
Revision Sinus Surgery
.pdf
Tips and Pearls in Revision Sinus Surgery |
83 |
Fig. 10.4 Coronal CT scan of a patient with isolated left frontal recess disease. The left middle turbinate has lateralized against the medial orbital wall, resulting in the obstruction (red circle)
cigarette smoking should be stopped at least 3 weeks prior to surgery as well as in the 6 weeks following surgery to limit its deleterious effects on the nasal mucosa.
Patient comorbidities should also be optimized prior to surgery. In the case of FESS, asthma is the most common comorbidity and should be well controlled prior to surgery with inhalers and/or systemic steroids. Systemic hypertension should also be under good control, as poorly controlled intraoperative blood pressures can certainly worsen mucosal bleeding and complicate endoscopic visualization.
Prior to surgery, the informed consent process should also be carefully addressed. Compared to primary FESS, revision FESS often carries a greater risk for cerebrospinal fluid leaks, orbital injury, and postoperative drug-re- sistant infections. Major as well as minor complications should be discussed with the patients and it is prudent to have an FESS-specific consent form signed and witnessed prior to surgery [1].
Preoperative Workup
Once the decision is made to perform a revision endoscopic procedure, it is imperative in the preoperative period to review each patient’s anatomy, the amount of disease, and underlying comorbidities. CT scan and nasal endoscopy are the best modalities to use to assess the remnant bony partitions and areas of scarring that are obstructing the natural ostium of each sinus. Revision FESS is most often performed for recurrent nasal polyposis, persistent mucosal inflammation, mucoceles, or iatrogenic sinus disease. In each of these cases, mucosal inflammation should be kept at a minimum prior to the case in order to decrease surgical bleeding and improve endoscopic visualization.
Systemic steroids and oral antibiotics are helpful in stabilizing mucosal inflammation prior to surgery. Oral prednisone is started 1 week prior to surgery and continued for at least 2 weeks after surgery or until mucosal inflammation has resolved, as assessed by endoscopic examination. A prednisone dose of 0.6 mg/kg/day for 3 days followed by 0.4 mg/kg for 3 days is given starting 1 week prior to surgery. Along with this, culture-directed antibiotics are started 1 week prior to surgery. If no purulence is present, an empiric antibiotic such as a fluoroquinolone or combination of clindamycin and bactrim can be used to help stabilize the mucosa.
Patients are also instructed to stop aspirin or nonsteroidal anti-inflammatory drug use 1 week prior to surgery. Other agents that can potentially increase surgical bleeding, such as vitamin E and the herbal supplements ginger, gingko, and ginseng, are encouraged to be discontinued at least 3 weeks prior to surgery. In addition, active
Tips and Pearls
1.Preoperative oral steroids and antibiotics can often decrease mucosal edema and intraoperative bleeding.
2.The patient should refrain from cigarette smoking at least 3 weeks prior to surgery.
3.Obtain FESS-specific informed consent.
Surgical Equipment
Once the decision is made to perform a revision procedure, specialized instruments should be used to achieve a sound surgical technique of sparing mucosa while performing a complete sphenoethmoidectomy that removes osteitic ethmoid bony partitions.
Surgical Navigation Systems
With the advent of surgical navigation in the late 1980s, endoscopic surgeons have been increasingly utilizing this technology for intraoperative localization and preoperative planning. Fine-cut axial CT scans, often 1 mm in section, are reformatted into coronal and sagittal views and allow for a greater understanding of the anatomy, which has been distorted by previous surgery, polypoid mucosa, and/or anatomical variants. Preoperatively, image-guid- ance workstations can be used to plan out the dissection and identify areas that need to be addressed. Specifically, the frontal recess and the cells obstructing the frontal recess are best visualized utilizing the three views made available by the workstations, providing the surgeon with the ability to truly conceptualize the three-dimensional surgical anatomy prior to the procedure. In addition, os-

84
teitic bony partitions along the skull base and medial orbital wall are best seen using multiple views.
Intraoperatively, image guidance is useful in dissecting out remnant bony partitions while identifying the skull base and medial orbital wall. In many cases, previously operated and diseased ethmoid bone becomes osteitic and hardened, thus taking away the tactile difference between the skull base and ethmoid bone that is often appreciated and relied upon in primary surgery.
Some image-guidance systems also offer a virtual image update. Using computer software packages, a surgeon can trace their dissection and “virtually” eliminate the bony partitions and mucosal disease seen in the preoperative images (Fig. 10.5). This device can aid in perform-
Alexander G. Chiu and David W. Kennedy
ing a complete sphenoethmoidectomy and skeletonizing the skull base and medial orbital wall of bony ethmoid partitions.
Angled Endoscopes and Instruments
Angled instruments are essential in revision endoscopic sinus surgery. Endoscopes of 30 °, 45 °, and 70 ° allow direct visualization of the natural ostium of the maxillary sinus, frontal recess, and anterior skull base.
Revision surgery along the skull base necessitates the use of angled through-cutting instruments. Powered instrument companies have devised angled debrider
10
Fig. 10.5 An intraoperative endoscopic view with triplanar CT imaging of a patient with a left supraorbital ethmoid mucocele and corresponding skull base erosion (a). Upon entering the mucocele, the Eraser software program begins to digitally “erase” the mucocele based on the path of the instrument (b)

Tips and Pearls in Revision Sinus Surgery
blades, as well as drills, diamond and cutting, that may be attached to hand-held microdebriders. The 70 diamond suction irrigation drill has, in particular, made a dramatic difference for revision frontal sinus surgery. In particular, the drill reduces the amount of trauma and exposed bone during an extended frontal recess approach [2]. There is a variety of 90 ° instruments designed to reach around the nasofrontal beak and into the frontal recess. These instruments, specifically designed for the frontal recess, are also useful in accessing pathology within the floor and anterior portion of the maxillary sinus. Fungal debris or mucus retention cysts along the floor of the sinus can be irrigated and instrumented out by using angled giraffe forceps and curettes. Revision procedures often become a methodic process of cut, remove, suction, and reexamine. These specialized instruments allow for the preservation of mucosa and removal of fine bony fragments, which if left behind, can serve as a nidus for scarring and infection along the maxillary sinus and anterior skull base.
Intraoperative CT Scans
A more recent development that is currently limited in accessibility but may become more prevalent in the future is the use of an intraoperative CT scanner to assess the thoroughness of revision surgery. Xoran Technologies (Ann Arbor, MI, USA) has developed a mobile intraoperative CT scanner that is able to perform 0.4-mm slice thickness scans of the paranasal sinuses while the patient is on the operating room table (Fig. 10.6). This allows the opportunity to assess the extent and thoroughness of the
Fig. 10.6 Intraoperative CT with the xCAT™ – a mobile conebeam scanner. A special head rest allows rotation of the gantry for image acquisition. CT scans are then transferred into the surgical navigation system of preference for a real-time update of the images
85
revision procedure, and these real-time pictures can be used to update image-guidance systems if additional surgery is needed.
Surgical Technique
General Principles
In revision surgical procedures the anatomy can be significantly distorted and landmarks such as the middle turbinate may be partially resected, making them unreliable for anatomic localization. Image guidance is a helpful tool, but should not be trusted blindly. Accurate identification of static anatomical landmarks that cannot be altered from previous surgery can help guide the revision procedure and minimize the risk of complications.
Tips and Pearls – I
1.Landmarks to be used include the roof of the maxillary sinus, the medial orbital wall, and the skull base.
2.The skull base is usually most easily identified in the posterior ethmoid or sphenoid sinuses, where it is more horizontal and the cells are larger.
3.The roof of the maxillary sinus is closely related to the approximate height of the sphenoid sinus. Staying at this vertical level of the maxillary sinus roof during dissection posteriorly keeps the surgeon from venturing into the anterior skull base.
4.Be careful where the skull base slopes down medially toward the attachment of the middle turbinate in the region of the anterior ethmoid artery. The ethmoid roof is at its thinnest in this area, and may even be membranous in part, making it particularly vulnerable to injury. As this area is approached, it is important to stay close and parallel to the medial orbital wall while remembering that the opening of the frontal sinus is most frequently medial, close to the attachment of the middle turbinate to the skull base.
Since revision FESS is most often performed for persistent mucosal edema and mucoceles, it is recommended that the procedure should be a complete sphenoethmoidectomy. For isolated mucus recirculation of the maxillary sinus or frontal sinus mucoceles, the procedure can be tailored to the specific sinus. But for the most part, a complete and thorough dissection removing remnant ethmoid bony partitions and skeletonizing the medial orbital wall and anterior skull base gives the patient their best opportunity for a lasting surgical procedure. Removing remnant osteitic bony partitions requires the use of through-cutting instruments. Additional mucosal

86 |
Alexander G. Chiu and David W. Kennedy |
|
stripping in a revision procedure can result in even more |
|
neo-osteogenesis, which can be particularly crippling in |
|
the sphenoid and frontal sinus. The steps to performing |
|
a revision FESS are identical to those of a primary FESS. |
|
The maxillary sinus is first identified, and the medial or- |
|
bital wall is skeletonized free of ethmoid bony partitions. |
|
Dissection is then carried low and posterior through the |
|
inferior ethmoids and basal lamellae into the posterior |
|
ethmoids. The superior turbinate is then identified along |
|
with the natural os of the sphenoid sinus. Once the sphe- |
|
noidotomy is enlarged, the skull base is identified and |
|
dissection is carried posterior to anterior along the skull |
|
base with angled endoscopes and instrumentation. Eth- |
|
moid bony partitions along the skull base are removed. |
|
The anterior ethmoid artery is identified and preserved |
|
and the frontal recess is dissected out. |
10 |
Tips and Pearls – II |
1. Be methodical in revision surgery. Remove all |
ethmoid bony partitions along the skull base and medial orbital wall.
2. Spare the mucosa and use through-cutting instruments.
3. Identify the skull base within the sphenoid sinus or posterior ethmoids.
4. Make a large antrostomy within the maxillary, sphenoid, and frontal sinus in patients with polypoid disease.
Maxillary Sinus
■Identifying a remnant uncinate process is the most critical step in revising a maxillary antrostomy.
Mucus recirculation is a common cause of recurrent symptoms following FESS and is due to a mucosal or bony partition between the natural ostium and the surgical antrostomy. Scarring can be a cause, as well as a remnant uncinate process. A back biter or angled balltip probe is a useful instrument to reach posterior to the uncinate process and resect anteriorly to its attachment along the lacrimal bone. Once the hard bone of the lacrimal is palpated, no further resection is performed to prevent a nasolacrimal duct injury. The superior attachment of the uncinate should also be removed. This attachment is often fused to the anterior face of the agger nasi cell, and can be a source for frontal recess obstruction following initial sinus surgery.
■Infraorbital ethmoid or Haller cells can also be a source of persistent obstruction of the maxillary sinus.
These cells are easily missed because they are often more anterior than expected. Using an angled endoscope, the
bony walls of the infraorbital ethmoid cell can be visualized and removed using the combination of a ball tip probe, 90 ° forceps, and back-biter. Keep in mind its relation to the inferior orbital wall and infraorbital nerve as it courses along the roof of the maxillary sinus.
The floor of the maxillary sinus should be addressed in any revision procedure. Remnant fungal debris or mucus retention cysts should be identified and removed. Angled endoscopes such as the 45 ° or 70 ° are often needed to visualize these areas. Instruments normally used for the frontal recess, such as a 90 ° curette or giraffe forceps, are helpful in reaching down to the floor and removing debris. Irrigation with normal saline is also useful in removing thick allergic mucin or fungal debris from the sinus.
Inferior Ethmoid Sinus
Once the maxillary sinus antrostomy is revised, remnant ethmoid bony partitions off the medial orbital wall should be removed flush to the bone. The roof of the maxillary sinus can serve as a guide to the height of dissection through the ethmoid cavity as the surgeon moves posteriorly through the basal lamellae and into the posterior ethmoid cells. The basal lamella has an inferior, horizontal segment and a superior, vertical segment.
■Care should be taken to preserve the horizontal segment of the basal lamella to prevent postoperative lateralization of the middle turbinate.
Another specific area to address is the medial border of the ethmoid bulla. Often, this bony segment can be left untouched from a previous surgery in which the bulla was entered centrally and “cored” out with a microdebrider. The medial border can become osteitic, serve as a platform for polyp formation, and is best removed with a J-curette placed medial and posterior to the bony segment.
Once the dissection is carried through the basal lamellae and into the posterior ethmoid air cells, care should be taken to identify and remove any remaining ethmoid cells behind the maxillary sinus and against the posterior medial orbital wall or orbital apex. Often these retro maxillary cells can be left behind from a previous surgery and can again serve as a nidus for inflammation and polyposis.
The superior turbinate is then addressed. The superior turbinate serves as an anatomical landmark for the natural sphenoid os. Resecting the lower half of the superior turbinate allows visualization of the os while preserving olfactory function found in the superior portion of the superior turbinate. The basal lamellae of the superior turbinate as it attaches to the medial orbital wall can also be removed and debrided of inflammation and polyps.

Tips and Pearls in Revision Sinus Surgery
Sphenoid Sinus
■The sphenoid sinus can be safely entered through the natural os of the sinus.
The os is located medially and inferior and can be entered with a straight J-curette. The thin bone of the anterior face of the sphenoid sinus often turns into hardened, osteitic bone in revision surgery. In addition, dissection in this area can be highly vascular and bleeding can easily obscure the visual field. Sphenopalatine artery injections of lidocaine with epinephrine can aid in decreasing mucosal bleeding and improving endoscopic visualization. This may be performed prior to dissection through a transpalatal injection through the greater palatine foramen, or transnasally by injecting in a region near the lateral insertion of the middle turbinate. In either case, sphenopalatine injections can greatly aid in performing a sphenoidotomy.
With bleeding under control, the anterior face of the sphenoid should be removed superiorly to the skull base and inferiorly to the level of the septal branch of the sphenopalatine artery.
■It is advantageous to create a large sphenoidotomy especially in a highly inflamed field, since the small surface area of the sphenoid makes it more likely to stenose in the postoperative period.
Heavy through-cutting hand instruments, such as the straight mushroom punch and Kerrison forceps, are often needed to cut through the thick bone of the sphenoid. The anterior face of the sphenoid should then be removed laterally to the orbital apex and medial orbital wall. An angled endoscope can then be used to view the floor of the sphenoid. Any remaining fungal debris or mucin should then be removed or irrigated from the sinus.
Sphenoethmoidal (Onodi) cells are ethmoid cells that lie superior and lateral to the sphenoid sinus. There is a typically an oblique bony partition that separates the cell from the sphenoid sinus. In maximally enlarging the sphenoid, this horizontal bony partition should be removed with a through-cutting forceps, while bearing in mind that it may be attached posteriorly to either the optic or carotid canal.
Superior Ethmoid Sinus
■The skull base can be safely identified along the roof of the sphenoid sinus. Once identified, dissection is carried from a posterior to anterior direction along the skull base to remove the superior ethmoid bony remnants attached to the skull base.
87
Again, the use of angled endoscopes and through-cutting forceps is recommended to improve visualization and remove ethmoid bony fragments while preserving the skull-base mucosa.
As the dissection along the skull base is carried forward, the anterior ethmoid artery typically lies in a superior extension of the anterior wall of the bulla ethmoidalis at, or somewhat below, the skull base, and courses anteriorly as it travels medially (Fig. 10.7). The openings of one, or more frequently two, supraorbital ethmoid cells often lie anterior to the vessel and extend laterally and superiorly.
Frontal Sinus
The opening to the frontal sinus is frequently not immediately evident, so it is important that the surgeon has conceptualized the location of the drainage pathway prior to the surgical procedure. Very fine malleable probes have been developed that can be utilized to gently probe the openings and help determine which of these ostia truly passes superiorly into the frontal sinus. Once the opening has been clearly identified, adjacent bony partitions may be fractured with specialized frontal sinus instruments to open the frontal sinus. Bony fragments are then teased out and redundant mucosa is trimmed with through-cut- ting instruments. Common areas of attention for revision surgery include the agger nasi cell and superior uncinate process. In addition, a lateralized superior segment of the middle turbinate can cause iatrogenic frontal sinusitis. This can be addressed by either resecting the middle turbinate to its insertion along the skull base or by cutting the synechiae and medializing the middle turbinate with a stitch or a middle meatal spacer.
Packing and Stenting
■As long as there is at least 180 ° of intact frontal recess mucosa, stents are rarely needed in the frontal recess.
In extended frontal sinus procedures in which a drill is used to denude the mucosa and remove bone more than 180 around the frontal recess, stents can be employed to try to keep the recess open. However, there is not good evidence that they are better than local postoperative care, even in this situation. There are commercially available silicone stents, but we prefer using a thin silastic stent rolled into a tube and placed within the frontal recess, when for one reason or another, a decision to use a stent is made. These are easily removed in the clinic and can unfurl to cover the entire recess.
■Medializing the middle turbinate following surgery is often critical to the success of the procedure.

88 |
Alexander G. Chiu and David W. Kennedy |
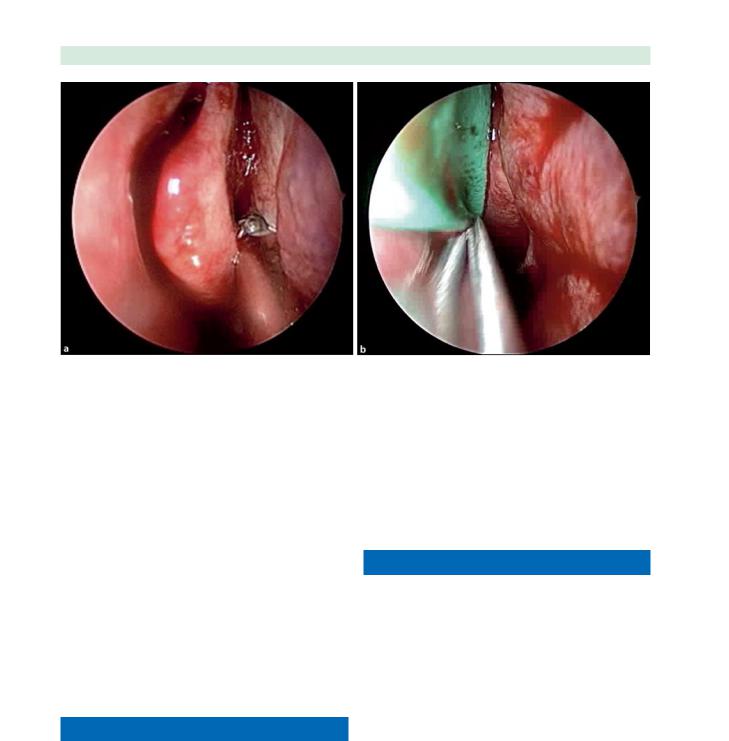
This allows easier postoperative access for debridements and helps prevent iatrogenic frontal recess obstruction from a lateralized middle turbinate. Often in revision cases, the horizontal portion of the basal lamellae has been removed, making the middle turbinate less stable. Medializing the middle turbinate against the septum can be done in a variety of methods. Scarring the medial surface of the middle turbinate and the opposing surface of the septum while placing a spacer in the middle meatus can create a controlled synechia, keeping the middle turbinate medialized (Fig. 10.8). Other techniques involve suturing both middle turbinates together through the septum.
10
Fig. 10.7 Coronal CT scan views of an anterior ethmoid artery within the skull base (a) and traveling below the skull base (b). c An endoscopic view with a 30 ° endoscope. The black arrowhead is pointing to an anterior ethmoid artery below the skull base and without a bony mesentery
Postoperative Care
■In the case of revision sinus surgery, postoperative care is often as important as the actual surgery itself.
Revision sinusotomies must be carefully and diligently examined following surgery. A failure to actively debride the recess and ostia, ensure its patency, and suction contaminated blood and mucus from the sinus is a recipe for restenosis and failure. To do this in a setting of an awake, often anxious patient, with topical analgesia alone makes this portion of the process very challenging, but can be aided by the careful application of topical ponticaine or even cocaine solution to the site. Where local debridements are necessary, 1% lidocaine with 1:100,000 epinephrine can be injected using a fine bent needle and a small syringe.
The timing of the first postoperative debridement varies with the individual surgeon’s preference. Some debride on postoperative day one, while others wait for an additional 3–7 days. It is advantageous to have a full set of sinus instruments available in the clinic. This is coupled with angled suctions that are long and curved enough to reach into the frontal sinus and lateral and inferior regions of the maxillary sinus. Debridements should be aimed at clearing away fibrin debris and any loose bony fragments, while keeping trauma to the surrounding mucosa to a minimum.
While the mechanical care of revision antrostomies are important to prevent restenosis, medical management of the disease state is essential to long-term success. In a patient with significant polypoid edema, postoperative oral steroids can be used to keep the edema to a minimum. Intranasal steroids sprayed in the Moffit or head-down position, can help with delivery to the frontal recess. Postoperative antibiotics should also be given in an infectious setting, and antibiotics with good bone penetration should be used in patients with evidence of neoosteogenesis. Other considerations in the management of these difficult patients includes local or oral antihis-
Tips and Pearls in Revision Sinus Surgery |
89 |
Fig. 10.8 Endoscopic views of creating a controlled synechiae between the right middle turbinate and nasal septum to prevent middle turbinate lateralization. The microdebrider is used to de-
tamines, antileukotriene medications, long-term irrigations, or an even a trial of itraconazole. In the latter situation, the patient’s liver function tests should be evaluated prior to therapy and monitored during the treatment, and the patient should be aware of the off-label usage of this medication in this situation.
This routine of mechanical debridement and postoperative medication should be continued on a weekly basis until the mucosa of maxillary, ethmoids, and frontals are healed. The medical treatment is guided almost entirely by the endoscopic appearance of the cavity and the presence of residual, typically asymptomatic, disease. Once the cavity is secure, routine surveillance by nasal endoscopy should continue for the life of the patient.
Conclusion
Revision endoscopic sinus surgery remains a great challenge to all who practice sinus surgery. Surgical technique aside, the most important decisions are still made in the office. These entail assessing whether or not the patient is a good surgical candidate, identification of the patient’s
nude opposing surfaces of the middle turbinate and septum (a). A merocel sponge is then placed lateral to the middle turbinate and kept in place for 1–3 days (b)
anatomy that is contributing to patient’s symptoms and disease process, and the institution of aggressive adjuvant medical therapy.
References
1.Bowden MT, Church CA, Chiu AG, et al. (2004) Informed consent in functional endoscopic sinus surgery: the patient’s perspective. Otolaryngol Head Neck Surg 131:126–132
2.Chandra RK, Schlosser R, Kennedy DW (2004) Use of the 70-degree diamond burr in the management of complicated frontal sinus disease. Laryngoscope 114:188–192
3.Glicklich RE, Metson R (1997) Effects of sinus surgery on quality of life. Otolaryngol Head Neck Surg 117:12–17
4.King JM, Caldarelli DD, Pigato JB (1994) A review of revision functional endoscopic sinus surgery. Laryngoscope 104:404–408
5.Senior BA, Kennedy DW, Tanabodee J, et al. (1998) Longterm results of functional endoscopic sinus surgery. Laryngoscope 108:151–157

Chapter 11 |
11 |
Septal and Turbinate Surgery |
|
in Revision Sinus Surgery |
Joseph Raviv and Peter H. Hwang
Core Messages
■Septal deviation and turbinate variants can encroach on sinus drainage pathways and may benefit from correction in the revision surgical patient.
■Preoperative nasal endoscopy is critical to formulating a surgical plan regarding treatment of the septum and turbinates.
■Endoscopic septoplasty provides excellent visualization and facilitates dissection in cases of revision septoplasty.
■Surgical release of the lateralized middle turbinate may require adjunctive medialization techniques to prevent reformation of scarring.
Introduction
Functional endoscopic sinus surgery has a long-term success rate of approximately 90% for improving symptoms in patients with medically refractory chronic rhinosinusitis [10]. In the evaluation of patients with persistent postoperative disease, the paranasal sinuses are typically scrutinized for causative factors, but the closely related nasal septum and turbinates may often be overlooked. This chapter will discuss the role of the nasal septum and turbinates in chronic sinus disease and the specific treatment techniques available for successful outcomes in the revision surgical treatment of patients with chronic rhinosinusitis.
Anatomy
Septum
The nasal septum separates the two nasal cavities, provides structural support for the nose, influences airflow in
Contents |
|
Introduction . . . . . . . . . . . . . . . . . |
. 91 |
Anatomy . . . . . . . . . . . . . . . . . . . |
91 |
Septum . . . . . . . . . . . . . . . . . . |
. 91 |
Turbinates . . . . . . . . . . . . . . . . . . 92 |
|
Association with Rhinosinusitis . . . . . . . . . |
. 93 |
Preoperative Workup . . . . . . . . . . . . . . |
93 |
Surgical Technique . . . . . . . . . . . . . . . |
93 |
Endoscopic Septoplasty . . . . . . . . . . . |
. 93 |
Incision Design . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 95 |
Surgical Technique Principles . . . . . . . . . . . . . . . . . . |
. 95 |
Inferior-Turbinate Reduction . . . . . . . . . |
. 95 |
Advantages of Microdebrider Inferior-Turbinate |
|
Reduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 95 |
Excision of Middle-Turbinate Concha Bullosa . . |
96 |
Lateralized Middle-Turbinate Release . . . . . |
. 97 |
Superior-Turbinate Resection . . . . . . . . . |
97 |
the nasal cavity, and expresses olfactory neuroepithelium. The membranous septum connects the columella to the caudal margin of the quadrangular cartilage, which comprises the majority of the anterior septum. The posterior septum is bony, consisting of the perpendicular plate of the ethmoid bone posterosuperiorly and the vomer posteroinferiorly. Finally, the nasal, frontal, maxillary, and palatine bones each contribute nasal crests to the periphery of the septum. The cartilaginous and bony portions of the septum align during fetal development and fuse to form bony cartilaginous junctions. Displacement of the bony–cartilaginous interfaces results in deviations or deflections of the septum; this may occur congenitally as well as during periods of rapid facial growth, or consequent to external trauma.

92
Turbinates
The turbinates are intrinsic to the normal functioning of the nasal airway. They facilitate the filtering, warming, and humidification of inspired air. The middle and superior turbinates also participate in olfaction.
■In light of the functional nature of the turbinates, decision making in turbinate surgery requires a judicious consideration of the potential impact of surgical modification on nasal function.
The precursors of the nasal turbinates can be identified during the 8–10th week of fetal development as outgrowths from the lateral nasal wall [12]. These outgrowths, or ethmoturbinals, ultimately yield the middle and superior turbinates. The inferior turbinate is derived from the maxilloturbinal, a separate structure located inferior to the ethmoturbinals. Given its distinct embryonic derivation, the inferior turbinate is subject to fewer of the
11 anatomic variations commonly seen in the middle and superior turbinates.
The three scroll-like turbinate bones divide the nasal lumen into meati. The inferior meatus is defined by the space between the inferior turbinate and the floor of the
Joseph Raviv and Peter H. Hwang
nose; the middle meatus by the space between the inferior and middle turbinates; and the superior meatus by the space between the middle and superior turbinates.
■The turbinate bones are encased by pseudostratified ciliated columnar respiratory epithelium, which contributes to mucociliary clearance of the nose. The inferior turbinate also possesses a vasoactive submucosal stroma that may hypertrophy and contribute to nasal obstruction.
Anatomic variations of turbinate structure are common, with pneumatization being the most commonly observed variant (Fig. 11.1). Concha bullosa of the middle turbinate is perhaps the most commonly encountered anatomic variant during surgery. A study reviewing 202 consecutive computed tomography (CT) scans found pneumatization of the vertical lamella of the middle turbinate in 46.2%, of the inferior or bulbous segment in 31.2%, and both the lamella and bulbous portion in 15.7% of cases [1]. Septal deviation and middle-turbinate concha bullosa often occur concurrently [11]. Nearly 80% of patients with a dominant concha bullosa have a concurrent deviated septum. There is also a strong association between unilateral concha bullosa and contralateral septal deviation. Pneu-
Fig. 11.1 Middle-turbinate pneumatization variants. a Pneumatization of the bulbous segment of the middle turbinate. b Pneumatization of the vertical lamella of the middle turbinate

Septal and Turbinate Surgery in Revision Sinus Surgery |
93 |
matization of the superior turbinate is not uncommon [15, 17], and pneumatization of the inferior turbinate is a rare but acknowledged variant as well.
Association with Rhinosinusitis
■Variations in septal and turbinate anatomy may play a role not only in nasal obstruction, but also in the development of chronic sinus disease. This is believed to be due to both anatomic narrowing of the ostiomeatal complex (OMC) as well as to disruption of mucociliary function.
For example, septal deviation may displace the middle turbinate laterally, encroaching upon the maxillary ostium. Likewise, a pneumatized middle turbinate or superior turbinate may narrow the middle meatus or superior meatus, respectively [16]. Middle-turbinate concha bullosa has been associated with anterior ethmoid disease, while septal deviation has been associated with disease of the ostiomeatal complex, anterior ethmoid, and posterior ethmoid [2, 6].
In addition to contributing to anatomic narrowing of the OMC, septal deviation may also contribute to rhinosinusitis by impairing mucociliary clearance. Septal deviation has been associated with significantly longer mucociliary clearance times than in normal controls. Notably, normalization of mucociliary clearance has been observed after septoplasty [4, 13].
Preoperative Workup
Assessment of the revision surgical patient should include a historical review of previous septal or turbinate surgical procedures. Evidence of previous surgical treatment of the turbinates and/or septum should be correlated with findings on nasal endoscopy.
Diagnostic nasal endoscopy is an essential component of the evaluation of the revision surgical patient, and inspection of the turbinates and septum forms a key component of the endoscopy. The inferior turbinates should be examined before and after application of topical decongestant, in order to adequately assess the degree of soft tissue hypertrophy. Evidence of previous surgical treatment of the inferior turbinate can often be identified, including resection or out-fracture.
■If inspection of the inferior meatus reveals an accessory surgical antrostomy, the region should be carefully evaluated for possible recirculation around the inferior turbinate between the inferior meatal and middle meatal antrostomies.
The middle turbinate should be examined carefully for evidence of previous middle-turbinate resection or scarring to the lateral nasal wall. Several studies of patients requiring revision endoscopic sinus surgery found that the most common surgical alteration associated with recurrent sinus disease was partial middle turbinectomy resulting in middle meatal scarring and lateralization of the middle turbinate [4, 9].
■Lateralization and adhesions of the superior aspect of the middle turbinate may indicate underlying iatrogenic frontal sinus obstruction.
The superior turbinate and sphenoid ostia should be visualized as well if possible. Recirculation around the superior turbinate may occur if there is discontinuity between the surgical sphenoidotomy and the natural ostium of the sphenoid sinus. Finally, an endoscopic assessment of the nasal septum should be performed. Difficulty accessing the middle meatus during the office endoscopy indicates a strong likelihood that septoplasty would be beneficial.
Septoplasty is often a necessary complement to endoscopic sinus surgery for both intraoperative and postoperative access [3]. During surgery, endoscopic access to the middle meatus can be impaired by otherwise asymptomatic deflections of the septum. Postoperatively, examination and debridement of the sinus cavities can be more difficult if obstructive septal deflections are left uncorrected. In postsurgical failures, it is important to assess whether either lack of or incomplete correction of septal deviation contributed to the poor outcome. Evidence of prior septoplasty should be recognized prior to revision septal surgery and may be easier to assess endoscopically by palpation of the septum with a probe or suction as opposed to simple inspection.
Surgical Technique
Endoscopic Septoplasty
Please refer to Video 11.1. Traditional techniques for correction of septal deformity have relied on headlight illumination. The application of endoscopic techniques to septoplasty was initially described in 1992 [8], and offers several important advantages [5, 7] .
■Utilization of the endoscope allows excellent illumination and visualization, including enhanced identification of dissection planes, better assessment of posterior septal deflections, and earlier recognition of flap tears.
In addition, the endoscopic technique permits limited, minimally invasive approaches for isolated deviations by
