
- •Foreword
- •Preface
- •Contents
- •About the Editors
- •Contributors
- •1: Tracheobronchial Anatomy
- •Trachea
- •Introduction
- •External Morphology
- •Internal Morphology
- •Mucous Layer
- •Blood Supply
- •Anatomo-Clinical Relationships
- •Bronchi
- •Main Bronchi
- •Bronchial Division
- •Left Main Bronchus (LMB)
- •Right Main Bronchus (RMB)
- •Blood Supply
- •References
- •2: Flexible Bronchoscopy
- •Introduction
- •History
- •Description
- •Indications and Contraindications
- •Absolute Contraindications
- •Procedure Preparation
- •Technique of FB Procedure
- •Complications of FB Procedure
- •Basic Diagnostic Procedures
- •Bronchoalveolar Lavage (BAL)
- •Transbronchial Lung Biopsy (TBLB)
- •Transbronchial Needle Aspiration (TBNA)
- •Bronchial Brushings
- •Advanced Diagnostic Bronchoscopy
- •EBUS-TBNA
- •Ultrathin Bronchoscopy
- •Transbronchial Lung Cryobiobsy (TBLC)
- •Therapeutic Procedures Via FB
- •LASER Bronchoscopy
- •Electrocautery
- •Argon Plasma Coagulation (APC)
- •Cryotherapy
- •Photodynamic Therapy
- •Airway Stent Placement
- •Endobronchial Valve Placement
- •Conclusion
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •Procedure Description
- •Procedure Planning
- •Target Approximation
- •Sampling
- •Complications
- •Future Directions
- •Summary and Recommendations
- •References
- •4: Rigid Broncoscopy
- •Innovations
- •Ancillary Equipment
- •Rigid Bronchoscopy Applications
- •Laser Bronchoscopy
- •Tracheobronchial Prosthesis
- •Transbronchial Needle Aspiration (TBNA)
- •Rigid Bronchoscope in Other Treatments for Bronchial Obstruction
- •Mechanical Debridement
- •Pediatric Rigid Bronchoscopy
- •Tracheobronchial Dilatation
- •Foreign Bodies Removal
- •Other Indications
- •Complications
- •The Procedure
- •Some Conclusions
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •Preprocedural Evaluation and Preparation
- •Physical Examination
- •Procedure-Related Indications
- •Application of the Technique
- •Topical Anesthesia
- •Anesthesia of the Nasal Mucosa and Nasopharynx
- •Anesthesia of the Mouth and Oropharynx
- •Superior Laryngeal Nerve Block
- •Recurrent Laryngeal Nerve Block (RLN)
- •Conscious Sedation
- •Monitored Anesthesia Care (MAC)
- •General Anesthesia
- •Monitoring the Depth of Anesthesia
- •Interventional Bronchoscopy Suites
- •Airway Devices
- •Laryngeal Mask Airway (LMA)
- •Endotracheal Tube (ETT)
- •Rigid Bronchoscope
- •Modes of Ventilation
- •Spontaneous Ventilation
- •Assisted Ventilation
- •Noninvasive Positive Pressure Ventilation (NIV)
- •Positive Pressure Controlled Mechanical Ventilation
- •Jet Ventilation
- •Electronic Mechanical Jet Ventilation
- •Postprocedure Care
- •Special Consideration
- •Anesthesia for Peripheral Diagnostic and Therapeutic Bronchoscopy
- •Anesthesia for Interventional Bronchoscopic Procedures During the COVID-19 Pandemic
- •Summary and Recommendations
- •Conclusion
- •References
- •Background
- •Curricular Structure and Delivery
- •What Is a Bronchoscopy Curriculum?
- •Tradition, Teaching Styles, and Beliefs
- •Using Assessment Tools to Guide the Educational Process
- •The Ethics of Teaching
- •When Learners Teach: The Journey from Novice to Mastery and Back Again
- •The Future Is Now
- •References
- •Interventional Procedure
- •Assessment of Flow–Volume Curve
- •Dyspnea
- •Analysis of Pressure–Pressure Curve
- •Conclusions
- •References
- •Introduction
- •Adaptations of the IP Department
- •Environmental Control
- •Personal Protective Equipment
- •Procedure Performance
- •Bronchoscopy in Intubated Patients
- •Other Procedures in IP Unit
- •References
- •Introduction
- •Safety
- •Patient Safety
- •Provider Safety
- •Patient Selection and Screening
- •Lung Cancer Diagnosis and Staging
- •Inpatients
- •COVID-19 Clearance
- •COVID Clearance: A Role for Bronchoscopy
- •Long COVID: A Role for Bronchoscopy
- •Preparing for the Next Pandemic
- •References
- •Historical Perspective
- •Indications and Contraindications
- •Evidence-Based Review
- •Summary and Recommendations
- •References
- •Introduction
- •Clinical Presentation
- •Diagnosis
- •Treatment
- •History and Historical Perspectives
- •Indications and Contraindications
- •Benign and Malignant Tumors
- •Tumors with Uncertain Prognosis
- •Application of the Technique
- •Evidence Based Review
- •Summary and Recommendations
- •References
- •12: Cryotherapy and Cryospray
- •Introduction
- •Historical Perspective
- •Equipment
- •Cryoadhesion
- •Indications
- •Cryorecanalization
- •Cryoadhesion and Foreign Body Removal
- •Cryoadhesion and Mucus Plugs/Blood Clot Retrieval
- •Endobronchial Cryobiopsy
- •Transbronchial Cryobiopsy for Lung Cancer
- •Safety Concerns and Contraindications
- •Cryoablation
- •Indications
- •Evidence
- •Safety Concerns and Contraindications
- •Cryospray
- •Indications
- •Evidence
- •Safety Concerns and Contraindications
- •Advantages of Cryotherapy
- •Limitations
- •Future Research Directions
- •References
- •13: Brachytherapy
- •History and Historical Perspective
- •Indications and Contraindications
- •Application of the Technique
- •Evidence-Based Review
- •Adjuvant Treatment
- •Palliative Treatment
- •Complications
- •Summary and Recommendations
- •References
- •14: Photodynamic Therapy
- •Introduction
- •Photosensitizers
- •First-Generation Photosensitizers
- •M-Tetrahidroxofenil Cloro (mTHPC) (Foscan®)
- •PDT Reaction
- •Tumor Damage Process
- •Procedure
- •Indications
- •Curative PDT Indications
- •Palliative PDT Indications
- •Contraindications
- •Rationale for Use in Early-Stage Lung Cancer
- •Rationale
- •PDT in Combination with Other Techniques for Advanced-Stage Non-small Cell Lung Cancer
- •Commentary
- •Complementary Endoscopic Methods for PDT Applications
- •New Perspectives
- •Other PDT Applications
- •Conclusions
- •References
- •15: Benign Airways Stenosis
- •Etiology
- •Congenital Tracheal Stenosis
- •Iatrogenic
- •Infectious
- •Idiopathic Tracheal Stenosis
- •Distal Bronchial Stenosis
- •Diagnosis Methods
- •Patient History
- •Imaging Techniques
- •Bronchoscopy
- •Pulmonary Function Test
- •Treatment
- •Endoscopic Treatment
- •Dilatation
- •Laser Therapy
- •Stents
- •How to Proceed
- •Stent Placement
- •Placing a Montgomery T Tube
- •The Rule of Twos for Benign Tracheal Stenosis (Fig. 15.23)
- •Surgery
- •Summary and Recommendations
- •References
- •16: Endobronchial Prostheses
- •Introduction
- •Indications
- •Extrinsic Compression
- •Intraluminal Obstruction
- •Stump Fistulas
- •Esophago-respiratory Fistulas (ERF)
- •Expiratory Central Airway Collapse
- •Physiologic Rationale for Airway Stent Insertion
- •Stent Selection Criteria
- •Stent-Related Complications
- •Granulation Tissue
- •Stent Fracture
- •Migration
- •Contraindications
- •Follow-Up and Patient Education
- •References
- •Introduction
- •Overdiagnosis
- •False Positives
- •Radiation
- •Risk of Complications
- •Lung Cancer Screening Around the World
- •Incidental Lung Nodules
- •Management of Lung Nodules
- •References
- •Introduction
- •Minimally Invasive Procedures
- •Mediastinoscopy
- •CT-Guided Transthoracic Biopsy
- •Fluoroscopy-Guided Transthoracic Biopsies
- •US-Guided Transthoracic Biopsy
- •Thoracentesis and Pleural Biopsy
- •Thoracentesis
- •Pleural Biopsy
- •Surgical or Medical Thoracoscopy
- •Image-Guided Pleural Biopsy
- •Closed Pleural Biopsy
- •Image-Guided Biopsies for Extrathoracic Metastases
- •Tissue Acquisition, Handling and Processing
- •Implications of Tissue Acquisition
- •Guideline Recommendations for Tissue Acquisition in Mediastinal Staging
- •Methods to Overcome Challenges in Tissue Acquisition and Genotyping
- •Rapid on-Site Evaluation (ROSE)
- •Sensitive Genotyping Assays
- •Liquid Biopsy
- •Summary, Recommendations and Highlights
- •References
- •History
- •Data Source and Methodology
- •Tumor Size
- •Involvement of the Main Bronchus
- •Atelectasis/Pneumonitis
- •Nodal Staging
- •Proposal for the Revision of Stage Groupings
- •Small Cell Lung Cancer (SCLC)
- •Discussion
- •Methodology
- •T Descriptors
- •N Descriptors
- •M Descriptors
- •Summary
- •References
- •Introduction
- •Historical Perspective
- •Fluoroscopy
- •Radial EBUS Mini Probe (rEBUS)
- •Ultrasound Bronchoscope (EBUS)
- •Virtual Bronchoscopy
- •Trans-Parenchymal Access
- •Cone Beam CT (CBCT)
- •Lung Vision
- •Sampling Instruments
- •Conclusions
- •References
- •History and Historical Perspective
- •Narrow Band Imaging (NBI)
- •Dual Red Imaging (DRI)
- •Endobronchial Ultrasound (EBUS)
- •Optical Coherence Tomography (OCT)
- •Indications and Contraindications
- •Confocal Laser Endomicroscopy and Endocytoscopy
- •Raman Spectrophotometry
- •Application of the Technique
- •Supplemental Technology for Diagnostic Bronchoscopy
- •Evidence-Based Review
- •Summary and Recommendations, Highlight of the Developments During the Last Three Years (2013 on)
- •References
- •Introduction
- •History and Historical Perspective
- •Endoscopic AF-OCT System
- •Preclinical Studies
- •Clinical Studies
- •Lung Cancer
- •Asthma
- •Airway and Lumen Calibration
- •Obstructive Sleep Apnea
- •Future Applications
- •Summary
- •References
- •23: Endobronchial Ultrasound
- •History and Historical Perspective
- •Equipment
- •Technique
- •Indication, Application, and Evidence
- •Convex Probe Ultrasound
- •Equipment
- •Technique
- •Indication, Application, and Evidence
- •CP-EBUS for Malignant Mediastinal or Hilar Adenopathy
- •CP-EBUS for the Staging of Non-small Cell Lung Cancer
- •CP-EBUS for Restaging NSCLC After Neoadjuvant Chemotherapy
- •Complications
- •Summary
- •References
- •Introduction
- •What Is Electromagnetic Navigation?
- •SuperDimension Navigation System (EMN-SD)
- •Computerized Tomography
- •Computer Interphase
- •The Edge Catheter: Extended Working Channel (EWC)
- •Procedural Steps
- •Planning
- •Detecting Anatomical Landmarks
- •Pathway Planning
- •Saving the Plan and Exiting
- •Registration
- •Real-Time Navigation
- •SPiN System Veran Medical Technologies (EMN-VM)
- •Procedure
- •Planning
- •Navigation
- •Biopsy
- •Complications
- •Limitations
- •Summary
- •References
- •Introduction
- •Image Acquisition
- •Hardware
- •Practical Considerations
- •Radiation Dose
- •Mobile CT Studies
- •Future Directions
- •Conclusion
- •References
- •26: Robotic Assisted Bronchoscopy
- •Historical Perspective
- •Evidence-Based Review
- •Diagnostic Yield
- •Monarch RAB
- •Ion Endoluminal Robotic System
- •Summary
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •General
- •Application of the Technique
- •Preoperative Care
- •Patient’s Position and Operative Field
- •Incision and Initial Dissection
- •Palpation
- •Biopsy
- •Control of Haemostasis and Closure
- •Postoperative Care
- •Complications
- •Technical Variants
- •Extended Cervical Mediastinoscopy
- •Mediastinoscopic Biopsy of Scalene Lymph Nodes
- •Inferior Mediastinoscopy
- •Mediastino-Thoracoscopy
- •Video-Assisted Mediastinoscopic Lymphadenectomy
- •Transcervical Extended Mediastinal Lymphadenectomy
- •Evidence-Based Review
- •Summary and Recommendations
- •References
- •Introduction
- •Case 1
- •Adrenal and Hepatic Metastases
- •Brain
- •Bone
- •Case 1 Continued
- •Biomarkers
- •Case 1 Concluded
- •Case 2
- •Chest X-Ray
- •Computerized Tomography
- •Positive Emission Tomography
- •Magnetic Resonance Imaging
- •Endobronchial Ultrasound with Transbronchial Needle Aspiration
- •Transthoracic Needle Aspiration
- •Transbronchial Needle Aspiration
- •Endoscopic Ultrasound with Needle Aspiration
- •Combined EUS-FNA and EBUS-TBNA
- •Case 2 Concluded
- •Case 3
- •Standard Cervical Mediastinoscopy
- •Extended Cervical Mediastinoscopy
- •Anterior Mediastinoscopy
- •Video-Assisted Thoracic Surgery
- •Case 3 Concluded
- •Case 4
- •Summary
- •References
- •29: Pleural Anatomy
- •Pleural Embryonic Development
- •Pleural Histology
- •Cytological Characteristics
- •Mesothelial Cells Functions
- •Pleural Space Defense Mechanism
- •Pleura Macroscopic Anatomy
- •Visceral Pleura (Pleura Visceralis or Pulmonalis)
- •Parietal Pleura (Pleura Parietalis)
- •Costal Parietal Pleura (Costalis)
- •Pleural Cavity (Cavitas Thoracis)
- •Pleural Apex or Superior Pleural Sinus [12–15]
- •Anterior Costal-Phrenic Sinus or Cardio-Phrenic Sinus
- •Posterior Costal-Phrenic Sinus
- •Cost-Diaphragmatic Sinus or Lateral Cost-Phrenic Sinus
- •Fissures18
- •Pleural Vascularization
- •Parietal Pleura Lymphatic Drainage
- •Visceral Pleura Lymphatic Drainage
- •Pleural Innervation
- •References
- •30: Chest Ultrasound
- •Introduction
- •The Technique
- •The Normal Thorax
- •Chest Wall Pathology
- •Pleural Pathology
- •Pleural Thickening
- •Pneumothorax
- •Pulmonary Pathology
- •Extrathoracic Lymph Nodes
- •COVID and Chest Ultrasound
- •Conclusions
- •References
- •Introduction
- •History of Chest Tubes
- •Overview of Chest Tubes
- •Contraindications for Chest Tube Placement
- •Chest Tube Procedural Technique
- •Special Considerations
- •Pneumothorax
- •Empyema
- •Hemothorax
- •Chest Tube Size Considerations
- •Pleural Drainage Systems
- •History of and Introduction to Indwelling Pleural Catheters
- •Indications and Contraindications for IPC Placement
- •Special Considerations
- •Non-expandable Lung
- •Chylothorax
- •Pleurodesis
- •Follow-Up and IPC Removal
- •IPC-Related Complications and Management
- •Competency and Training
- •Summary
- •References
- •32: Empyema Thoracis
- •Historical Perspectives
- •Incidence
- •Epidemiology
- •Pathogenesis
- •Clinical Presentation
- •Radiologic Evaluation
- •Biochemical Analysis
- •Microbiology
- •Non-operative Management
- •Prognostication
- •Surgical Management
- •Survivorship
- •Summary and Recommendations
- •References
- •Evaluation
- •Initial Intervention
- •Pleural Interventions for Recurrent Symptomatic MPE
- •Especial Circumstances
- •References
- •34: Medical Thoracoscopy
- •Introduction
- •Diagnostic Indications for Medical Thoracoscopy
- •Lung Cancer
- •Mesothelioma
- •Other Tumors
- •Tuberculosis
- •Therapeutic Indications
- •Pleurodesis of Pneumothorax
- •Thoracoscopic Drainage
- •Drug Delivery
- •Procedural Safety and Contraindications
- •Equipment
- •Procedure
- •Pre-procedural Preparations and Considerations
- •Procedural Technique [32]
- •Medical Thoracoscopy Versus VATS
- •Conclusion
- •References
- •Historical Perspective
- •Indications and Contraindications
- •Evidence-Based Review
- •Endobronchial Valves
- •Airway Bypass Tracts
- •Coils
- •Other Methods of ELVR
- •Summary and Recommendations
- •References
- •36: Bronchial Thermoplasty
- •Introduction
- •Mechanism of Action
- •Trials
- •Long Term: Ten-Year Study
- •Patient Selection
- •Bronchial Thermoplasty Procedure
- •Equipment
- •Pre-procedure
- •Bronchoscopy
- •Post-procedure
- •Conclusion
- •References
- •Introduction
- •Bronchoalveolar Lavage (BAL)
- •Technical Aspects of BAL Procedure
- •ILD Cell Patterns and Diagnosis from BAL
- •Technical Advises for Conventional TLB and TLB-C in ILD
- •Future Directions
- •References
- •Introduction
- •The Pediatric Airway
- •Advanced Diagnostic Procedures
- •Endobronchial Ultrasound
- •Virtual Navigational Bronchoscopy
- •Cryobiopsy
- •Therapeutic Procedures
- •Dilation Procedures
- •Thermal Techniques
- •Mechanical Debridement
- •Endobronchial Airway Stents
- •Metallic Stents
- •Silastic Stents
- •Novel Stents
- •Endobronchial Valves
- •Bronchial Thermoplasty
- •Discussion
- •References
- •Introduction
- •Etiology
- •Congenital ADF
- •Malignant ADF
- •Cancer Treatment-Related ADF
- •Benign ADF
- •Iatrogenic ADF
- •Diagnosis
- •Treatment Options
- •Endoscopic Techniques
- •Stents
- •Clinical Results
- •Stent Complications
- •Other Available Stents
- •Other Endoscopic Methods
- •References
- •Introduction
- •Anatomy and Physiology of Swallowing
- •Functional Physiology of Swallowing
- •Epidemiology and Risk Factors
- •Types of Foreign Bodies
- •Organic
- •Inorganic
- •Mineral
- •Miscellaneous
- •Clinical Presentation
- •Acute FB
- •Retained FB
- •Radiologic Findings
- •Bronchoscopy
- •Airway Management
- •Rigid Vs. Flexible Bronchoscopy
- •Retrieval Procedure
- •Instruments
- •Grasping Forceps
- •Baskets
- •Balloons
- •Suction Instruments
- •Ablative Therapies
- •Cryotherapy
- •Laser Therapy
- •Electrocautery and APC
- •Surgical Management
- •Complications
- •Bleeding and Hemoptysis
- •Distal Airway Impaction
- •Iron Pill Aspiration
- •Follow-Up and Sequelae
- •Conclusion
- •References
- •Vascular Origin of Hemoptysis
- •History and Historical Perspective
- •Diagnostic Bronchoscopy
- •Therapeutic Bronchoscopy
- •General Measures
- •Therapeutic Bronchoscopy
- •Evidence-Based Review
- •Summary
- •Recommendations
- •References
- •History
- •“The Glottiscope” (1807)
- •“The Esophagoscope” (1895)
- •The Rigid Bronchoscope (1897–)
- •The Flexible Bronchoscope (1968–)
- •Transbronchial Lung Biopsy (1972) (Fig. 42.7)
- •Laser Therapy (1981–)
- •Endobronchial Stents (1990–)
- •Electromagnetic Navigation (2003–)
- •Bronchial Thermoplasty (2006–)
- •Endobronchial Microwave Therapy (2004–)
- •American Association for Bronchology and Interventional Pulmonology (AABIP) and Journal of Bronchology and Interventional Pulmonology (JOBIP) (1992–)
- •References
- •Index
Brachytherapy |
13 |
|
|
Sara Shadchehr and Ileana Iftimia |
|
Introduction and Defnition of the Procedure
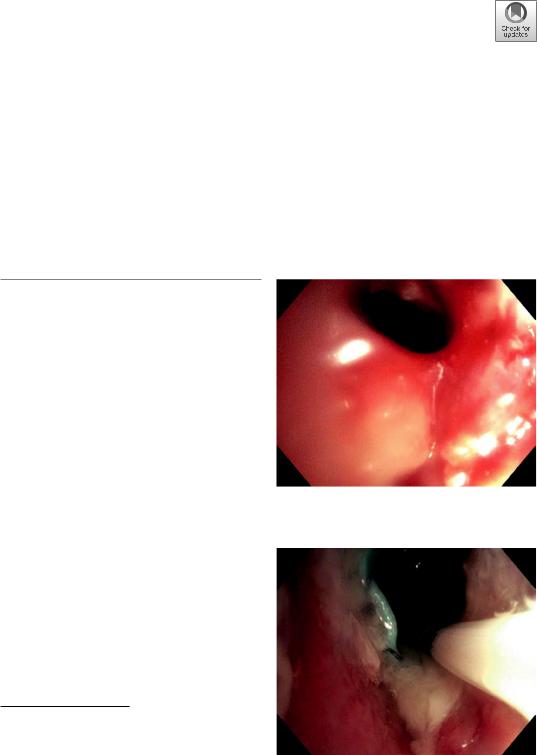
Patients with advanced stage lung cancer with endoluminal disease have limited treatment options for relief of dyspnea, or cough resulting from obstructing tumor. Advanced therapeutic bronchoscopy utilizing a combination of rigid and exible bronchoscopic techniques allows for local tumor destruction using hot modalities (i.e., electrocautery, argon plasma, or laser) or cold modalities such as cryotherapy (Fig. 13.1). Aforementioned techniques which have been discussed fully in other chapters can be used in combination with stenting if appropriate to maintain airway patency. Endobronchial brachytherapy (EBBT) is a localized form of radiation which, in combination with above techniques, can help restore airway patency over a longer period of time. It involves the placement of an afterloading catheter under direct visualization using the exible bronchoscope (Fig. 13.2). Once the catheter is in place, the bronchoscope is then removed, and the catheter is secured (Fig. 13.3a,
Fig. 13.1 Restoration of airway patency after tumor debulking
S. Shadchehr (*)
Interventional Pulmonology, TUSM, Lahey Hospital and Medical Center, Burlington, MA, USA
e-mail: Sara.shadchehr@lahey.org
I. Iftimia
Radiation Oncology, TUSM, Lahey Hospital and Medical Center, Burlington, MA, USA
e-mail: ileana.n.iftimia@lahey.org
Fig. 13.2 Airway after multimodality treatment (stent and brachytherapy catheter shown)
© The Author(s), under exclusive license to Springer Nature Switzerland AG 2023 |
189 |
J. P. Díaz-Jiménez, A. N. Rodríguez (eds.), Interventions in Pulmonary Medicine, https://doi.org/10.1007/978-3-031-22610-6_13

190 |
S. Shadchehr and I. Iftimia |
|
|
a |
b |
Fig. 13.3 (a) Pre-bronchoscopy preparation of brachytherapy catheter with tip marked at 5, 10, and 11 cm. (b) Patient after bronchoscopic placement of brachytherapy catheter
b). Marker seeds are inserted into the catheter for tumor localization and treatment planning, which will subsequently be replaced by the radioactive source when the patient undergoes local radiation. The EBBT treatment is usually repeated several times in sequence based on a predetermined plan set in place by the radiation oncologist.
History and Historical Perspective
Since the discovery of X-ray by Wilhelm Roentgen and Radium by Marie Curie, both modalities have since been extensively investigated and utilized by the medical feld to treat malignancies. The delivery of X-ray to the target sites through machines like linear accelerators is called teletherapy (or External Beam Radiation Therapy—EBRT), meaning to treat at a long distance. On the other hand, the delivery of radiation by applying radio-isotopes to the tumor site is known as brachytherapy (BT), meaning to treat at a short distance. In 1922, Yankauer described two cases of lung tumors treated endoscopically with radium [1]. Since then there have been innumerable publications on the application of various radio-isotopes in the treatment of malignancies in the form of brachytherapy which further advanced the use of this technique in treating intraluminal lung cancer.
The mainstay of treatment for intrathoracic malignancies usually involves the combination
of EBRT with systemic treatment such as chemotherapy or immunotherapy. However, with the help of signifcant advancement in technology such as computerized tomography (CT), 3D treatment planning, and high dose rate (HDR) afterloading delivery system, the use of EBBT plays a more important role in the treatment of lung cancer. It has been utilized as adjuvant treatment postoperatively for positive margins in the stump, boost dose to the endobronchial disease site to enhance local control when combined with EBRT, and symptomatic relief in patients with symptoms such as bleeding or bronchial obstructions [2, 3]. Though EBBT is not routinely recommended as the frst line defnitive treatment for early stage lung cancer, it remains a useful alternative treatment in a highly selected group of surgically inoperable patients with limited or occult endobronchial cancer less than 1 cm without metastatic lymphadenopathy or disease [2, 4].
Description of the Equipment
Needed
EBBT is the procedure of bringing the radioactive source intraluminally to the target site by a multidisciplinary team consisting of the pulmonologist, radiation oncologist, anesthesiologist, medical physicist, dosimetrist, radiation therapist, and nurses. It requires meticulous communi-
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

13 Brachytherapy |
191 |
|
|
cation between all specialties. CT scanning and simulation for planning and treatment is highly recommended.
The advantage of brachytherapy is that it can deliver a higher relative dose to the tumor site as compared to the surrounding normal structures because of the physical phenomenon known as the “inverse square law.” The radiation dose rate falls off rapidly, inversely as a function of the square of the distance from the radioactive source, so that the contacting tumor can be treated to a higher relative dose while sparing the further distant normal tissues. Historically, brachytherapy was done by manual application of low dose rate (LDR) radio-isotopes such as Iodine 125 seeds to the tumor. Because the dose rate is less than 2 Gray (Gy) per hour, it will take a longer time to treat, which in turn can cause patient discomfort, seed migration, and unnecessary radiation exposure to personnel [5, 6]. Nowadays, nearly all EBBT is delivered by high dose rate (HDR) radio-isotopes such as Iridium 192 with a dose rate of over 2 Gy per minute through an afterloading machine. When not in use, the HDR Ir192 source located at the tip of a exible wire is housed by the remote afterloader lead shielded unit. The unit has a system to deliver the source from the storage location to the patient via a catheter and to return it back to the storage at the end of the treatment and during power failure or emergencies. During treatment the radioactive source is maintained in various planned positions (called dwell positions) for a planned amount of time (called dwell time), so as to provide optimal dose coverage to the target. The delivery of radiation is performed with only the patient in a radiation bunker while a physicist can remotely control the afterloader safely outside. After treatment planning and dose calculation are performed, the computer within the machine will control the movement of the source and the wire, the dwell time and position of the source along the length of the active treatment, so as to deliver the designed cylindrical-like dose volume to the target. Figure 13.4 shows a photo of the Varian GammaMediX remote afterloader. The GammaMediX unit has 24 channels and can
Fig. 13.4 Varian GMEDiX HDR remote afterloader
extend the radioactive source to a fxed distance of 130 cm.
The afterloader uses an HDR Ir192 source with a nominal activity of 10 Ci. The source radioactivity is decaying in time. Consequently, the source needs to be replaced at least quarterly to ensure the treatment time does not become excessively long.
The HDR afterloading technique has the advantage of:
\(a)\ Providing 3D customized dose plan to the specifc target volume with maximal tumor dose and minimal normal tissue dose
\(b)\ Shortening treatment time per session and reducing patient discomfort
\(c)\ Reducing unnecessary radiation exposure to caregivers and personnel since there is no direct handling of the radioactive source

192 |
S. Shadchehr and I. Iftimia |
|
|
Table 13.1 The ABS dose recommendations for endobronchial brachytherapy
Brachytherapy alone
Pulsed dose |
30 Gy in one insertion (using pulses |
rate |
that offer biological equivalence to low |
|
dose rate) |
High dose |
10 Gy in one fraction |
rate |
15 Gy in one fraction |
|
|
|
14.2–20 Gy in two fractions |
|
|
|
22.5 Gy in three fractions |
|
|
|
24 Gy in four fractions |
|
30 Gy in six fractions (high dose |
|
palliation) |
Brachytherapy as a boost following EBRT
Pulsed dose |
15–20 Gy in one insertion (using pulses |
rate |
that offer biological equivalence to low |
|
dose rate) |
High dose |
10–15 Gy in two to three fractions |
rate (HDR) |
(following up to 60 Gy in 30 fractions) |
|
|
There is no consensus on the optimal dose fractionation for EBBT and it depends on the intention of treatment, condition of the patient, and institutional experience. Frequently it is given in two or three fractions of 5–10 Gy requiring repeated bronchoscopies [2]. The American Brachytherapy Society (ABS) published a list of guidelines on dose fractionation as listed in Table 13.1.
Indications and Contraindications
EBBT is considered an adjunctive procedure for palliative management of endobronchial tumor burden. Patient selection is essential as the patient must be able to tolerate several bronchoscopy procedures in sequence (i.e., 1 week apart) for the therapy to be effective. ABS Consensus guidelines recommend suitable patients who have the following characteristics:
•\ Signifcant intraluminal disease
•\ Predicted survival over 2 months to allow time for treatment response
•\ Patient who are unable to undergo EBRT or who have already received EBRT
Contraindications to bronchoscopy such as severe hypoxic respiratory failure, irreversible
coagulopathy, or recent MI exclude the possibility of EBBT.
Application of the Technique
The ABS recommends exible bronchoscopy via the transnasal approach to evaluate the anatomic location and length of the lesion with the patient comfortable. The treatment area should be photographed for documentation and comparison on follow-up examination. The majority of procedures can be performed under moderate sedation; however, general anesthesia may be required to keep patients from coughing excessively and dislodging the catheter. A pre-marked exible polyethylene catheter is inserted via the working channel. Graduated markings on the catheter help determine its location relative to the tumor (Fig. 13.3a). If pre-marked catheters are not available, marking the distal portion of the catheter at 5-cm intervals before insertion provides these visual reference points for catheter placement and treatment planning. Although the exact length to be treated depends on the extent of bronchial or tracheal involvement, lengths of 5–7 cm are commonly irradiated. The catheter may need to be introduced over a guidewire in stenotic areas too tight for the bronchoscope. The catheter tip should be at least 2 cm beyond the most distal aspect of the tumor when possible. Localizing the tip in a segmental bronchus can help hold the catheter in place [7]. It should be noted that the endobronchial lesion is usually well visualized under bronchoscopy but not under uoroscopy. Conversely, the catheter tip with the guidewire in place can usually be clearly seen under uoroscopy, but the tip’s position in relation to the distal end of the tumor is harder to verify on bronchoscopy. Therefore, both methods should be used to ensure accurate position of the catheter. The catheter is then secured to the patient’s nose and the catheter exit (at the nostrils) is marked (Fig. 13.3b).
High-dose-rate endobronchial brachytherapy (HDR-EBBT) is a multi-disciplinary procedure. After the catheter is placed by the pulmonologist, the patient is transferred to the Radiation
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

13 Brachytherapy |
193 |
|
|
Oncology Department, where a team (radiation oncologist, nurse, radiation therapist, physicist, and dosimetrist) is involved in the patient care. The HDR-EBBT procedure consisting of imaging, treatment planning, and treatment should be competently and effciently performed in order to minimize the amount of time the patient has the bronchial catheter(s) in place. Before imaging, the catheter length is checked using a gauge wire and assessed for kinks or obstruction along its pathway. The physicist should check the catheter exit marking to ensure that the catheter has maintained its proper position (i.e., not shifted out). Then, a dummy wire (i.e., a thin plastic wire with 1 cm apart small metal markers) is placed inside the catheter. The metal markers having a high atomic number are clearly visible on the X-ray and CT images, which in turn may help with catheter digitization during the treatment planning process. The images used for treatment planning are acquired using a breath hold technique to reduce blurriness and to help with digitizing the catheter(s).
Based on the tumor location, two or more catheters may be needed to achieve good dosimetric coverage. There are various ways to generate the treatment plans, such as using CT or orthogonal X-ray images to identify the catheter(s), or using pre-calculated treatment plans (for single catheter cases). This last approach is less accurate for curved catheters [8].
Based on the current ABS guidelines [3], the orthogonal X-ray imaging approach can be used,
but whenever possible CT scan of the patient to identify the catheter(s) should be performed. Figure 13.5 shows an anterior-posterior X-rayuoroscopic image with the catheter and dummy wire in place. Figure 13.6a, b show anterior- posterior digitally reconstructed radiographs from CT images, with the applicator and dummy wire in place, for a right and a left bronchial tumor, respectively.
Images are then imported to the Treatment Planning System (TPS) and used to generate a treatment plan. The catheter(s) are digitized, and based on the information received from the Interventional pulmonologist, the distal and proximal extents of the tumor are marked on the digitized catheter(s). If necessary, the radiation
Fig. 13.5 Anterior-posterior X-ray uoroscopic image with the catheter and dummy wire in place
a |
b |
Fig. 13.6 (a) Anterior-posterior digitally reconstructed radiograph from CT images (R side tumor), showing the applicator and dummy wire. (b) Anterior-posterior digi-
tally reconstructed radiograph from CT images (L side tumor), showing the applicator and dummy wire
194 |
S. Shadchehr and I. Iftimia |
|
|
oncologist will contour the tumor. A 1–2 cm margin should be added to each end of the tumor, in order to account for microscopic extension, as well as placement and dosimetric uncertainties [7]. Subsequently, the catheter(s) are after-loaded with radioactive sources (i.e., HDR dwell positions) to cover the tumor plus margins.
If the tumor is located in close proximity to organs at risk, the areas of concern can also be contoured as avoidance areas on the CT images and dose adjusted to ensure the organ at risk dose is within limits. This may help to avoid subsequent complications, such as major hemoptysis [7, 9].
There is some variation on how lung brachytherapy is performed, but practitioners should follow the current guidelines [3, 10]. The HDR- EBBT treatment often consists of multiple fractions. The treatment can be performed in a same day single insertion with the fractions spaced at least 6 h apart or consecutive weekly insertion usually over 2–3 weeks. For a single insertion approach, a single plan can be generated and used for all fractions, but X-ray or CT images should be performed before each fraction to ensure the catheter is in the same position as used for the treatment plan (no displacements or kinks). For the multi-insertion approach, imaging should be acquired and a new treatment plan should be generated for each fraction. There are two methods to prescribe the dose for the HDR- EBBT: (a) to a fxed depth from the center of the catheter throughout (typically 10 mm) or (b) to 10 mm from the center of the catheter in the area of trachea/main bronchus, and gradually to a smaller depth distally, ranging from 5 to 10 mm, depending on the bronchus position [3, 10]. For CT-based planning and curved catheters, the dose at 10 mm should be averaged over 4 points (located in 2 orthogonal directions). If the tumor extent is known and contoured, the CT-based plan can be designed to adequately cover the tumor with the prescribed dose. When using a 5F catheter the distance to the airway walls may not be symmetric, unless a centering device is used. This may increase the dose to normal tissue, or could be a beneft for tumor coverage [3].
For palliative cases, the HDR-EBBT can be used as monotherapy or as a boost treatment, in combination with EBRT. The common prescription doses recommended by the ABS are 7.5 Gy*3 and 10 Gy*2 fractions for HDR-EBBT monotherapy, and 7.5 Gy*2 and 5 Gy*3 fractions for boost HDR-EBBT treatments (see Table 13.1), at 10 mm from the center of the catheter [3, 7]. The fractions are usually weekly. These dose/fractionations regimes have similar biological effects [7]. The prescription dose for the HDR-EBBT will be reduced if patients are treated concurrently with chemotherapy. If possible, concomitant HDR- EBBT-chemotherapy should be avoided.
For selected patients (with predominantly endobronchial tumor) the HDR-EBBT (monotherapy or boost) can be used as a curative approach, with similar doses as listed above for the palliative cases. This approach is still under exploration [11–13].
Figure 13.7a, b show the dose cloud around the catheter for an HDR-EBBT CT-based boost plan, for a left and a right bronchial tumor, respectively. Yellow line is the prescription dose of 6 Gy per fraction. Tumor with margins is shown in red. Figure 13.7c shows the Dose Volume Histogram (DVH) plot for the left bronchial tumor, 100% covered with the prescribed dose of 6 Gy. The tumor is surrounding the catheter in close proximity to the HDR source dwell positions. This results in a high dose to some parts of the tumor, re ected in the long tail of the DVH plot. Prior to the HDR treatment, this patient was treated bilaterally using an EBRT approach. The dose cloud for this bilateral bronchus EBRT plan is shown in Fig. 13.8. Yellow line is the prescribed dose of 45 Gy, delivered in 25 fractions. The tumor is shown in color wash.
Dose cloud around a catheter for an HDR- EBBT monotherapy CT-based plan is shown in Fig. 13.9. Yellow line is the prescription dose of 7 Gy per fraction.
The HDR-EBBT procedure is performed in a very compressed time and the entire treatment is delivered in only a few fractions. After the plan generated by the planner (dosimetrist or physi-
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/

13 Brachytherapy |
195 |
|
|
a
b
c
Fig. 13.7 (a) L side tumor: dose cloud around the catheter showed on CT images for an HDR-EBBT boost plan. Yellow line is the prescribed dose. Tumor with margins is shown in red. Prescription depth is 10 mm from the center of the catheter. (b) R side tumor: dose cloud around the
catheter showed on CT images for an HDR-EBBT boost plan. Yellow line is the prescribed dose. Tumor with margins is shown in red. (c) Dose Volume Histogram plot for a left bronchial tumor showing 100% coverage at the prescribed dose of 6 Gy
