathy; not the same as the L-type dihydropyridine calcium channel that is mutated in hypokalemic periodic paralysis.
Box 10.5
Myasthenia crisis
Occurs in patients with a baseline respiratory impairment who develop a second respiratory disorder (usually infection); typically defined as a forced vital capacity (FVC) 15 cc/kg
Distinguish from respiratory failure due to acetylcholinesterase inhibitor overdose by challenging the patient with edrophonium after the patient is intubated
Myasthenic Syndromes
235

10 Diseases of the Muscles
236
iii.thymus removal for thymoma or thymic hyperplasia: thymus removal is useful only for paraneoplastic myasthenia and is not usually effective acutely, in severe disease, or in patients with anti-MuSK antibodies
(1)thymomas are generally encapsulated, but 35% may be invasive; thymoma invasion of surrounding tissues may require irradiation and/or chemotherapy
(2)in postthymectomy patients, rising antibody titers may indicate thymoma recurrence
f.prognosis: slow progression of symptoms over months with periods of exacerbation
i.no clear association exists between exacerbations and pregnancy
ii.no association between antibody titers and severity of disease
2.Pediatric myasthenia syndromes
a.transitory neonatal myasthenia—caused by transplacental passive transfer of antibodies from a myasthenic mother to the fetus; occurs in 20% of children of myasthenic mothers
i.symptoms are present at birth, and include hypotonia and poor feeding
ii.treatment: may require plasmapheresis if severe
iii.prognosis: self-limited course usually lasting 2 weeks
b.congenital myasthenia—caused by abnormalities in acetylcholine release, neuromuscular junction acetylcholinesterase function, or postsynaptic acetylcholine receptors; usually exhibits autosomal recessive inheritance
i.symptoms develop at birth, and include ptosis, ocular motor weakness, and facial weakness; limb weakness is mild
ii.treatment: as per adult myasthenia gravis
c.familial infantile myasthenia—associated with polymorphisms on chromosome 17
i.symptoms are present at birth, and include poor feeding and respiratory failure often leading to sudden death
ii.treatment: as per adult myasthenia
iii.prognosis: symptoms generally improve with age
3.Lambert-Eaton myasthenia
a.pathophysiology: a paraneoplastic syndrome caused by antibodies to the P/Q calcium channel on presynaptic motoneuron terminals, thereby reducing acetylcholine release; rarely involves antibodies against the N-type calcium channels
b.subtypes: both occur with approximately equal frequencies
i.Lambert-Eaton myasthenia without associated cancer: average age of onset 50 years of age; often associated with other autoimmune conditions (i.e., myasthenia gravis, pernicious anemia, vitiligo, diabetes, lupus)
ii.Lambert-Eaton myasthenia with associated cancer: average age of onset 60 years of age; classically associated with small-cell lung cancer or rarely associated with leukemia or lymphoma
(1)3% of small-cell lung cancer patients with develop Lambert-Eaton myasthenia
(2)symptomatic onset of myasthenia precedes the diagnosis of cancer in 80%
c.symptoms: usually is subacute at onset, but may be acutely precipitated by exposure to calcium channel blockers, iodinated contrast agents, and muscle paralytic agents
i.weakness in the proximal lower extremities upper extremities oropharyngeal muscles respiratory muscles
(1)weakness improves with brief exercise but then worsens with sustained exercise; similarly, patients are initially hyporeflexic but the reflexes improve with repetition
(2)ocular and facial muscles are generally spared, although 40% will complain of diplopia
ii.autonomic dysfunction (Box 10.6): dry mouth, constipation, impotency
d.diagnostic testing: EMG exhibits transient, incremental compound muscle action potential (CMAP) response to rapid nerve stimulation (20–50 Hz) and a decremental response to slow nerve stimulation (1–5 Hz), similar to botulism; also exhibits small CMAP amplitudes (Box 10.7)
e.treatment: inconsistently responsive to acetylcholinesterase inhibitors
i.Lambert-Eaton myasthenia without associated cancer: chronic immunosuppression
(1)requires careful and frequent evaluation for development of a neoplasm
ii.Lambert-Eaton myasthenia with associated cancer
(1)treatment of neoplasm
(2)3, 4-diaminopyridine; immunosuppression; plasma exchange, IVIg
IV. Congenital Myopathies
1.X-linked myopathies: Mostly symptomatic in males; however, female carriers may develop mild symptoms at later ages due to random inactivation of the X chromosome
a. Duchenne’s muscular dystrophy
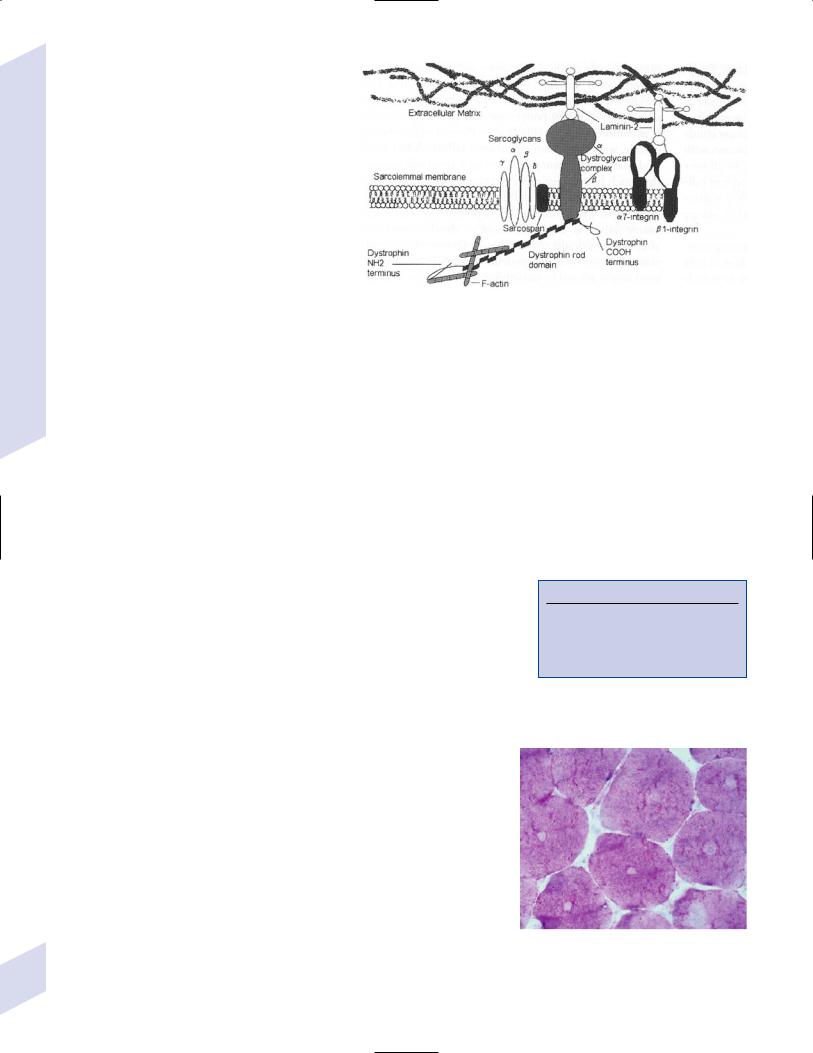
i.pathophysiology: caused by mutation of the dystrophin gene, which encodes a protein that anchors the cytoskeleton to actin in the myocontractile apparatus
(1)the majority of mutations are frameshift; 30% of cases are novel mutations, and 15% of novel mutations are gonadal mosaics
ii.symptoms
Box 10.6
Autonomic dysfunction does not occur with myasthenia gravis.
Box 10.7
CMAP is a summation of multiple MUPs
(1)progressive symmetric weakness beginning at 3–5 years of age as cramping and fatigue of the proximal muscles that progresses to the inability to walk by 13 years of age
(a)the waddling gait with toe walking is due to weakness of the gluteus muscles and shortness of the ankle tendon
(b)proximal muscle weakness necessitates that the patient stands up with the hands pushing on the knees {Gower’s sign}, which is nonspecific for Duchenne’s muscular dystrophy
(2)progressive generalized muscle hypertrophy that is most pronounced in calf muscles
(3)joint contractures, particularly in lower extremities
(4)dilated cardiomyopathy: 60% of cases are symptomatic by 18 years of age
(5)mild mental retardation
iii.diagnostic testing
(1)elevated CK (100-times normal levels) that declines with muscle loss over time
(2)genetic testing for dystrophin mutations
(3)muscle biopsy demonstrates endomysial fibrosis and clumps of variable-sized, irregular muscle fibers in a contracted state but with normal internal muscle architecture (Fig. 10–6)
(a)immunohistochemical analysis demonstrates absent staining for dystrophin
iv.treatment: avoid exercise, which likely worsens muscle injury
Figure 10–6 Duchenne’s muscular dystrophy. Endomysial fibrosis is noted in the region at the top of the figure. Courtesy of Dr. C. Yamada.
Congenital Myopathies
237
10 Diseases of the Muscles
(1)weekly glucocorticoid treatment delays loss of ability to walk by 2–3 years
(2)androgenic steroids to increase muscle bulk
(3)splints and release surgery for contractures
v.prognosis: death by 25 years of age from respiratory or cardiac failure
b.Becker’s muscular dystrophy
i.pathophysiology: caused by mutations of dystrophin gene that do not completely eliminate its functional activity; 70% of mutations are point mutations, and novel mutations are rare (unlike Duchenne’s muscular dystrophy)
ii.symptoms: as for Duchenne’s muscular dystrophy; the severity of symptoms correlates with the residual level of dystrophin activity
(1)onset of symptoms 7 years of age, progressing slowly to the loss of the ability to walk in late adolescent or even adulthood
(2)cardiomyopathy and mental retardation are not as common as they are in Duchenne’s muscular dystrophy
iii.diagnostic testing
(1)elevated CK ( 10-fold normal levels)
(2)muscle biopsy exhibits high levels of muscle regeneration and reduced (but present) dystrophin staining
(a)must rule out reduced levels of sarcoglycans as a cause for decreased dystrophin levels, as occurs in limb-girdle muscular dystrophies
(3)cannot rely on genetic testing to establish the diagnosis because decreased expression of an otherwise normal dystrophin protein may cause the disease
iv.treatment: as for Duchenne’s muscular dystrophy
c.Emery-Dreifuss muscular dystrophy
i.subtypes
(1)Emery-Dreifuss muscular dystrophy type 1: caused by an X-linked mutation of the emerin gene, which encodes a protein located on the inner surface of the nuclear membrane that (in association with lamin A/C and nuclear actin) forms the nucleoskeleton
(2)Emery-Dreifuss muscular dystrophy type 2: caused by an autosomal mutation of the gene for lamin A/C (Box 10.8)
ii.symptoms: onset in childhood or adolescence
(1)weakness of the shoulders, brachium, and calf muscles (a distinctive pattern for Emery-Dreifuss muscular dystrophy)
(2)contractures, which develop earlier than would be expected given the weakness
(3)cardiomyopathy with conduction block
iii.diagnostic testing: elevated CK ( 10-fold normal levels)
iv.treatment: cardiac pacing; contracture release
2.Autosomal-dominant congenital myopathies
a.myotonic dystrophy
i.subtypes
(1)myotonic dystrophy type I (98% of cases): caused by an expanded trinucleotide repeat in the 3 untranslated region of the myotonin protein kinase gene, which encodes a protein that regulates actinmyosin interaction and voltage-gated ion channel activity
(a)disease usually develops with 100 trinucleotide repeats
(2)myotonic dystrophy type II: mutation on chromosome 3
Box 10.8
Lamin A/C
Other mutations cause autosomal-recessive Charcot-Marie-Tooth disease type 2A and autosomal-dominant limb-girdle dystrophy.
238
ii.symptoms: an earlier age of onset is associated with increased number of trinucleotide repeats {anticipation}
(1)congenital-onset myotonic dystrophy
(a)severe hypotonia, dysphagia, and facial diplegia; no myotonia is present
(b)multiple joint contractures {arthrogryposis}
(c)mental retardation, which is often associated with hydrocephalus ex vacuo, hypoplasia of the corpus callosum, and abnormal myelination
(2)adult-onset myotonic dystrophy
(a)weakness of face and neck muscles, wrist and finger extensors, and foot extensors (a distinctive pattern for myotonic dystrophy)
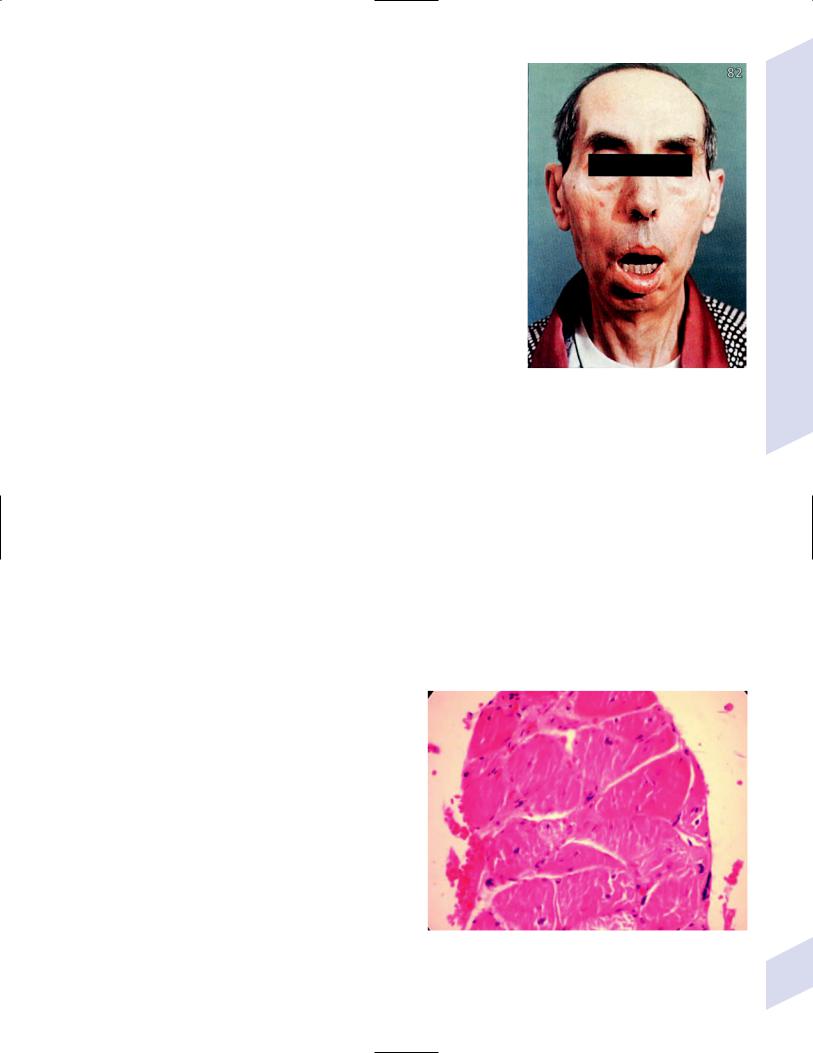
(i)facial weakness involves the levator palpebrae, masseters, and temporalis muscles, causing a characteristic sleepy, slack-jawed facial appearance with a narrow forehead (“hatchet head” appearance [Fig. 10–7])
(ii)palate weakness produces a nasal voice
(b)delayed relaxation of a muscle after contraction (e.g., grasping a doorknob) or after tapping on a muscle {myotonia}
(c)muscle flaccidity after cold exposure
(d)frontal balding
(e)hypersomnia
(f)cardiac conduction defects with an increased risk of tachyarrhythmias
(g)gonadal atrophy leading to infertility in males
(h)cataracts
(i)insulin resistance or type II diabetes mellitus
iii.diagnostic testing
(1)normal to mildly elevated CK
(2)EMG displays spontaneous bursts of high-amplitude discharges {myotonic discharges} that are usually of longer duration than those of myotonia congenita
(3)genetic testing
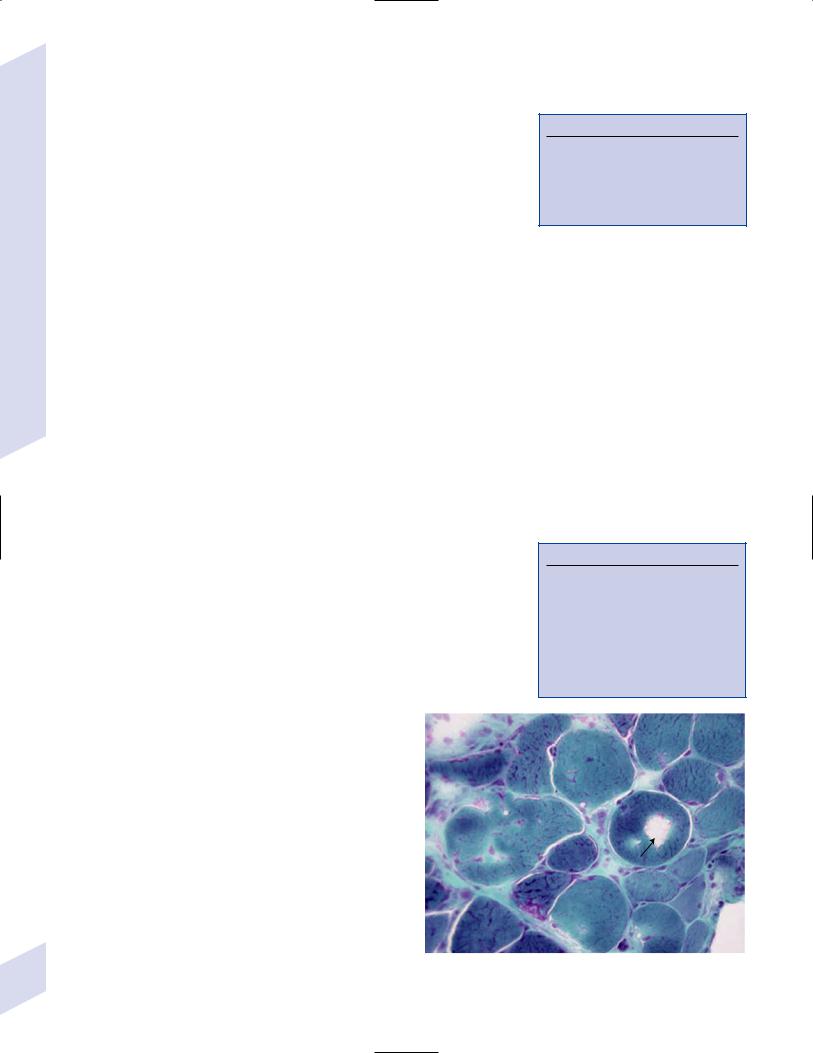
(4)muscle biopsy demonstrates myocytes with numerous internal nuclei, and rarely a reduced size of the type I (fast twitch) muscle fibers (Fig. 10–8)
iv.treatment: patients are generally not bothered by the myotonia, and the weakness has no effective treatment
(1)prophylactic antiarrhythmic medications or cardiac pacemaker implantation
(2)acetazolamide or mexiletine for myotonia
v.prognosis: average lifespan with adult onset 60 years of age, but 50% are wheelchair bound before their death
Figure 10–7 Myotonic muscular dystrophy. (From McKhann GM et al. Q&A Color Review of Clinical Neurology and Neurosurgery. Stuttgart, Germany: Georg Thieme; 2003:89, Fig. 82. Reprinted by permission.)
b.myotubular/centronuclear myopathy
i.subtypes
(1)autosomal dominant form: caused by mutation of the MYF6 gene protein, a myogenic factor
(2)autosomal recessive form: caused by an un-
known mutation |
Figure 10–8 Myotonic dystrophy. Courtesy of Dr. C. Yamada. |
Congenital Myopathies
239

10 Diseases of the Muscles
240
(3) X-linked form: caused by mutation of the myotubularin gene, the |
|
Box 10.9 |
|
protein of which may function in myocyte maturation (Box 10.9) |
|
||
|
|
|
|
ii. symptoms: onset in infancy (X-linked form), childhood (autosomal |
|
Mutations of myotubularin-related protein |
|
recessive form), or adulthood (autosomal dominant form) |
|
occur in Charcot-Marie-Tooth type 4B. |
|
|
|
|
|
(1)diffuse weakness lacking the typical proximal-distal gradient of a myopathy
(2)generalized atrophy, or rarely muscle hypertrophy (e.g., calf pseudohypertrophy)
(3)mild ophthalmoplegia, usually ptosis limitation of upgaze
iii.diagnostic testing: muscle biopsy demonstrates myocytes with centrally located nuclei associated with radial extensions of the sarcoplasmic reticulum from the nuclei that are observable on NADH staining; these abnormal myocytes resembles neonatal myocytes {myotubules} (Fig. 10–9)
(1)nonspecific findings include type I fiber predominance and hypotrophy
iv.treatment: none specific
v.prognosis: severity is dependent upon the age of onset
(1)onset in infancy leads to death within 1 year
(2)childhood onset progresses to wheelchair Figure 10–9 Centronuclear myopathy. Note the central location of
dependency by adulthood |
the nuclei, radial distribution of the sarcoplasmic reticulum, and the |
(3) adult onset generally progresses only to mild |
predominance of hypertrophic (type 1) fibers. (From Jeannet PY et al. |
Clinical and histologic findings in autosomal centronuclear myopathy. |
|
gait impairment in old age |
Neurology 2004, 62:1488. Reprinted by permission.) |
c. nemaline rod myopathy |
|
i.pathophysiology: caused by mutations in -actin; rarely by mutations of troponin
ii.symptoms: symptoms are apparent at birth
(1)weakness leading to feeding difficulties and respiratory failure; hypotonia
(2)arthrogryposis
(3)mental retardation
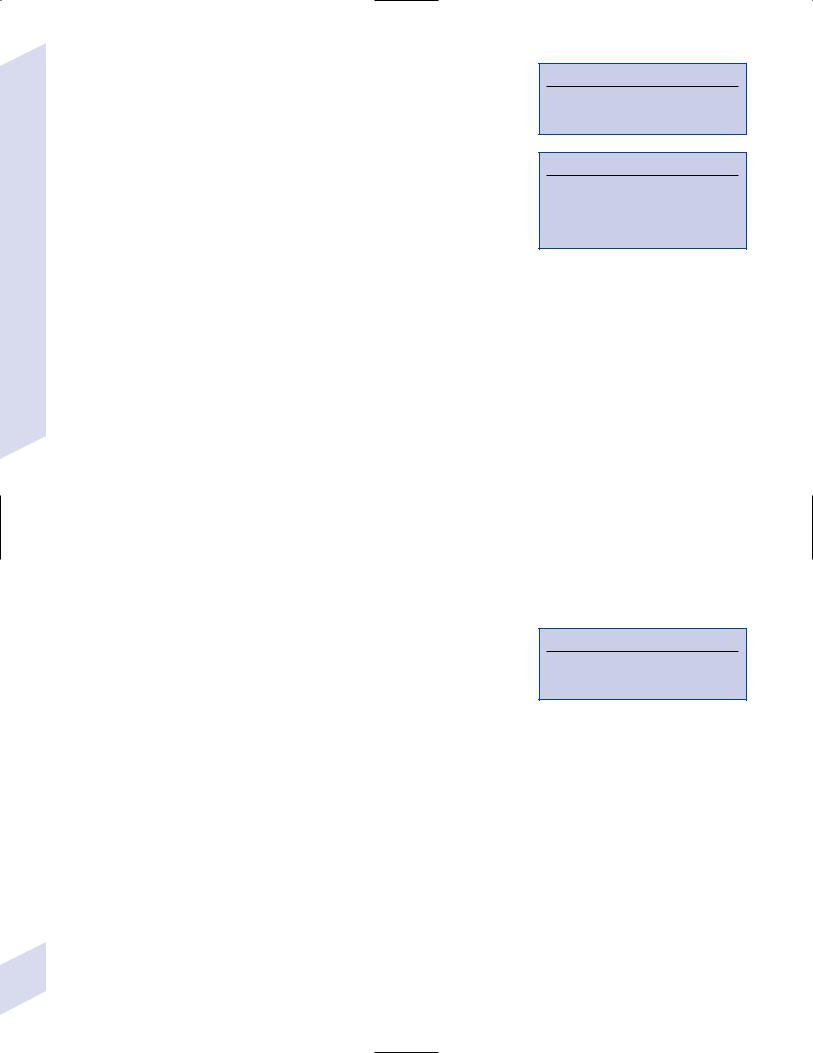
iii.diagnostic testing: muscle biopsy demonstrates red-blue-stained structures on Gomori trichrome stain in type I muscle fibers {nemaline rods} (Fig. 10–10)
(1)electron microscopy demonstrates nemaline rods as electron densities radiating from the Z-lines along the lengths of the thin filaments
iv.prognosis: death from respiratory failure d. facioscapulohumeral muscular dystrophy
i.pathophysiology: caused by partial deletion of a repeat sequence near a telomere of chromosome 4 that controls expression of the nearby gene for polyA-binding protein 2, the protein of which participates in mRNA turnover and initiation of translation
(1)large deletions produce more severe symptoms and an earlier age of onset
(2)40% of cases involve novel mutations that often present with mild symptoms because of mosaic expression (i.e., every cell does not contain the mutation)
ii.symptoms
(1)weakness of facial (particularly orbicularis oris and oculi muscles), scapular, and brachial muscles that is typically
A
B
Figure 10–10 Nemaline rods under light microscopy
(A) and electron microscopy (B), which shows the rods to be aligned in parallel with the myofibers. (From Lamont PJ et al. Nemaline rods and complex I deficiency in three infants with hypotonia, motor delay, and failure to thrive. Neuropediatrics 2004, 35:303, Fig. 1; 304, Fig. 2. Reprinted by permission.)

asymmetric; the weakness progresses to involve the distal extremities
(a)facial weakness produces a characteristic transverse smile and pouting lips as well as an inability to close the eyes while sleeping; patients are dysarthric for labial consonants and have difficulty using straws
(b)drooping shoulders from trapezius weakness gives a triangular appearance to the neck
(c)deltoids, oropharyngeal, and respiratory muscles are typically spared
(2)mental retardation (90%); seizures (40%)
(3)pectus excavatum
(4)retinal telangiectasias and detachment {Coat’s syndrome}
(5)cardiac arrhythmia (5%)
iii.diagnostic testing
(1)normal or mildly elevated CK
Figure 10–11 Facioscapulohumeral muscular dystrophy. Courtesy of Dr. C. Yamada.
(2)muscle biopsy demonstrates endomysial and perivascular inflammation, and hypertrophied muscle fibers with pale centers that are mixed together with atrophied muscle fibers (Fig. 10–11)
iv.treatment: scheduled albuterol increases muscle mass but not strength
v.prognosis: progression of the weakness may arrest around 50 years of age, leaving 20% of patients wheelchair bound; normal lifespan
e. limb-girdle dystrophies (LGMD) types 1A–E (autosomal dominant forms)
i.general symptoms: symmetric proximal weakness
ii.common subtypes (Table 10–1)
(1)myotilin: an actin-binding protein located in the I-band of the sarcomere
(2)lamin A/C: located on the inner nuclear membrane and forms the nucleoskeleton (Box 10.10)
(3)caveolin-3: forms invaginations of the sarcolemma {caveolae} that act as aggregation sites for transmembrane signaling proteins
Box 10.10
Other mutations of lamin A/C cause EmeryDreifuss muscular dystrophy type 2 and Charcot-Marie-Tooth disease type 2A
Congenital Myopathies
Table 10–1 Subtypes of Limb-Girdle Muscular Dystrophy
Subtype |
Gene product |
Notes |
|
|
|
LGMD 1A |
Myotilin |
|
LGMD 1B |
Lamin A/C |
Cardiomyopathy; onset 10 years of age |
LGMD 1C |
Caveolin-3 |
Onset 5 years of age; slowly progressive |
LGMD 2A* |
Calpain-3 |
Pronounced gluteus muscle involvement |
|
|
sparing the quadriceps |
LGMD 2B |
Dysferlin |
|
LGMD 2C |
-sarcoglycan |
|
LGMD 2D* |
-sarcoglycan |
Pronounced quadriceps involvement |
LGMD 2E |
-sarcoglycan |
|
LGMD 2F |
-sarcoglycan |
|
LGMD 2G |
Telethonin |
|
|
|
|
*common subtypes of the autosomal recessive form. |
|
Note: Light background autosomal dominant forms; dark background autosomal recessive |
241 |
forms. |
10 Diseases of the Muscles
242
iii.diagnostic testing
(1)mildly increased CK, in comparison with autosomal recessive LGMDs
(2)muscle biopsy
(3)genetic testing
3.Autosomal-recessive congenital myopathies a. limb-girdle dystrophies (LGMD) types 2A–H
i.general symptoms: symmetric proximal weakness
ii.subtypes (Table 10–1) (Fig. 10–12)
(1)calpain-3: a cytosolic calciumactivated protease that may regulate expression of transcription factors for myocyte differentiation; interacts with the myoskeletal protein titan
Figure 10–12 The dystrophin–sarcoglycan complex. (From Gilchrist JM. Overview of neuromuscular disorders affecting respiratory function. Semin Respir Crit Care Med 2002, 23:197, Fig. 2. Reprinted by permission.)
(2)dysferlin: located on the cytoskeletal membrane and may be involved in membrane resealing caused by the trauma of muscle contraction
(3)sarcoglycans: may function to translate mechanical stresses into biochemical signaling reactions, particularly with nitric oxide synthase
(4)telethonin: involved in sarcomere formation; interacts with myoskeletal protein titan
iii.diagnostic testing
(1)greatly increased CK, unlike autosomal dominant LGMDs
(2)muscle biopsy with specific immunohistochemistry
(3)genetic testing b. central core disease
i.pathophysiology: caused by mutations of the ryanodine-sensitive calcium channel (Box 10.11), which normally releases calcium from the sarcoplasmic reticulum by means of a physically connection between the L-type/dihydropyridine calcium channels in the t-tubule
ii.symptoms
(1)congenital form: reduced fetal movements, hypotonia; congenital hip dislocation, scoliosis, arthrogryposis
(2)childhood form: mild proximal weakness sparing the face, usually resulting in delayed acquisition of motor milestones and clumsiness; weakness is associated with muscle cramps
(a)may develop episodes of malignant hyperthermia (see p. 202) by 20 years of age
Box 10.11
Other mutations of the ryanodininesensitive calcium channel cause malignant hyperthermia; antibodies to ryanodine calcium channel are implicated in myasthenia gravis.
iii.diagnostic testing
(1)increased CK
(2)muscle biopsy demonstrates
(a)variable-sized muscle fibers with internalized nuclei (like myotonic dystrophy)
(b)lucent central core on NADH staining particularly in type I muscle fibers (like facioscapulohumeral muscular dystrophy) (Fig. 10–13)
iv.treatment: avoid anesthetics and paralytic agents that can cause malignant hyperthermia
v.prognosis: weakness is not progressive c. Fukuyama congenital myopathy
Figure 10–13 Central core disease. (From Neudecker S. Klassifikation und benennung von myopathien. Psychoneuro 200, 29:86. Reprinted by permission.)

i.pathophysiology: caused by a transpositional insertion into the fukutin gene, the protein of which may be necessary for proper extracellular glycosylation of the protein dystroglycan that is necessary for cell–cell adhesions
(1)fukutin protein is highly expressed in the developing brain but is not apparently expressed in muscle
(a)abnormal function of fukutin protein in the brain likely explains the frequently associated developmental abnormalities (polymicrogyria, lissencephaly, disruption of gray matter lamination, hypomyelination)
ii.symptoms: onset 1 year of age
(1)hypotonia and weakness
(2)mental retardation, seizures, hydrocephalus
(3)retinal hypoplasia
iii.diagnostic testing
(1)neuroimaging of the brain exhibits multiple developmental abnormalities
(2)muscle biopsy demonstrates internal nuclei, and disrupted and increased connective tissue
iv.treatment: none specific
v.prognosis: death by 10 years of age
4.Inherited metabolic diseases of the muscle
a.carnitine palmitoyl transferase deficiency
i.pathophysiology: autosomal recessive mutations of carnitine palmitoyl transferase that links fatty acids to carnitine for transportation into the mitochondria where they are oxidized
ii.symptoms: fatigue and muscle pain developing well after the onset of exercise; episodes may present in childhood or adulthood
iii.diagnostic testing: significant myoglobinuria; increased respiratory
exchange ratio ( expired [CO2]/inspired [O2]) at rest, reflecting the use of glucose for resting metabolism rather than fatty acids
iv.treatment: glucose intake during periods of exercise
b.McArdle’s disease
i.pathophysiology: autosomal recessive mutations in myophosphorylase, which cleaves glycogen into free glucose for glycolysis
ii.symptoms: rapid-onset fatigue that can be overcome with continuing activity; muscle pain with forced muscle contraction or exercise; normal acute muscle strength and bulk
iii.diagnostic testing
(1)mild myoglobinuria
(2)EMG during ischemic contraction demonstrates no electrical activity
(3)increased CK occur only with significant muscle contractions
(4)minimal rise in serum lactate levels with anaerobic exercise
iv.treatment: none specific
c.phosphofructokinase deficiency
i.pathophysiology: caused by autosomal recessive mutations of phosphofructokinase, which phosphorylates fructose-6-phosphate prior to its cleavage into glyceraldehyde that then enters the Krebs cycle
(1)the rate-limiting enzyme for metabolism of glucose for the Krebs cycle
ii.symptoms: as per McArdle’s disease, although more severe; also hemolytic anemia
Congenital Myopathies
243
10 Diseases of the Muscles
244
V. Acquired Muscle Diseases
1.Inclusion body myositis
a.pathophysiology: likely an autoimmune reaction since CD8 T lymphocytes are found in affected muscles (Box 10.12)
i.associated with HLA-DR and –DQ
ii.20% of cases occur in the setting of a monoclonal gammopathy (i.e., paraproteinemia)
b.symptoms: typically develops in patients 50 years of age
i.weakness of the lower extremities upper extremities; the weakness spares the face, deltoids, and interossei muscles, but frequently involves the oropharyngeal muscles producing dysphagia (a characteristic pattern for this disease)
(1)the weakness is atypical for a disease of the muscle because it
(a)is asymmetric, to the degree that it appears to be focal
(b)may be distal proximal in 20% of cases
c.diagnostic testing
i.mildly increased CK
ii.muscle biopsy demonstrates endomysial inflammation, rimmed vacuoles on Congo Red stain, and muscle fiber hypertrophy; myocytes demonstrates accumulations of -amyloid, transglutaminases, and ubiquitin as inclusion bodies (similar to the inclusions of Alzheimer’s disease) (Fig. 10–14)
Box 10.12
Autoimmune diseases with myopathy—
Mixed connective tissue disorders; scleroderma; lupus
Myopathies with autoimmune diseases—
Polymyositis (40%); inclusion body myopathy (15%)
iii.nerve conduction studies often demonstrate a subclinical sensorimotor neuropathy
iv.elevated M protein levels (20%)
d.treatment: none specific; IVIg has been proven ineffective, and glucocorticoids are likely ineffective
e.prognosis: progressive weakness
2.Dermatomyositis (Box 10.13)
a.pathophysiology: an inflammatory myopathy with multiple causes
b.subtypes
i.childhood dermatomyositis
(1)often exhibits a viral prodrome (Coxsackie B, parvovirus, echovirus); also associated with hepatitis B vaccination, growth hormone treatment, and excessive sun exposure
(2)associated with HLA-DQ (90% of cases)
ii.adult dermatomyositis: may or may not have antibodies against the Mi-2 protein, which is a transcription regulatory factor
(1)Mi-2 antibody-positive cases are highly associated with underlying malignancy, particularly advanced ovarian cancers
(2)Mi-2 antibody-negative cases are associated with rheumatoid arthritis, scleroderma, and the CREST syndrome, also with an increased risk of underlying malignancy
c.symptoms
Box 10.13
CKs in Diseases of the Muscle
Really high CKs—Duchenne’s muscular dystrophy; autosomal recessive LGMDs; central core disease; glycogen storage diseases; acid maltase deficiency; hypothyroidism
Surprisingly low CKs—childhood dermatomyositis; hyperthyroidism; rheumatologic diseases; glucocorticoid myopathy
i.proximal muscle weakness and pain; dysphagia is common
ii.erythematous, edematous rash predominantly on the face and eyelids, extensor joint surfaces, and neck {shawl sign}; the rash occurs in sunexposed areas {heliotrope rash} and is exacerbated by sun exposure
Figure 10–14 Inclusion body myositis. Rimmed inclusion bodies are demonstrated at the arrow. Courtesy of Dr. C. Yamada.

iii.keratotic thickening of the skin on the extensor surfaces of the joints {Gottron’s papules}
iv.serositis and arrhythmias, in the adult Mi-2 antibody-negative subtype
d.diagnostic testing
i.increased CK ( 30-fold normal levels)
ii.muscle biopsy demonstrates perivascular inflammation, atrophy around the perimysium, microvascular IgM and complement deposits, and necrosis and regeneration (Fig. 10–15)
iii.serology for the Mi-2 antibody
(1)adult Mi-2 antibody-positive subtype uniformly is antinuclear antibody (ANA-) positive
(2)adult Mi-2 antibody-negative subtype often exhibit Jo-1 antibodies that target a t-RNA synthetase; these antibodies are fairly specific for inflammatory myopathies (polymyositis dermatomyositis)
e.treatment
Figure 10–15 Dermatomyositis. Note atrophy around the perimysium (arrow) and perivascular inflammation (double arrow). Courtesy of Dr. C. Yamada.
i.solumedrol acutely followed by prednisone tapered over several months or maintained chronically if needed
ii.immunosuppressants: azathioprine, methotrexate
(1)all immunosuppressants typically have a 2–3-month delay before symptomatic improvement becomes evident
iii.IVIg
3.Polymyositis
a.pathophysiology: an inflammatory myopathy associated with autoimmune disorders (lupus, Sjögren’s syndrome, antiphospholipid antibody syndrome), hyperthyroidism, or underlying malignancy (10%)
b.symptoms
i.symmetric proximal weakness, particularly of the oropharyngeal, posterior neck, and quadriceps muscles; involvement of the proximal third of the esophagus and the respiratory muscles is common
ii.pain in affected muscles (20%)
iii.inflammatory cardiomyopathy producing arrhythmias
c.diagnostic testing
i.increased CK
ii.antimyosin antibodies (90%)
iii.Jo-1 antibodies against the histidine t-RNA synthetase (95%)
iv.EMG demonstrates small-amplitude, shortduration MUPs; fibrillation potentials and sharp waves
v.muscle biopsy demonstrates endomysial and perivascular monocytic inflammation, and necrosis with regeneration (Fig. 10–16)
d.treatment
i.glucocorticoids as per dermatomyositis
ii.immunosuppressants: azathioprine, methotrexate, cyclosporine
iii.plasmapheresis, IVIg
e.prognosis: progressive course over a period of several months
4.Isaac’s syndrome/neuromyotonia
Figure 10–16 Polymyositis. Note the intense perivascular and endomysial leukocyte infiltration. (From Gonzalez PP, Richter HW. Akute polymyositis under glatiramerazetat (copaxone). Aktuelle Neurologie 2001, 28: 392, Fig. 1. Reprinted by permission.)
Acquired Muscle Diseases
245
10 Diseases of the Muscles
a.subtypes
i.paraneoplastic neuromyotonia: exhibits antibodies against voltagegated potassium (Box 10.14) channels and the nicotinic acetylcholine receptor that are located on peripheral nerves (Box 10.15)
(1)associated with small cell lung cancer and lymphoma as well as myasthenia gravis
ii.neuromyotonia associated with human immunodeficiency virus (HIV) infection and penicillamine use
b.symptoms: onset typically 60 years of age; the severity of symptoms fluctuates with the patient’s level of physical activity
i.neuromyotonia: prolonged muscle contraction induced by physical stimulation
ii.myokymia: rhythmic rippling of the muscles that is not sensitive to physical stimulation (e.g., percussion) as is myotonia
iii.stimulation-independent muscle cramping that may involve the face and oropharynx
iv.stiffness but little weakness (if any); normal reflexes
v.abnormal limb posturing similar to dystonias
vi.hyperhidrosis
vii.insomnia
c.diagnostic testing
i.EMG: shows spontaneous motor unit potentials, fasciculations, and myokymia and neuromyotonia potentials
ii.nerve conduction studies demonstrates axonal motor neuropathy
iii.CK is not reliably elevated
iv.muscle biopsy: demonstrates type I fiber predominance and hypertrophy
d.treatment: antiepileptics (phenytoin, carbamazepine); plasmapheresis
Box 10.14
Antibodies against voltage-gated potassium channels are also seen in limbic encephalitis paraneoplastic syndrome
Box 10.15
Other diseases of potassium channels—
Paraneoplastic limbic encephalitis; Hashimoto’s encephalopathy; Morvan syndrome ( Isaac’s syndrome encephalopathy)
VI. Channelopathies of Muscle
1.Diseases of sodium channels
a. hyperkalemic periodic paralysis
i.pathophysiology: caused by mutations in a voltage-gated sodium channel (Box 10.16) that increase its open time and slow its inactivation once it is open, thereby allowing prolonged membrane depolarization
(1)an increased extracellular potassium concentration depolarizes the cell and activates the abnormal channels, which then causes efflux of potassium from the cell that further depolarizes the cell and maintains the sodium channel in an open state
(2)exhibits autosomal dominant inheritance with complete penetrance
ii.symptoms: provoked by cold exposure, by resting after exercise, or by
potassium-rich |
foods (e.g., fruit juices); attacks develop before |
10 years of age |
|
(1)acute onset of symmetric proximal weakness that is generally less severe and of shorter duration than in hypokalemic periodic paralysis, but that occur more frequently than in hypokalemic periodic paralysis
(2)myotonia (an uncommon feature)
iii.diagnostic testing: provoke an attack with oral potassium supplements or exercise
(1)genetic testing
(2)increased serum and urinary potassium during attacks
246 |
(3) muscle biopsy demonstrates internalized nuclei and fibrosis |
Box 10.16
Other mutations in the voltage-gated sodium channel cause paramyotonia congenita.

iv.treatment
(1)avoid provocative stimuli, but during an attack
(a)consume glucose, which drives cellular potassium uptake to be absorbed
(b)administer intravenous calcium gluconate or sodium chloride
(2)chronic prophylaxis with acetazolamide or thiazide diuretics
b.paramyotonia congenita
i.pathophysiology: caused by mutations in a voltage-gated sodium channel (the same one as in hyperkalemic periodic paralysis)
ii.symptoms: stiffness provoked by cold exposure, exercise, or hypokalemia; severe cold exposure or strenuous exercise can also evoke weakness that outlasts the stiffness
iii.diagnostic testing
(1)EMG demonstrates fibrillation potentials or repetitive MUPs induced by cooling or exercise that disappears with further cooling or exercise
(2)muscle biopsy demonstrates only variable-sized muscle fibers
iv.treatment
(1)avoid provocative stimuli, but during attacks treat as hyperkalemic periodic paralysis
(2)prophylaxis with mexiletine in severe cases
2.Diseases of calcium channels
a.hypokalemic periodic paralysis
i.caused by
(1)mutations in the L-type (dihydropyridine) voltage-gated calcium channel (Box 10.17) that excessively depolarizes the myocyte
(a)voltage-gated calcium channel mutations are associated with reduced expression of voltage-gated sodium channels for unknown reasons
(b)exhibits autosomal dominant inheritance with incomplete penetrance particularly in women; onset of symptoms in early childhood
(2)hyperthyroidism, which increases expression of the sodiumpotassium ATPase in muscle that would excessively hyperpolarize the myocyte; exhibits an autosomal dominant pattern of inheritance
(3)hypokalemia from metabolic acidosis, hyperaldosteronism, or urine alkalinization
ii.symptoms: provoked by resting after exercise, carbohydrate consumption, or the use of aminergic agents or steroids
(1)acute attacks involve soreness in back and thighs that progresses to the extremities; muscle swelling, firmness, and weakness then develop
(a)episodes may last several hours, but have an acute onset and resolution
(2)chronic symptoms: residual weakness following an acute attack accumulates to symptomatic level by 40 years of age
iii.diagnostic testing: potassium levels during an attack are not reliable for the diagnosis
(1)muscle biopsy demonstrates vacuolation and tubular aggregates in the myocytes
iv.treatment
(1)correct underlying hypokalemia-inducing disorders, if any
(2)oral potassium supplementation
Channelopathies of Muscle
Box 10.17
Not the same as the ryanodine calcium channel, which is mutated in central core disease and malignant hyperthermia.
247

10 Diseases of the Muscles
Table 10–2 Comparison of Myotonic Conditions
|
Myotonic |
Paramyotonia |
Myotonia |
|
dystrophy |
congenita |
congenita |
|
|
|
|
Distribution of myotonia |
Distal proximal |
Diffuse |
Diffuse |
Effect of repetitive |
Mild decrement |
No effect |
Significant decrement |
stimulation |
|
|
|
MUAP morphology |
Myopathic changes |
Normal |
Normal |
|
|
|
|
(3)low sodium, low carbohydrate diet
(4)spironolactone for prophylaxis; avoid acetazolamide unless a calcium channel mutation is known to be the cause
3.Diseases of chloride channels
a. myotonia congenita/Thomsen’s disease (Table 10–2)
i.pathophysiology: caused by mutations in a chloride channel that decrease chloride conductance; the reduced chloride efflux during membrane depolarization allows accumulation of potassium in the T tubules, which induces repetitive membrane depolarizations by activation of the voltage-gated sodium channels
(1)exhibits autosomal dominant inheritance
ii.symptoms
(1)muscle stiffness and myotonia that is pronounced after inactivity but that improves after exercise
(2)normal strength and tone that temporarily decreases with exercise before returning to normal
(3)muscle hypertrophy, in the autosomal recessive form
iii.diagnostic testing: EMG demonstrates myotonic discharges
iv.treatment: mexiletine; quinine; phenytoin
VII. Diseases of Absent Muscles (Table 10–3)
Table 10–3 Diseases of Absent Muscles
Disease |
Pathology |
Mutation |
Inheritance |
|
|
|
|
Congenital |
Developmental absence of the |
Kinesin |
Autosomal |
fibrosis of |
superior division of CN III, causing |
|
dominant |
extraocular |
bilateral atrophy of the levator |
|
|
muscles |
palpebrae and superior rectus |
|
|
|
muscles; anomalous origins and |
|
|
|
insertions of the remaining muscles |
|
|
|
are common |
|
|
Duane’s |
Absent or hypoplastic CN VI causes |
Chromosome 2, 8 |
Sporadic or |
syndrome |
atrophy of lateral rectus muscle; may |
|
autosomal |
|
be bilateral |
|
dominant |
Mobius |
Absence or hypoplasia of the facial |
Chromosome 1, 3, |
Autosomal |
syndrome |
and/or abducens nuclei, usually |
10, 13 |
dominant |
|
bilateral; also may have hypoglossal |
|
|
|
palsy, dysarthria, dysphagia, irregular |
|
|
|
respiration, and/or hypoplasia of the |
|
|
|
lower extremities |
|
|
Poland |
Absence of the sternal head of the |
? |
Sporadic |
syndrome |
pectoralis muscle, possibly with |
|
|
|
absence of rotator cuff muscles; |
|
|
|
hand and spine deformations; |
|
|
|
dextrocardia |
|
|
|
|
|
|
248
