
Revision Sinus Surgery
.pdf
60 |
Adam J. Folbe and Roy R. Casiano |
Fig. 7.9 Sagittal view showing the relationship between the sinuses. From anterior to posterior, the nasolacrimal duct (white oval) is parallel to the direction toward the frontal sinus from the natural ostium of the maxillary sinus (white arrow), just behind the anterior attachment of the middle turbinate. More posteriorly are seen the ethmoid bullae (B), posterior ethmoids (PE), and the sphenoid (S)
7
moid cells, anterior superior attachments of the middle turbinate, and the uncinate process. However, in revision surgery, these structures are usually altered or removed. As a result, other landmarks have been sought after to safely enter the frontal sinus.
The key to revision frontal surgery is to have some landmark directing the surgeon in the general direction and appropriate superior trajectory of the frontal infundibulum, and not toward the anterior skull base, which is located slightly posterior to this area. These are:
1.Natural ostium or most anterior portion of maxillary antrostomy.
2.Remnant of the anterior attachment and vertical lamella of the middle turbinate.
3.Convexity of the nasolacrimal duct [5].
Starting at the anterior maxillary antrostomy (or preferably the natural ostium of the maxillary sinus), and following a line parallel to the nasolacrimal duct, the frontal recess can be confidently accessed using a small probe, a few millimeters behind the anterior attachment of the middle turbinate (Figs. 7.4c and 7.9). In well-pneuma- tized frontal sinuses, a supraturbinal (laterally) or transeptal (medial) penetration, anterior to the coronal plane of the frontal infundibulum and anterior attachment of the middle turbinate, may be possible. Posterior landmarks also exist. By following the roof of the ethmoids anteriorly, the surgeon can identify the anterior ethmoid artery as it runs along the posterior aspect of the frontal recess and infundibulum. Once the anterior ethmoid artery is seen, the surgeon identifies the area of the frontal infundibulum by also following the medial orbital wall superiorly, as it defines the lateral extent of this opening into the frontal sinus. Medially, the border of the frontal infundibulum is the vertical lamella of the middle turbinate. Anteriorly lie the agger nasi cell and variable frontal sinus
cells, as described later. As mentioned earlier, the amount of pneumatization of the agger nasi can affect the outflow of the frontal sinus [12]. Posteriorly, supraorbital cells can obscure the recess. In previous surgery, the suprabullar cells may be damaged and the frontal recess can become scarred with bone and debris. The recognition and removal of these cells is critical for confident entry into the frontal sinus and resolution of the patient’s symptoms.
In the frontal sinus itself, there can be variable structures. The intersinus septum can be midline or laterally
Fig. 7.10 Type I is a single air cell above the agger nasi. Type II is a group of small air cells above the agger nasi, but below the orbital roof. Type III is a single air cell extending from the agger nasi into the frontal sinus. Finally, type IV is an isolated air cell within the frontal sinus not contiguous with the agger nasi. A Agger nasi cell, IS inner sinus septum
Surgical Anatomy in Revision Sinus Surgery
displaced in either direction. It can also be pneumatized and become an isolated air cell. Bent and Kuhn classified frontal cells into four types [1]. During revision surgery, some or all of these intersinus cells can be present. Type I is a single air cell above the agger nasi. Type II is a group of small air cells above the agger nasi, but below the orbital roof. Type III is a single air cell extending from the agger nasi into the frontal sinus. Finally, type IV is an isolated air cell within the frontal sinus not contiguous with the agger nasi (Fig. 7.10).
Conclusion
With a sound understanding of the intricate sinus anatomy, a surgeon can confidently perform revision sinus surgery. The navigational systems are not a substitute for anatomical knowledge. They can provide a false sense of security and may lead the surgery beyond the limits at which the surgeon feels comfortable. However, the landmarks discussed in this chapter are reliable, and recognizable by any surgeon, and should supersede any navigational system.
References
1.Bent J, Kuhn FA, Cuilty C (1994) The frontal cell in frontal recess obstruction. Am J Rhinol 8:185–191
2.Bodino C, Jankowski R, Gringnon B, et al. (2004) Surgical anatomy of the turbinal wall of the ethmoidal labyrinth. Rhinology 42:73–80
61
3.Bolger WE (2001) Anatomy of the paranasal sinuses. In: Kennedy DW, Bolger WE, Zinreich J (eds) Diseases of the Sinuses, Diagnosis and Management. Decker, Hamilton, Ontario, pp 1–12
4.Bolger WE, Keyes A (2001) Use of superior meatus/turbinate in the endoscopic approach to the sphenoid sinus. Otolaryngol Head Neck Surg 120:308–313
5.Casiano RR (2001) A stepwise surgical technique using the medial orbital floor as the key landmark in performing endoscopic sinus surgery. Laryngoscope 111: 964–974
6.Castelnuovo P, Dallan I, Pistochini A, et al. (2007) Endonasal endoscopic repair of Sternberg’s canal cerebrospinal fluid leaks. Laryngoscope 117:345–349
7.Graney DO, Rice DH (1998) Paranasal sinus anatomy. In: Cummings CW, Fredrickson JM, Harker LA, et al. (eds) Otolaryngology – Head and Neck Surgery, 3rd edn. Mosby, St. Louis, pp 1059–1064
8.Herzallah IR, Casiano RR (2007) Endoscopic endonasal study of the internal carotid artery course and variations. Am J Rhinol 21:262–270
9.May M, Schaitkin B, Kay SL(1994) Revision endoscopic sinus surgery: six friendly surgical landmarks. Laryngoscope 104:766–767
10.Polavaram R, Devaiah AK, Sakai O, et al. (2004) Anatomic variants and pearls – functional endoscopic sinus surgery. Otolaryngol Clin North Am 37: 221–242
11.Stammberger HR, Kennedy DW (1995) Paranasal sinuses: anatomic terminology and nomenclature. The Anatomic Terminology Group. Ann Otol Rhinol Laryngol Suppl 167:7–16
12.Wormald PJ (2003) The agger nasi cell: the key to understanding the anatomy of the frontal recess. Otolaryngol Head Neck Surg 129:497–507

Chapter 8 |
8 |
Surgical Instruments in Revision |
|
Endoscopic Sinus Surgery |
Vijay R. Ramakrishnan and Todd T. Kingdom
Core Messages
■Continued development and refinement of available surgical instrumentation for endoscopic sinus surgery (ESS) remains important.
■The patient undergoing revision ESS may present a unique set of anatomic and surgical challenges requiring unique instrument solutions.
■New developments and refinements have improved visualization (videoscopic equipment), our ability to precisely handle soft tissue (microdebriders, through-cutting instruments), our ability to remove bone efficiently (high-speed burs), and our access (angled and malleable instrumentation) during ESS.
■The revision ESS surgeon must be experienced with these current and evolving instruments in order to optimize outcome and safety.
Introduction and Background
On a basic level, surgical instrumentation and surgical concepts are similar for both primary endoscopic sinus surgery (ESS) and revision ESS. However, unique situations and challenges will present during revision ESS, requiring the surgeon to be familiar with a wide range of surgical techniques and instrumentation. The surgeon must be knowledgeable, comfortable, and facile with the available surgical equipment options. The goal of this chapter is to review current surgical instruments of value for use in revision ESS.
Historically, surgical concepts behind the management of sinus disease have centered around “radical” therapy for “irreversible” mucosal disease. Messerklinger’s work, which began in the 1950s, introduced new concepts of reversible mucosal disease, mucosal sparing approaches,
Contents |
|
|
|
|
Introduction and Background . . . . . |
. . |
. . |
. |
63 |
Videoscopy and Visualization . . . . . |
. . |
. . |
. |
64 |
Mucosal-Sparing Instrumentation . . . . . . . |
. . . |
. . . . |
. . . 64 |
|
Powered Instrumentation . . . . . . . |
. . |
. . |
. |
66 |
Modifications of Traditional Instruments |
. . |
. . |
. |
67 |
Image-Guided Instrumentation . . . . . |
. |
. . |
. |
. 68 |
Summary . . . . . . . . . . . . . . |
. . |
. . |
. |
68 |
and the development of functional surgical techniques. The stated goals of the Messerklinger technique were to remove the obstructing anatomic variations causing disease and to resect only the most severely diseased mucosa in key locations [7]. To achieve these goals, the surgeon must be able to accurately visualize and diagnose endonasal disease, limit the scope of dissection as indicated, and precisely manipulate variable anatomy. This required a basic change in instrument design concepts that have only recently been widely available. Refinements in the videoscopic chain have enhanced visualization of the surgical field, the development of fine through-cutting instruments has provided the opportunity for mucosal sparing procedures, and the introduction of powered instrumentation has expanded the rhinologist’s armamentarium greatly. These developments have been critical to the evolution of both primary and revision ESS.
Mucosal preservation has been emphasized as an important objective for both successful primary and revision sinus surgery. Much of the technology presented in this chapter focuses on this important concept – precision dissection and mucosal preservation. Clinical experience suggests that surgical efficiency, mucosal recovery, and patient outcomes are improved with mucosal preservation. Meticulous removal of soft tissue and attempted mucosal preservation ought to be goals of the sinus surgeon in both primary and revision cases.
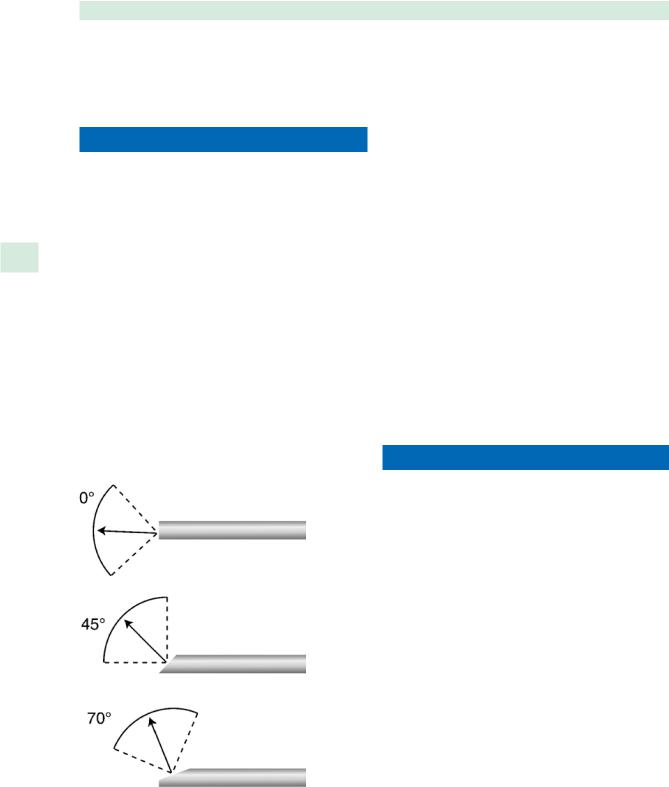
64 |
Vijay R. Ramakrishnan and Todd T. Kingdom |
■Mucosal preservation is an important objective for both primary and revision ESS.
■New instrument design focuses on the surgeon’s need for precision in a variety of clinical situations.
Videoscopy and Visualization
With the use of assorted endoscopes, the surgeon is capable of direct visualization of most sites within the surgical field. The zero-degree endoscope is preferred because of its optimal lighting and minimal optical distortion and disorientation; however, it may be inadequate in meeting the needs of many revision procedures. Angled en-
8 doscopes, including 30, 45, and 70°, have been designed to facilitate visualization around “corners” and to extend the surgeon’s “reach” (Fig. 8.1). Current technology has improved the width of view and degree of illumination for angled telescopes, but the operating surgeon must still consider the increasing distortion and disorientation associated with increasing telescopic angle [3]. Revision ESS often requires extended visualization and access, thus surgeon comfort with angled endoscopes is essential. Several attempts at creation of a three-dimensional endoscope have provided a superior stationary image, but can be disorienting when rotated laterally, and have not been widely utilized [4].
Fig. 8.1 Rigid nasal endoscopes with angles ranging from 0 to 70° (drawing by Scott Baird, adapted from Karl Storz Endoscopy America, Culver City, California)
■The operating surgeon must be aware of increasing distortion and disorientation and decreasing illumination with increasing telescopic angle of the endoscope.
Improvements in camera technology have addressed concerns of illumination, color rendition, and weight. Color rendition and depth perception on the videoscopic projection are less accurate than that of the direct endoscopic view, yet current technology has mitigated these concerns. In addition, operating from the projection screen facilitates the four-handed technique, which may be of benefit in difficult revision cases and endoscopic skull-base procedures [4]. High-definition (HD) video technology is the latest evolution in endoscopic visualization. HD-compatible video camera systems provide 1920 × 1080 pixels of resolution in a native 16:9 aspect ratio. Potential advantages include higher input resolution delivering more detail and depth of focus, larger viewing format with the 16:9 aspect ratio supported by widescreen monitors, and enhanced color brilliance. Though in its infancy, HD videoscopic technology should prove valuable in the differentiation between soft-tissue types and further augment extended rhinologic surgical approaches.
Mucosal-Sparing Instrumentation
Although mucosal preservation has been a tenet of primary and revision ESS since Messerklinger’s early work, appropriate instrumentation had not been widely available for many years. Until recently, ESS surgical instruments were too large and promoted more of a “rip and tear” technique when removing mucosal disease. Mucosal loss and bone exposure has been associated with increased scarring and mucosal sequestration, decreased ciliary function, chronic inflammation, neo-osteogenesis, and a propensity toward persistent pain [5,8]. Although intuitive, the true value of mucosal sparing techniques has not been objectively established. A prospective, randomized, controlled study in 24 patients with chronic rhinosinusitis comparing the use of microdebriders to conventional instrumentation found no statistical difference in symptoms, saccharin transport times, or endoscopic findings of synechiae and ostial reocclusion at 3-, 6-, and 13-month follow up [9]. Vauterin et al. performed a prospective, randomized, double-blinded experiment comparing the use of through-cutting forceps to noncutting forceps. At 3-week and 12-month follow up, there was no statistical difference in patient symptoms or endoscopic findings of parameters including adhesions, polyps, edema, crusting, and secretions [11]. Certainly, further studies are needed to define the effects

Surgical Instruments in Revision Endoscopic Sinus Surgery |
65 |
of mucosal preservation on microscopic and clinical outcome. As of today, however, mucosal preservation and meticulous soft-tissue removal ought to be fundamental goals of the sinus surgeon. Consequently, instrument design and technique development focuses on the concept of mucosal preservation and minimally-invasive surgical principles.
A wide array of instruments for ESS is now available from several manufacturers. Fine, through-cutting instruments with straight and up-biting jaws have been designed for both mucosal dissection and removal of thin bone. The cutting width of these tools ranges from submillimeter to 4 mm in size. These instruments also come in a variety of angles, with the operating portion angled up, down, or curved to the side (Fig. 8.2). The design of the cutting end of these instruments can include a microscissor blade and linear or circular cutting punches (Fig. 8.3). In addition, there are differences in the strength of these cutting instruments.
■Instrument shaft and handle lengths vary depending on the intended objective: 13–15 cm for standard ESS, extending to 18 cm for endoscopic skull-base approaches.
The current selection of mucosal sparing instrumentation allows the surgeon to match the instrument to the tissue type to be removed and the access needed to accomplish the task. Quite literally, there are numerous instruments to choose from to fit the surgeon’s goals and objectives.
Several chapters within this textbook will be devoted to revision maxillary, ethmoid, sphenoid, and frontal sinus surgery. To avoid redundancy only brief comments on frontal sinus instrumentation will be included in this section. The evolution of mucosal sparing frontal sinus instruments represents one of the most significant advances in revision ESS. The frontal sinus surgeon can now select from a variety of fine, angled instruments to manipulate the frontal recess and to access the frontal sinus. Circular punches and through-cutting forceps
Fig. 8.2 Through-cutting, mucosal sparing forceps: a straight, b upturned 45° (Karl Storz Endoscopy America, Culver City, California, USA)
Fig. 8.3 a Microscissors, curved. b Curved through-cutting forceps. c Circular cutting punches (Karl Storz Endoscopy America)

66
with shaft angles ranging from 45 to 110° facilitate precise mucosa-sparing dissection (Fig. 8.4). More robust punches with 70 and 90° angled shafts are available for precise yet aggressive bone removal in the frontal recess region (Fig. 8.5). In addition, a variety of angled curettes, picks, and probes are available to augment this type of dissection.
■Wide selections of mucosal sparing ESS instruments are available to the surgeon, including options in shaft length, angle, size, and cutting action.
|
|
Powered Instrumentation |
8 |
|
|
|
|
|
Powered instrumentation has aided tremendously in |
||
|
|
the challenges of revision sinus surgery, especially in the |
|
|
treatment of recurrent polyposis, synechiae, and osteitic |
|
|
bone. Microdebriders have been used intranasally for over |
|
|
10 years [10]. Their use allows for rapid sharp dissection, |
|
|
reduced bleeding, constant suction and irrigation, and |
|
|
immediate tissue removal from the field. This provides ex- |
|
|
cellent continuous visualization, allowing the surgeon to |
|
|
operate without removing the instrument from the nose. |
|
|
■ When used properly, microdebrider technology has |
|
|
reduced mucosal stripping, which has been shown to |
|
|
accelerate wound healing and decrease synechiae for- |
|
|
mation. |
|
|
In a retrospective, nonblinded analysis, Krouse reported |
|
|
more rapid mucosal healing, with reduction in crusting, |
Vijay R. Ramakrishnan and Todd T. Kingdom
synechiae formation, and ostial occlusion, in patients who underwent ESS with a microdebrider when compared to standard techniques [6]. Bernstein’s findings echoed these conclusions in a retrospective, uncontrolled analysis with a mean follow-up of 16 months [1].
The basic components of a microdebrider system are the power source, irrigation system, suction source, handpiece, and interchangeable disposable blades. The irrigation flow rate is adjustable to suit the surgeon’s preference, and the linear suction path has been tailored to reduce the amount of clogging. Blade speed may be adjusted to maximize tissue cutting without system plugging. Microdebrider blades contain a fenestrated, blunt outer sheath to minimize tissue trauma, and a mobile inner blade with the cutting surface. This design eliminates avulsion of adjacent tissue to be preserved, which may occur with traditional instrumentation. Angled microdebrider blades up to 120 ° are now available and have facilitated surgery in areas of difficult access, such as the far reaches of the frontal and maxillary sinuses (Fig. 8.6). In addition, short-ra- dius curved blades and rotating blade heads have made surgery in these areas easier. Video 8.1 demonstrates the use of the microdebrider for the removal of nasal polyps.
Microdebrider technology has been extended to include the development of high-speed burs and drills utilizing the same platform. Handheld suction-irrigation drills allow the surgeon to drill, irrigate, and utilize suction while under direct visualization of the target. Revision cases often include situations that require aggressive removal of osteitic bone or removal of bone for extended access. Angled burs allow for access to the frontal sinus with focused removal of bone from the frontal process
Fig. 8.4 Mucosal sparing and cutting frontal sinus instruments 65° circular cutting punch (Karl Storz Endoscopy America)
Fig. 8.5 70° cutting frontal sinus punches (Karl Storz Endoscopy America)

Surgical Instruments in Revision Endoscopic Sinus Surgery |
67 |
Fig. 8.6 Microdebrider blades with rotating cutting openings and shaft angles of 40, 60, 90, and 120° (Medtronic, Jacksonville, Florida, USA)
of the maxilla while keeping the amount of postoperative bone exposure to a minimum (Fig. 8.7). Chandra et al. reported on their experience using a 70 ° diamond bur for Draf IIb and Draf III procedures [2]. They emphasized the precise and efficient bone removal afforded with this device. The continuous irrigation helps to maintain bone viability by minimizing thermal damage, while the drill design resists skipping in such a tight area. Recent developments have focused on the design of high-speed burs for extended skull-base approaches in the region of the sella, sphenoid sinus, and clivus. Devices with a variety of shaft lengths and angles, and bur sizes and types are currently under development (Fig. 8.8).
The application of powered instrumentation for softtissue and bone removal in revision ESS has proven to be an invaluable advancement. However, one must remember that extensive removal of mucosa, skull-base penetra-
tion, and orbital injury are possible and potentially disastrous with the use of powered instrumentation. Video 8.2 demonstrates the use of a high-speed diamond bur for bone removal during an optic nerve decompression.
■A variety of blades for soft-tissue removal and burs for bone removal are available based on the same power platform.
■The surgeon must be aware of the potential hazards associated with the use of powered instrumentation in sinus surgery.
Modifications of Traditional Instruments
Traditional instruments have also been modified to increase their utility and efficacy. Visualization is fundamental to all intranasal procedures, and thus it seems intuitive to modify traditional instruments with the addition of suction.
■Suction instrumentation facilitates surgical precision and efficiency.
Suction elevators are particularly useful for endoscopically limited or revision septoplasty. A selection of suction curettes, elevators, punches, probes, hooks, and picks exist, and are extremely useful for difficult frontal
Fig. 8.7 70° degree high-speed diamond bur (Medtronic)
Fig. 8.8 High-speed skull-base burs with shaft angles ranging from 15 to 90° degrees. Diamond and cutting burs are available with fluted or round designs (Medtronic)

68
recess dissections, tumor removal, and extended revision procedures. Malleable suction probes, elevators, and curettes have been created to aid surgical access; these instruments have a working length of 11 cm, with the last 4 cm being of a malleable nature. Malleable instruments each have a predetermined strength appropriate for their individual uses, but have a finite lifespan due to intrinsic metallic properties.
The ability to control hemorrhage becomes more relevant in revision ESS cases and extended rhinologic procedures. Endoscopic cautery devices have been developed to assist with this challenge. Bipolar cautery, combined with small size, suction, and angled tips are ideal design goals. Several options are available that satisfy many of
8 these objectives and have proven useful during endoscopic procedures (Fig. 8.9). In addition, endoscopic vascular clip appliers have been developed by several manufacturers to aid in the control of intranasal hemorrhage (Fig. 8.10). Although still of limited value due to design limitations, these instruments can be quite useful in select situations. Video 8.3 demonstrates the use of bipolar cautery, the vascular clip applier, and microscissors during endoscopic tumor removal.
Vijay R. Ramakrishnan and Todd T. Kingdom
Image-Guided Instrumentation
The use of image guidance technology is discussed in great detail in Chap. 30. The currently available imageguided surgery (IGS) systems can track a wide variety of instruments. Commonly used tracked instruments include straight and angled suctions, forceps, and probes. Powered instrumentation – microdebriders and drills
– may also be tracked, although higher levels of caution must be exercised with these instruments. Suffice it to say, IGS has assumed an important role in revision ESS and extended rhinologic procedures.
Summary
The challenges presented by revision endoscopic sinus surgery – difficult anatomy, synechiae formation, bone removal, and precision soft-tissue removal – may be assuaged by the use of refined instrumentation. Mucosal preservation is stressed, and is also enhanced with the surgeon’s knowledge of currently available technology and instrumentation. Visualization may be improved
Fig. 8.9 a Stammberger endoscopic bipolar device (Karl Storz Endoscopy America). b Wormald endoscopic bipolar device (Medtronic)

Surgical Instruments in Revision Endoscopic Sinus Surgery |
69 |
with use of angled telescopes, improved cameras and monitors, and the introduction of HD technology. Speed, hemostasis, and precision are hallmarks of powered instrumentation. Through-cutting instruments and refinements of existing instruments also help to address the unique challenges presented in difficult revision cases. The sinus surgeon who embarks upon complicated revision cases must have a complete knowledge of the available instruments, and feel comfortable with their use in the appropriate circumstance.
References
1.Bernstein JM, Lebowitz RA, Jacobs JB (1998) Initial report on postoperative healing after endoscopic sinus surgery with the microdebrider. Otolaryngol Head Neck Surg. 118:800–803
2.Chandra RK, Schlosser R, Kennedy DW (2004) Use of the 70-degree diamond burr in the management of complicated frontal sinus disease. Laryngoscope 114:188–192
3.Kang SK, White PS, Lee MS, et al. (2002) A randomized control trial of surgical task performance in frontal recess surgery: zero degree versus angled telescopes. Am J Rhinol 16:33–36
Fig. 8.10 Endoscopic clip applier (Karl Storz Endoscopy America; recommended clips: ligating clip cartridge, medium titanium clips. Ligaclip Extra by Ethicon, Cincinnati, OH, USA)
4.Kennedy DW (2007) Technical innovations and the evolution of endoscopic sinus surgery. Ann Otol Rhinol Laryngol Suppl 196:3–12
5.Kennedy DW (2000) Functional endoscopic sinus surgery: concepts, surgical indications, and instrumentation. In: Kennedy DW, Zinreich SJ, Bolger W (eds) Diseases of the Sinuses: Diagnosis and Endoscopic Management. Decker, Hamilton, Canada, pp 197–210
6.Krouse JH, Christmas DA Jr (1996) Powered instrumentation in functional endoscopic sinus surgery. II: A comparative study. Ear Nose Throat J 75:42–44
7.Messerklinger W (1978) Endoscopy of the Nose. Baltimore: Urban Schwarzenberg
8.Moriyama H, Yanagi K, Ohtori N, et al. (1996) Healing process of sinus mucosa after endoscopic sinus surgery. Am J Rhinol 10:61–66
9.Selivanova O, Kuehnemund M, Mann WJ, et al. (2003) Comparison of conventional instruments and mechanical debriders for surgery of patients with chronic rhinosinusitis. Am J Rhinol 17:197–202
10.Setliff RC III, Parsons DS (1994) The “hummer”: new instrumentation for functional endoscopic sinus surgery. Am J Rhinol 8:275–278
11.Vauterin T, Vander Poorten V, Jorissen M (2006) Long term effects of cutting forceps in endoscopic sinus surgery. Rhinology 44:123–127

Chapter 9 |
9 |
Anesthetic Choices, |
|
Techniques, and Injections |
W. Derek Leight and Brent Senior
Core Messages
■General anesthesia using total intravenous anesthesia (TIVA) with propofol and remifentanyl may improve endoscopic vision in the surgical field.
■TIVA may provide patients with a better postoperative recovery period due to the antiemetic effects of propofol and the decreased hangover effect due to the fast metabolism of these two drugs.
■If TIVA is unavailable, the use of inhaled anesthetics should be minimized to avoid vasodilation with reflex tachycardia, which may worsen the surgical field.
■Perioperative beta-blockers may also be useful in improving the quality of vision in the surgical field. The use of these drugs should be discussed preoperatively with an anesthesiologist.
■Both inhaled anesthetics and propofol infusions degrade platelet function, which may contribute to an overall worsening of the quality of vision in the surgical field over time.
■Local injections are safe and reliable methods to enhance visualization in the surgical field.
Introduction
Since its inception in the 1980s, functional endoscopic sinus surgery (FESS) has become an important tool in the treatment of sinonasal disease. Technological advances have improved surgical techniques, which in turn have led to a widening of the scope of surgically treatable diseases. In comparison to traditional otolaryngologic surgery, methods of anesthetic administration for FESS are more influential in determining the quality of the surgical field.
Contents |
|
Introduction . . . . . . . . . . . . . . . . . |
. 71 |
Anesthetic Choices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 71 |
Pharmacology of General Anesthesia . . . . . . . |
72 |
Volatile Anesthetics . . . . . . . . . . . . . |
. 72 |
Intravenous Anesthesia . . . . . . . . . . . . |
72 |
Propofol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 72 |
Opioid Agonists . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 72 |
Balanced Anesthesia Versus TIVA . . . . . . . . |
. 73 |
Anesthesia for Sinus Surgery . . . . . . . . . |
. 73 |
Pharmacology of Local Anesthetics . . . . . . . |
. 73 |
Injections . . . . . . . . . . . . . . . . . . . |
74 |
Techniques . . . . . . . . . . . . . . . . . . |
75 |
General Anesthesia . . . . . . . . . . . . . |
. 75 |
Local Anesthesia . . . . . . . . . . . . . . |
. 75 |
Conclusions . . . . . . . . . . . . . . . . . . |
76 |
Anesthetic Choices
Local anesthesia with sedation has been a widely employed method for endoscopic sinus surgery. Several authors have reported this as the anesthetic method of choice, reporting fewer side effects, shorter operative times, and less perioperative bleeding.[5, 8] In addition, it is thought of as being theoretically safer, due to the fact that dissection in high-risk areas such as the anterior and posterior ethmoid bundles and lamina papyracea is more likely to induce pain and therefore allow the patient to alert the surgeon before an injury occurs [13]. Local anesthesia with sedation is more technically challenging for the anesthesiologist, as a balance must be achieved between patient comfort, respiratory drive, airway protection, surgical access, and positioning [13]. It is also depen-
