
- •Preface
- •Content
- •Contributors
- •2 Practicing Evidence-Based Surgery
- •5 Surgical Critical Care
- •7 Shock
- •8 Surgical Bleeding and Hemostasis
- •11 Head and Neck Lesions
- •16 Acute and Chronic Chest Pain
- •17 Stroke
- •18 Surgical Hypertension
- •19 Breast Disease
- •20 Gastrointestinal Bleeding
- •21 Abdominal Pain
- •23 Abdominal Masses: Vascular
- •24 Jaundice
- •25 Colon and Rectum
- •26 Perianal Complaints
- •28 The Ischemic Lower Extremity
- •29 The Swollen Leg
- •30 Skin and Soft Tissues
- •31 Trauma Fundamentals
- •33 Musculoskeletal Injuries
- •34 Burns
- •36 Neonatal Intestinal Obstruction
- •37 Lower Urinary Tract Disorders
- •38 Evaluation of Flank Pain
- •39 Scrotal Disorders
- •40 Transplantation of the Kidney
- •41 Transplantation of the Pancreas
- •42 Transplantation of the Liver
- •Index

17
Stroke
Rocco G. Ciocca
Objectives: Altered Neurologic Status
1.To describe the evaluation and management of a patient with an acute focal neurologic deficit.
2.To differentiate transient ischemic attack (TIA), reversible ischemic neurologic deficit (RIND), and cerebral vascular accident (CVA).
3.To differentiate anterior versus posterior circulation symptoms.
4.To outline the diagnostic tests and monitoring of carotid occlusive disease, including the role of angiography and noninvasive methods.
5.To discuss medical versus surgical management of carotid artery disease.
Case
A 68-year-old man with a history of hypertension, elevated cholesterol, type 2 diabetes, and a 50-pack-per-year smoking history notices that he cannot see out of his right eye. It is as if a “shade” had been pulled down over the eye.
Introduction
Stroke and its complications can be devastating. The term stroke and cerebral vascular accident (CVA) are used interchangeably in this chapter. Approximately 500,000 people develop new strokes annually. It is the leading cause of neurologic death, and it is the third leading cause of death, preceded by myocardial infarction (MI) and cancer. The societal costs number in the billions of dollars. While not all strokes are related to large-vessel disease, the incidence is large enough to warrant attention.
305
306 R.G. Ciocca
This chapter discusses the pathophysiology of stroke, its workup, and the therapeutic options, and presents treatment recommendations and the available evidence to support them.
Pathophysiology
Definitions
The differentiation between the aforementioned entities generally is determined by timing and length of symptoms. A transient ischemic attack (TIA) is defined as an acute loss of cerebral function that persists for less than 24 hours. Most of these neurologic events are brief, lasting 15 minutes or less. Generally, these events are focal and specific. Symptoms associated with anterior or carotid bifurcation disease include sensory or motor deficits affecting the contralateral face, arms, or legs, aphasia, or alterations in higher cortical dysfunction. Patients with posterior or vertebrobasilar ischemia may present with vertigo, dizziness, gait ataxia, dysarthria, nystagmus, diplopia, bilateral visual loss, drop attacks (collapse caused by loss of control of extremities without loss of consciousness), as well as bilateral or alternating motor or sensory impairment. Nonfocal symptoms, such as syncope, confusion, and “light-headedness,” rarely are the result of cerebrovascular disease.
Reversible ischemic neurologic deficits (RINDs) are cerebral vascular symptoms that persist for more than 24 hours but less than 7 days. Symptoms that persist beyond 7 days usually are considered a stroke. Many would consider a stroke to have occurred if the symptoms persist beyond 24 hours. The RIND classification of symptoms seems to be used less commonly in clinical medicine.
Transient unilateral loss of vision is referred to as amaurosis fugax. This is the symptom described by the patient in the case presented at the beginning of this chapter. This symptom is described classically as the sensation of a shade coming down over the entire eye, half an eye, or a quadrant of an eye. This event is the consequence of a microembolus lodging in the ophthalmic artery or one of its retinal branches. A cholesterol crystal (Hollenhorst plaque) occasionally is observed on funduscopic examination as a bright refractive body in a branch of the retinal artery. The significance of the above-mentioned focal neurologic events is that they are markers of stroke potential. While only 10% of strokes are preceded by TIAs, the patient who experiences one has a 5% to 8% per year chance of developing a stroke. Within 5 years of the onset of TIAs, the patient has a 25% to 40% chance of developing a stroke.
A stroke also may be called a cerebral vascular accident (CVA) to distinguish it from the vascular nature of most strokes. Thirty-four percent of strokes are the result of large-artery disease as compared with embolism, which leads to 31% of strokes, lacunar infarctions (usually associated with hypertension and small-vessel disease), which leads to 19% of strokes, and hemorrhage, which leads to 16% of strokes. Causes of stroke other than large-vessel disease rarely are associated with TIAs.
17. Stroke 307
Anatomy
A thorough understanding of the arterial anatomy of the brain is critically important in understanding the pathology and treatment of stroke. The anatomy is divided into anterior and posterior, and these are connected via the circle of Willis.
Paired internal carotid arteries that provide approximately 80% to 90% of the total cerebral blood flow feed the anterior circulation. The left common carotid artery originates directly from the aortic arch, whereas the right common carotid artery originates from the innominate artery. The common carotid arteries bifurcate at the angle of the mandible into the external and internal carotid arteries. The external carotid artery has many divisions and primarily provides circulation to the face and neck. It supplies the cerebral circulation through collaterals. The internal carotid artery can be divided into the cervical (or extracranial), intrapetrosal, intracavernous, and supraclinoid segments. The cervical, intrapetrosal, and intracavernous portions of the internal carotid artery have no branches.
The posterior circulation is composed of paired vertebral arteries that supply 10% to 20% of the total cerebral circulation. Both vertebral arteries originate from the first portion of their respective subclavian arteries and then enter the vertebral canal at the transverse foramina of the sixth cervical vertebra. The vertebral arteries unite to form the basilar artery, which then branches into the right and left posterior cerebral arteries. The posterior circulation supplies the brainstem, cranial nerves, cerebellum, and the occipital and temporal lobes of the cerebrum.
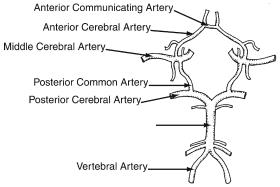
The circle of Willis (Fig. 17.1) is the term used to describe the interconnecting network of vessels that link the posterior and anterior circulations. The anterior communicating artery connects the two anterior cerebral arteries, while the posterior communicating artery connects the internal carotid arteries to the posterior cerebral arteries.
Basilar Artery
Figure 17.1. Configuration of the terminal branches of the vertebral and internal carotid arteries and their interconnections to form the circle of Willis. (Reprinted from Patel ST, Kent KC. Cerebrovascular disease. In: Norton JA, Bollinger RR, Chang AE, et al, eds. Surgery: Basic Science and Clinical Evidence. New York: Springer-Verlag, 2001, with permission.)
308 R.G. Ciocca
The circle is intact in 20% to 40% of individuals and allows for collateral flow between the hemispheres and the anterior and posterior circulation. The fact that the circle so infrequently is intact implies two things: first, there are other means of collateral circulation; second, the existence of collateral circulation cannot be assumed before surgical intervention.
Presentation
One of the most frequently misunderstood anatomic and pathophysiologic points is that carotid artery stenosis leads to atheroembolic events. The pathology usually is not secondary to decreased perfusion. The brain has a tremendously rich collateral circulation. The carotid circulation dominates the anterior circulation. The internal carotid artery is the main conduit to the brain, feeding the middle cerebral artery. The circle of Willis interconnects the anterior and posterior circulation. It is rare for people to have hypoperfusion secondary to carotid occlusive disease. The pathology is embolic and therefore focal. The “dizzies” and syncope rarely are caused by carotid disease. This is not hard to believe, since probably greater than 90% to 95% of the time carotid surgery is performed safely with a shunt.
Risk Factors and Pathology
The primary risk factors for stroke are similar to those for patients presenting with any other form of cardiovascular disease: smoking, hypertension, diabetes, hypercholesterolemia, advanced age, obesity, inactivity, and, to a lesser extent, family history.
The primary pathology leading to the development of extracranial carotid disease is atherosclerosis. This accounts for approximately 90% of lesions in the extracranial system seen in the Western world. The remaining 10% include such entities as fibromuscular dysplasia, arterial kinking because of arterial elongation, extrinsic compression, traumatic occlusion, intimal dissection, the inflammatory angiopathy, and migraines. Radiation-induced atherosclerotic change of the extracranial carotid artery has become a recognized entity. Other rare entities, usually involving intracranial vessels, include fibrinoid necrosis, amyloidosis, polyarteritis, allergic angitis, Wegener’s granulomatosis, granulomatious angiitis, giant cell arteritis, and moyamoya disease. Embolization from a cardiac source also is an important contributing factor to cerebral vascular disease.
The most likely etiology of the symptoms experienced by the patient in the case presented at the beginning of this chapter is the presence of atherosclerotic plaque at the ipsolateral carotid bifurcation.
Epidemiology
Incidence/Prevalence
As previously stated, approximately 500,000 patients in the United States develop new strokes each year. It is the third leading cause of

17. Stroke 309
death, but perhaps more disconcerting are the morbidity and potential loss of independence that result from stroke.
The overall incidence of new stroke is 160 per 100,000 per year. The incidence rises, however, as one ages. This has been borne out by several population-based studies designed to look at the incidence of stroke. The Rochester, Minnesota, population study (from 1955 to 1969) emphasized the influence of advancing age on the progressive incidence of cerebral infarction: the 55-year-old to 64-year-old age group had a cerebral infarction rate of 276.8 per 100,000 per year; the 65-year- old to 74-year-old age group had an incidence of 632 per 100,000 per year; and the 75-year-old and over age group had a stroke rate of 1786.4 per 100,000 per year.1
Analysis of the cerebral infarction rate by sex distribution indicated that the rate was approximately 1.5 times greater in men than in women of the same age.
The prognosis after a stroke is varied, but 6 months following the survival of a stroke only 29% of the patients in the Rochester study had normal cerebral function; 71% continued to have manifestations of neurologic dysfunction. In the latter group, 4% required total nursing care, 18% were disabled but capable of contributing to selfcare, and 10% were aphasic. Of the patients who suffered a fatal stroke, 38% died of the initial stoke, 10% died of a subsequent stroke, and 18% died from complications of coronary disease. The chance of recurrent stroke within 1 year of the initial stroke was 10%, and the chance of a recurrent stroke within 5 years of the initial attack was 20%.
The above data are somewhat dated, and yet, somewhat surprisingly, the incidence of stroke actually may have increased.2 The increased incidence may be due to greater awareness and imaging studies leading to diagnosis that is more accurate. The overall prognosis of stroke has changed little over time.
Workup
History and Physical Examination
The history taken and the physical exam performed on a patient with a change in neurologic status are no different from any other history and physical exam. They should be thorough, and they should include a head-to-toe evaluation of the patient. It is important to document clearly and precisely the patient’s neurologic status so that other healthcare professionals clearly can understand the neurologic status of the patient.
1 Matsumato N, Whisnant JP, Kurland LT, et al. Natural history of stroke in Rochester, Minnesota, 1955 through 1969: an extension of a previous study, 1945 through 1954. Stroke 1973;4:20.
2 Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke 1996; 27(3):373–380.
310 R.G. Ciocca
In verbal communication with the patient regarding the patient’s neurologic state, it is helpful to speak in terms of cerebral hemispheres rather than right or left sides of the body. Since the left cerebral hemisphere controls right-sided body function, it can be confusing as to just what a right-sided stroke means. Does it mean a right cerebral hemispheric event with associated left-sided bodily dysfunction or does it imply right-sided weakness? Therefore, speaking in terms of cerebral hemispheres provides a clearer understanding of the possible source of the problem.
The presence of a cervical bruit is an important physical finding to document in the evaluation of a patient with cerebrovascular disease.
In 20% of patients with bruits, hemodynamically significant stenosis can be documented. Conversely, it is estimated that 19% to 27% of patients with notable stenotic lesions of the carotid were reported to have no bruit. It also is important to recognize that internal carotid artery plaques cause the vast majority (75–90%) of cervical bruits. The external carotid artery accounts for approximately 10% of the bruits. While the presence of a carotid bruit may denote significant carotid disease in only a small minority of patients, it is an important marker for increased risk of death from coronary artery disease. Interestingly, a bruit may disappear as the degree of stenosis increases beyond 85% to 90%.
In addition to focusing on the patient’s neurologic status and whether or not a cervical bruit is present, one also must focus attention on the overall health and physical findings of the patient, as these are of equal, if not of more, importance. Attention needs to be paid to the patients other comorbities, and their surgical risk should be assessed.
Carotid Duplex
Duplex ultrasound (DU) is the noninvasive test of choice when evaluating a patient for the presence of extracranial carotid artery disease.
It is a bimodal study employing B-mode ultrasound with Doppler waveform analysis. Evaluation of the Doppler waveform and the peak systolic and end diastolic velocities in the internal carotid artery determine the degree of internal artery within several relatively broad ranges. It is a relatively inexpensive exam that is safe and very well tolerated by the patient. It also is accurate approximately 90% of the time in experienced vascular diagnostic laboratories. Increasingly, DU is being used safely as the only diagnostic test in the workup of patients for carotid artery disease. Many experienced vascular surgeons have operated safely based on the results of a DU alone.
But DU does have several limitations. First, a skilled technician must perform the DU, and it must be read properly. In addition, it may be difficult to differentiate between a very high grade stenosis and complete occlusion. Also, DU interrogates only the extracranial carotid system, and therefore tells nothing about the presence or the absence of intracranial disease. The clinical significance of these so-

17. Stroke 311
called tandem lesions is open to debate. Even with these limitations, DU remains a useful diagnostic tool.
Computed Tomography Scan
Computed tomography (CT) scanning is a very useful tool in the evaluation of a patient who may have had a stroke. Axial images of the brain are obtained noninvasively, and anatomic abnormalities are visualized. It is useful to differentiate a mass lesion from an intracranial bleed. A CT scan generally is the primary diagnostic study in evaluating an individual for a stroke. It is essential to realize, however, that a CT scan initially may be read as normal in an individual who has had a stroke. It can take anywhere from 24 to 48 hours for the stroke-induced changes to be seen on a CT scan. Therefore, it is recommended to repeat a CT scan in 48 hours if clinically indicated.
Not too long ago in the history of carotid surgery, a CT scan was a routine study ordered prior to proceeding with surgery, even in asymptomatic patients. Prospective studies have shown that CT scans of the brain prior to carotid surgery are unnecessary and not cost-effective.3
Magnetic Resonance Imaging/Angiography
The advent of magnetic resonance imaging (MRI) of the brain has been a tremendous advance in neuroimaging due to increased sensitivity, flexibility, and greater variety of images. The MRI scans depend on several characteristics of body tissue being imaged. These characteristics include the density of hydrogen nuclei, whether the nuclei are moving or stationary (flow), and two magnetic properties of tissue called T1 and T2 relaxation. Scans can be generated that capitalize on tissue difference of T1, T2, hydrogen density, and flow. Techniques that are more advanced and software allow magnetic resonance angiography (MRA), a noninvasive means of assessing vascular anatomy, to be performed, thereby noninvasively providing anatomic delineation of vascular anatomy (Fig. 17.2).
Magnetic resonance angiography is used best in conjunction with a high-quality duplex scan. In a large study in which both techniques were evaluated, the accuracy of DU and MRA in predicting a greater than 70% carotid stenosis (86% and 88%, respectively) increased to 94% when the results of these two tests were combined.4
Contrast Angiography
Contrast angiography is the traditional “gold standard” for evaluating the carotid arteries and cerebral circulation. Conventional contrast
3 Martin JD, Valentine RJ, Myers SI, Rossi MB, Patterson CB, Clagett GP. Is routine CT scanning necessary in the preoperative evaluation of patients undergoing carotid endarterectomy? J Vasc Surg 1991;14(3):267–270.
4 Patel MR, Kuntz KM, Klufas RA, et al. Preoperative assessment of the carotid bifurcation: can magnetic resonance angiography and duplex ultrasonography replace contrast arteriography? Stroke 1995;26:1753–1758.

312 R.G. Ciocca
Figure 17.2. Magnetic resonance angiography (MRA) of the carotid bifurcation. MRA can provide a precise anatomic depiction of carotid bifurcation disease. (Reprinted from Patel ST, Kent KC. Cerebrovascular disease. In: Norton JA, Bollinger RR, Chang AE, et al, eds. Surgery: Basic Science and Clinical Evidence. New York: Springer-Verlag, 2001, with permission.)
angiography is performed by gaining access to the arterial system, usually through the femoral artery, and placing a catheter into the artery that needs to be studied. Radiopaque contrast material then is injected via the catheter, and x-rays are taken. It also can provide excellent images of the aortic arch and proximal great vessels, areas that are not imaged well by DU or MRA. The posterior and intracranial circulation can be visualized readily.
However, contrast angiography is invasive and is associated with a significant complication rate. In the Asymptomatic Carotid Atherosclerosis Study (ACAS), there was a 1.2% rate of stroke associated with angiography.5 Contrast angiography is reserved for the rare instances in which the noninvasive studies are inconclusive and the physician is unable to make a clinical decision based on their findings.
5 Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273:304–308.

17. Stroke 313
Positron Emission Tomography
Positron emission tomography (PET) is a technique that utilizes radioactive tracers to visualize the extent, intensity, and rate of biologic processes occurring within the brain. Positron-emitting isotopes are produced for carbon, nitrogen, oxygen, and fluorine; these can be utilized to label a wide variety of metabolic substrates and drug analogues. When a positron decays, two photons are emitted 180 degrees apart: these photons are detected electronically by detectors that record only the simultaneously occurring photons 180 degrees apart. Input to a ring of detector is reconstructed to a tomographic image similar to those of CT.
Tracer techniques are available for measuring cerebral blood flow, cerebral blood volume, cerebral metabolic rate for oxygen, and cerebral metabolic rate for glucose; in addition, a useful derived function is the fraction of oxygen extracted by tissue (oxygen extraction fraction).
While much useful data can be acquired via a PET scan, it rarely is used for the acute evaluation of a stroke patient and rarely is necessary in preparation for carotid surgery.
Treatment
The initial therapy for a patient who presents with a change in neurologic status is supportive. It is critical to take an accurate history, with particular attention to the onset of symptoms. There is increasing evidence that early intervention in a patient with stroke can affect the outcome positively. A thorough physical examination needs to be performed, and clear and concise documentation of any neurologic deficit needs to be made. Comorbid conditions, such as hypertension, breathing problems, and chest pain, need to be treated aggressively.
Once it has been determined that the patient has an acute neurologic deficit, a CT scan is a very useful first study. While the study frequently is interpreted as “normal” or “unchanged” initially in the evaluation of a patient presenting with a stroke, it also is helpful in ruling out other possible causes of a change in neurologic function, particularly an intracranial bleed or mass lesion. Ruling out a bleed particularly is important if the treating physician is contemplating the use of thrombolytic therapy for the treatment of acute stroke.
There is increasing interest, growing experience, and accruing evidence to suggest that there is a role for thrombolytic therapy in the acute management of stroke. The goal is to dissolve a clot that has formed in the cerebral circulation. Successful protocols have been developed for the use of both intraarterial and intravenous thrombolytic therapy. Multicentered trials have demonstrated a significant benefit to stroke patients if the therapy can be employed within 3 to 6 hours after the onset of symptoms.6 This benefit is at the cost of an
6 Lisboa RC, Jovanovic BD, Alberts MJ. Analysis of the safety and efficacy of intraarterial thrombolytic therapy for ischemic stroke. Stroke 2002;33(12):2866–2871.

314 R.G. Ciocca
increased rate of significant intracranial hemorrhage without a significant effect on overall mortality. In general, the benefit of thrombolysis decreases and the risks increase with time after the onset of symptoms. It is thought that, with increased awareness of the signs and symptoms of stroke and with more rapid response, employment of thrombolysis will prove to be safe and cost-effective.
The evidence does not support the use of systemic anticoagulation for either therapeutic or prophylactic treatment of stroke, the critical exception being for those patients who have cardiogenic sources of cerebral embolization (e.g., atrial fibrillation, atrial flutter, valvular disease, prosthetic valves, etc.).7 Patients with cardiogenic sources of embolization do benefit from anticoagulation, maintaining an international normalized ratio (INR) of 2.0 to 3.0. Higher ratios are associated with increased risks of bleeding.
There is level-one evidence to support the use of antiplatelet therapy in the management and prevention of patients with stroke.8 Aspirin [acetylsalicylic acid (ASA)], secondary to its low cost, availability, and good safety profile, generally is considered first-line antiplatelet therapy. There is some debate as to the optimal dose, with the range being between 81 and 325 mg daily. There is good evidence that clopidogrel (Plavix) is slightly better than ASA in preventing ischemic events, without the hematologic toxicity associated with ticlopidine (Ticlid). While clopidogrel is significantly more expensive than ASA, it may prove to be cost-effective if it can successfully prevent stroke and other ischemic events that carry significant morbidity.
One of the more controversial issues in the management of stroke has been the role of carotid surgery. The controversy has been abated with good evidence. The North American Symptomatic Carotid Endarterectomy Trial (NASCET) was a large prospective randomized trial designed to test the efficacy of carotid endarterectomy (CEA) in patients with symptomatic carotid stenosis.9 Fifty centers in the United States and Canada randomized 659 patients with greater than 70% symptomatic carotid stenosis to CEA or best medical management. The study was designed as a 5-year trial, but it was concluded at 18 months due to the markedly significant benefit of CEA. The study found the 30-day mortality and stroke morbidity was 5.8% in patients randomized to CEA. The cumulative 2-year risk of ipsilateral stroke was 26% in patients treated medically and 9% in patients treated with CEA, representing an absolute risk reduction of 17% and a relative risk reduction of 65%. The benefit of surgery has proven to be durable for at least 8 years. The benefit of surgery has shown to be greater in
7 VanWalraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs. aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA 2002;288(19): 2441–2448.
8 Straus SE, Majumber SR, McAlister FA. New evidence for stroke prevention: scientific review. JAMA 2002;288(11):1388–1395.
9 North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–453.

17. Stroke 315
patients with higher-grade stenosis, but subset analysis has confirmed statistically significant absolute risk reduction of CEA for symptomatic patients with greater than 50% carotid artery stenosis.
See Algorithm 17.1 for the management of extracranial carotid stenosis.
Prevention
Risk Reduction
Risk reduction is the cornerstone of prevention; it means the cessation of smoking, and the control of diabetes, hypertension, and cholesterol. In addition, as previously stated, there is good evidence to support antiplatelet therapy with either ASA or clopidogrel in the prevention of cerebral ischemic events. Anticoagulation with heparin and Coumadin has been shown to reduce the incidence of stroke in patients with cardiogenic sources of embolization.
Carotid Surgery
Just as the efficacy of CEA for the treatment of high-grade symptomatic carotid stenosis was challenged, its role in the management of asymptomatic patients with high-grade carotid stenosis required clinical trials to support its benefit. The Asymptomatic Carotid Atherosclerosis Study (ACAS) is the largest available randomized trial of patients with asymptomatic carotid stenosis.10 The study randomized 1662 asymptomatic patients with 60% to 90% carotid stenoses to receive CEA or medical management. The 5-year risk of stroke was 5.1% in patients treated surgically and 11% in patients treated medically, yielding a statistically significant 5.9% absolute risk reduction. This beneficial effect of surgery in asymptomatic carotid disease was in large part the result of a low 30-day operative risk (2.3%) for CEA. Interestingly, only half of the strokes were related to the surgical procedure; the remainder were due to contrast angiography. This finding has led to a significant decrease in the use of routine preoperative contrast angiography for patients with carotid stenosis.
The benefit of CEA was significantly less for women. This may be accounted for partly by the fact that the perioperative stroke rate in women was higher (3.6% versus 1.7%) than for men.
Although a statistical benefit for CEA in asymptomatic patients with 60% to 99% carotid stenoses was demonstrated by ACAS, skeptics argue that 17 operations were required to prevent one stroke over 5 years. This raised questions about the cost-effectiveness as well as the sensibility of treating asymptomatic patients with CEA. Subsequent studies, however, have demonstrated the cost-effectiveness of CEA.11 There are several caveats, however. The patient’s longevity
10 Executive Committee for the Asymptomatic Carotid Artherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273:304–308.
11 Back MR, Harward TRS, Huber TS, et al. Improving the cost-effectiveness of carotid endarterectomy. J Vasc Surg 1997;26:456–464.

|
|
Extracranial carotid stenosis |
|
|
|
|
Asymptomatic |
|
|
Symptomatic |
|
||
Stenosis |
Stenosis |
Mild stenosis |
Moderate stenosis |
Severe stenosis |
||
<60% |
≥60% |
|
(<50%) |
(50–69%) |
(≥70%) |
|
Age > 79 years |
|
Age £79 years |
Less severe stenosis |
More severe stenosis |
||
Unstable cardiac disease |
Stable cardiac disease |
Age <75 years |
|
Age ≥ 75 years |
||
Experienced surgeon |
|
Experienced surgeon |
Female sex |
|
Male sex |
|
unavailable |
|
available |
Stroke >3 mo earlier |
Stroke 3 mo earlier or less |
||
|
|
|
Visual symptoms alone |
Hemispheric symptoms |
||
|
|
|
No intracranial stenosis |
Intracranial stenosis |
||
|
|
|
Microvascular ischemia |
No microvascular ischemia |
||
Surgical risk |
|
Surgical risk |
Lower risk of |
|
Higher risk of |
|
>3% |
|
£3% |
carotid stroke |
|
carotid stroke |
|
|
|
Endarterectomy |
|
|
|
Endarterectomy |
Medical therapy
(risk-factor control, antiplatelet drugs, statins, and angiotensin-converting–enzyme inhibitors)
Algorithm 17.1. Algorithm for the management of extracranial carotid stenosis. The algorithm is partially based on the guidelines of the American Heart Association and the National Stroke Association.1 Other factors not included in the figure may also be relevant in risk stratification (e.g., the results of cardiac evaluation or hemodynamic testing). (Reprinted from Sacco RL. Extracranial carotid stenosis. N Engl J Med 2001:345(15), with permission. Copyright © 2001 Massachusetts Medical Society. All rights reserved.)
Ciocca .G.R 316

17. Stroke 317
must be taken into consideration. Generally, for patients to derive a benefit from CEA, they should be expected to live 5 years; degree of stenosis also may be important, with patients with high-grade stenosis (greater than 80%) deriving the greatest benefit.
There have been some concerns about performing CEA on patients in their 80s, the concern being that they may not live long enough to derive benefit from the surgery. There is increasing evidence to support a selectively aggressive approach in these patients as well.
While this chapter does not cover the surgical technique of carotid endarterectomy in detail, there are, however, several issues regarding this operation that do warrant brief consideration here.
The operation may be performed either under general anesthesia or via a regional block. While there are distinct advantages to both techniques, e.g., accurate cerebral monitoring under regional anesthesia, no consistent benefit has been found with either approach (Table 17.1).
The operation can be performed via standard operative technique or via an eversion endarterectomy. In the standard operation, the artery is opened longitudinally through the plaque, and the plaque is removed through the arteriotomy (Fig. 17.3). The eversion technique involves obliquely dividing the carotid bifurcation and everting the atherosclerotic plaque (Fig. 17.4). Both techniques have their champions. Both techniques may render excellent outcomes (Table 17.2).
Another hotly debated area of carotid surgery is the technique of arterial closure employed at the completion of the operation. If the carotid artery is of reasonable diameter, some surgeons advocate primary closure. Others, out of concern for restenosis, favor patch closure of the arteriotomy. The type of patch also has been debated, vein versus prosthetic material. Again, good results have been documented using any of a number of closure techniques. The evidence tends to support the bias toward patch closure.
Another issue with which the vascular surgeon must deal is cerebral protection. Surprisingly, the vast majority of patients tolerate having their carotid artery clamped for the period of the surgery. There is a small subset of patients who do not tolerate any significant period of cerebral ischemia. Those patients needed to be shunted during the
1 (Footnote to Algorithm 17.1) Goldstein LB, Adams R, Becker K, et al. Primary prevention of ischemic stroke: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke 2001;32:280–299; Wolf PA, Clagett GP, Easton JD, et al. Preventing ischemic stroke in patients with prior stroke and transient ischemic attack: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke 1999;30:1991–1994; Albers GW, Hart RG, Lutsep HL, Newell DW, Sacco RL. Supplement to the guidelines for the management of transient ischemic attacks: a statement from the Ad Hoc Committee on Guidelines for the Management of Transient Ischemic Attacks, Stroke Council, American Heart Association. Stroke 1999;30:2502–2511; Gorelick PB, Sacco RL, Smith DB, et al. Prevention of a first stroke: a review of guidelines and multidisciplinary consensus statement from the National Stroke Association. JAMA 1999;281:1112–1120.

Ciocca .G.R 318
Table 17.1. Influence of anesthetic technique on perioperative complications in patients undergoing CEA.
|
|
|
General anesthesia |
|
|
|
|
Regional anesthesia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stroke/death |
|
|
|
|
Stroke/death |
|
|
|
|
|
|
|
|
|
||
Author |
Year |
CEAs |
(%) |
MI |
CEAs |
(%) |
MI |
||
|
|
|
|
|
|
|
|
|
|
Fiorani et alb |
1997 |
337 |
5.0* |
0.6 |
|
683 |
2.0* |
0.3 |
|
Ombrellaro et alc |
1996 |
126 |
4.8 |
0.8 |
|
140 |
2.1 |
2.1 |
|
Rockman et ald |
1996 |
349 |
4.1* |
1.2 |
|
1414 |
2.1* |
0.6 |
|
Shah et ale |
1994 |
419 |
1.9 |
|
|
654 |
2.0 |
|
|
Allen et alf |
1994 |
361 |
3.9 |
2.5 |
|
318 |
2.5 |
0.6 |
|
Becquemin et alg |
1991 |
242 |
5.4 |
4.1* |
145 |
4.1 |
0* |
||
Bergeron et alh |
1991 |
250a |
4.4* |
|
|
114 |
1.8* |
0 |
|
Forssell et ali |
1989 |
55a |
3.6 |
1.8 |
|
|
56a |
5.4 |
3.6 |
Godin et alj |
1989 |
50 |
2.0 |
|
|
|
50a |
0 |
|
Palmer et alk |
1989 |
37a |
2.0 |
5.4 |
|
|
184a |
1.5 |
1.6 |
Corson et all |
1987 |
242 |
2.9* |
0.8 |
|
157 |
1.3* |
1.3 |
|
Muskett et alm |
1986 |
45a |
0 |
6.7 |
|
|
30a |
3.3 |
0 |
Gabelman et aln |
1983 |
46a |
6.5 |
2.2 |
|
|
54a |
3.7 |
0 |
Pietzman et alo |
1982 |
53 |
5.7 |
|
|
226 |
3.1 |
|
|
Anderson et alp |
1980 |
189 |
3.1 |
|
|
232 |
4.8 |
|
|
CEA, carotid endarterectomy; MI, myocardial infarction.
a |
Number of patients undergoing carotid endarterectomy (number of procedures performed not indicated). |
b |
Fiorani P, Sbarigia E, Speziale F, et al. General anaesthesia versus cervical block and perioperative complications in carotid artery surgery. Eur J Vasc Endovasc Surg |
1997;13:37–42. |
|
c |
Ombrellaro MP, Freeman MB, Stevens SL, et al. Effect of anesthetic technique on cardiac morbidity following carotid artery surgery. Am J Surg 1996;171:387–390. |
d |
Rockman CB, Riles TS, Gold M, et al. A comparison of regional and general anesthesia in patients undergoing carotid endarterectomy. J Vasc Surg 1996;24:946–956. |
e |
Shah DM, Darling RC, Chang BB, et al. Carotid endarterectomy in awake patients: its safety, acceptability, and outcome. J Vasc Surg 1994;19:1015–1020. |
f |
Allen BT, Anderson CB, Rubin BG, et al. The influence of anesthetic technique on perioperative complications after carotid endarterectomy. J Vasc Surg 1994;19:834–843. |
g |
Becquemin JP, Paris E, Valverde A, et al. Carotid surgery: is regional anesthesia always appropriate? J Cardiovasc Surg 1991;32:592–598. |
h |
Bergeron P, Benichou H, Rudondy P, et al. Stroke prevention during carotid surgery in high risk patients (value of transcranial Doppler and local anesthesia). J Car- |
diovasc Surg 1991;32:713–719. |
|
i |
Forssell C, Takolander R, Bergqvist D, et al. Local versus general anaesthesia in carotid surgery: a prospective, randomized study. Eur J Vasc Surg 1989;3:503–509. |
j |
Godin MS, Bell WH, Schwedler M, et al. Cost-effectiveness of regional anesthesia in carotid endarterectomy. Am Surg 1989;55:656–659. |
k |
Palmer MA. Comparison of regional and general anesthesia for carotid endarterectomy. Am J Surg 1989;157:329–330. |
l |
Corson JD, Chang BB, Shah DM, et al. The influence of anesthetic choice on carotid endarterectomy outcome. Arch Surg 1987;122:807–812. |
m Muskett A, McGreevy J, Miller M. Detailed comparison of regional and general anesthesia for carotid endarterectomy. Am J Surg 1986;691–694. |
|
n |
Gabelman CG, Gann DS, Ashworth CJ, et al. One hundred carotid reconstructions: local versus general anesthesia. Am J Surg 1983;145:477–482. |
o |
Peitzman AB, Webster MW, Loubeau J, et al. Carotid endarterectomy under regional (conductive) anesthesia. Ann Surg 1982;196:59–64. |
p |
Andersen CA, Rich NM, Collins GJ, et al. Carotid endarterectomy: regional versus general anesthesia. Am Surg 1980;48:323–327. |
* |
p value <.05 (general versus regional anesthesia). |
Source: Reprinted from Patel ST, Kent KC. Cerebrovascular disease. In: Norton JA, Bollinger RR, Chang AE, et al, eds. Surgery: Basic Science and Clinical Evidence. New York: Springer-Verlag, 2001, with permission.
Stroke .17
319

320 R.G. Ciocca
Figure 17.3. Technique of standard carotid endarterectomy. After adequate exposure is achieved, the internal, external, and common carotid arteries are clamped (A). A plane of dissection is created between the arterial wall and the atheromatous process (B). After the plaque is transected proximally, it can be reflected upward to aid in the distal portion of the endarterectomy (C). After completion of the endarterectomy, any remaining loose pieces of atheroma or strands of media are removed (D). The arteriotomy is closed primarily (E) or with a patch graft (F). (Reprinted from Patel ST, Kent KC. Cerebrovascular disease. In: Norton JA, Bollinger RR, Chang AE, et al, eds. Surgery: Basic Science and Clinical Evidence. New York: Springer-Verlag, 2001, with permission.)
Figure 17.4. Technique of eversion carotid endarterectomy. Internal carotid artery is transected obliquely at the carotid bifurcation (A). Medial and adventitial layers of the internal carotid artery are everted over the atheromatous core
(B). Completion of endarterectomy with the distal end point directly visualized (C). Internal carotid artery is reanastomosed to the common carotid artery
(D). (Reprinted from Patel ST, Kent KC. Cerebrovascular disease. In: Norton JA, Bollinger RR, Chang AE, et al, eds. Surgery: Basic Science and Clinical Evidence. New York: Springer-Verlag, 2001, with permission.)
17. Stroke 321
course of the procedure. There are many different types of shunts, but they all are some form of plastic tubing that is placed within the lumen of the carotid artery and maintains cerebral blood flow during the operation. The surgeon has several options regarding who needs to be shunted. The surgeon can shunt everyone. The surgeon can perform the operation under a regional anesthetic and selectively shunt only those patients who have a neurologic change upon carotid clamping. The surgical team also can monitor the patient’s electroencephalogram (EEG) and selectively shunt based on changes in the EEG. Another option is to measure the backpressure in the internal carotid artery. The pressure is a measurement of cerebral collateral blood flow. Shunting generally is recommended if the carotid stump pressure falls below 45 to 50 mm Hg.
Patients who have a carotid endarterectomy generally do exceedingly well postoperatively. They must be watched closely for bleeding, acute neurologic change, hypertension, hypotension, cardiac problems, and signs of cranial nerve injury. Many patients may be discharged home on the first postoperative day. They generally are followed periodically with carotid duplex scans to check for restenosis of the operative side and to evaluate the contralateral side.
Carotid Angioplasty and Stenting
As in most aspects of vascular surgery, endovascular techniques are being employed in the hope of decreasing patient discomfort, hospital length of stay, requirements for general anesthesia, and scarring. There are encouraging data for and a growing experience with balloon angioplasty for carotid stenosis. The overall experience, however, has resulted in a higher procedural stroke rate compared to open surgery, with no significant decrease in length of stay and with increased costs. With increased experience and with the advent of cerebral protection devices, the outcomes may improve. There currently are several ongoing multicentered clinical trials designed to compare the results of carotid angioplasty to open surgery. The results of these trials will clarify the role of carotid angioplasty in the future.
Case Conclusion
The patient in the case at the beginning of this chapter presents with signs and symptoms that certainly could be attributed to atherosclerotic disease at the carotid bifurcation. After a thorough history and physical and particularly if he has a carotid bruit, he would benefit from a carotid duplex exam. If that reveals significant stenosis greater than 70%, then surgical correction of the lesion should be considered. If his carotid duplex reveals minimal disease, then other sources of arte-

Ciocca .G.R 322
Table 17.2. Influence of the technique of carotid endarterectomy on rates of perioperative stroke/death and restenosis.
|
|
|
|
|
Standard endarterectomy |
|
|
|
Eversion endarterectomy |
|
|
||
|
|
|
|
|
Stroke/death |
Restenosis |
F/U |
|
|
Stroke/death |
Restenosis |
F/U |
|
Author |
Year |
CEAs |
(%) |
(%) |
(months) |
CEAs |
(%) |
(%) |
(months) |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ballotta et alc |
1999 |
167a |
4.2* |
4.9* |
34 |
|
169 |
0* |
0* |
34 |
|
||
|
Shah et ald |
1998 |
474 |
4.2 |
1 |
18 |
|
2249 |
1.9 |
0.3 |
18 |
|
|
Cao et ale |
1998 |
675 |
1.3 |
4.1 |
14.9 |
|
678 |
1.3 |
2.4 |
14.9 |
|
||
Entz et alf |
1997 |
715a |
4.0* |
|
|
|
739 |
1.4* |
|
|
|
||
|
Vanmaele et alg |
1994 |
98a |
6.1 |
2 |
12 |
|
102 |
2.9 |
1 |
12 |
|
|
|
Kieny et alh |
1993 |
156b |
|
13.5 |
44 |
|
212 |
2.4 |
1.9 |
27.1 |
|
|
|
CEA, carotid endarterectomy; F/U, mean follow-up time. |
|
|
|
|
|
|
|
|
||||
a |
All CEAs performed with patch closure. |
|
|
|
|
|
|
|
|
|
|||
b |
All CEAs performed with primary closure. |
|
|
|
|
|
|
|
|
|
|||
c |
Ballotta E, Da Giau G, Saladini M, et al. Carotid endarterectomy with patch closure versus carotid eversion endarterectomy and reimplantation: a prospective ran- |
||||||||||||
domized study. Surgery (St. Louis) 1999;125:271–279. |
|
|
|
|
|
|
|
|
|||||
d |
Shah DM, Darling RC, Chang BB, et al. Carotid endarterectomy by eversion technique: its safety and durability. Ann Surg 1998;228:471–478. |
|
|
||||||||||
e |
Cao P, Giordano G, De Rango P, et al. A randomized study on eversion versus standard carotid endarterectomy: study design and preliminary results: the Everest |
||||||||||||
Trial. J Vasc Surg 1998;27:595–605. |
|
|
|
|
|
|
|
|
|
|
|||
f |
Entz L, Jaranyi Z, Nemes A. Comparison of perioperative results obtained with carotid eversion endarterectomy and with conventional patch plasty. Cardiovasc Surg |
||||||||||||
1997;5:16–20. |
|
|
|
|
|
|
|
|
|
|
|
||
g |
Vanmaele RG, Van Schil PE, DeMaeseneer MG, et al. Division-endarterectomy-anastomosis of the internal carotid artery: a prospective randomized comparative study. |
||||||||||||
|
Cardiovasc Surg 1994;2:573–581. |
|
|
|
|
|
|
|
|
|
|
||
h |
Kieny R, Hirsch D, Seiller C, et al. Does carotid eversion endarterectomy and reimplantation reduce the risk of restenosis? Ann Vasc Surg 1993;7:407–413. |
|
|
||||||||||
* p value <.05 (standard versus eversion endarterectomy).
Source: Reprinted from Patel ST, Kent KC. Cerebrovascular disease. In: Norton JA, Bollinger RR, Chang AE, et al, eds. Surgery: Basic Science and Clinical Evidence. New York: Springer-Verlag, 2001, with permission.
17. Stroke 323
rial-to-arterial emboli must be considered. Risk management and an antiplatelet agent should be prescribed.
Summary
Stroke as a consequence of cerebral vascular disease is very prevalent, and, as the population continues to age, it most likely will become increasingly common. The individual and societal costs of strokes are very high. As a physician or healthcare provider, one must be aware of the pathophysiology as well as of the risk factors that may increase a patient’s risk of stroke. The signs and symptoms of cerebral vascular disease have been discussed in detail in this chapter. An overview of appropriate diagnostic studies has been provided as well as a discussion of appropriate prophylactic and therapeutic interventions.
Selected Readings
AbuRahma AF, Robinson PA, Saiedy S, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: long-term followup. J Vasc Surg 1998;27:222–234.
Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery (St. Louis) 1999;125: 85–91.
Barnett HJ, Taylor DW, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339: 1415–1425.
Dawson DL, Zieler RE, Strandness DE, et al. The role of duplex scanning and arteriography before carotid endarterectomy: a prospective study. J Vasc Surg 1993;18:673–683.
Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995; 273:1421–1428.
Jordan WD, Voellinger DC, Fisher WS, et al. A comparison of carotid angioplasty with stenting versus endarterectomy with regional anesthesia. J Vasc Surg 1998;28:326–334.
North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–453.
Patel ST, Kent KC. Cerebrovascular disease. In Norton JA, Bollinger RR, Chang AE, et al, eds. Surgery: Basic Science and Clinical Evidence. New York: Springer-Verlag, 2001.
Patel ST, Kuntz KM, Kent KC. Is routine duplex ultrasound surveillance after carotid endarterectomy cost-effective? Surgery (St. Louis) 1998;124:343–352.
Rockman CB, Riles TS, Gold M, et al. A comparison of regional and general anesthesia in patients undergoing carotid endarterectomy. J Vasc Surg 1996;24:946–956.
Salasidis GC, Latter DA, Steinmetz OK, et al. Carotid artery duplex scanning in preoperative assessment for coronary artery revascularization: the associ-
324 R.G. Ciocca
ation between peripheral vascular disease, carotid artery stenosis, and stroke. J Vasc Surg 1995;21:154–162.
Shah DM, Darling RC, Chang BB, et al. Carotid endarterectomy by eversion technique: its safety and durability. Ann Surg 1998;228:471–478.
Yadav JS, Roubin GS, Iyer S, et al. Elective stenting of extracranial carotid arteries. Circulation 1997;20:S83.
