
5 курс / Пульмонология и фтизиатрия / Clinical_Tuberculosis_Friedman_Lloyd_N_,_Dedicoat
.pdf
100 Tuberculosis Transmission Control
and disease progression. Critical early host pathogen interactions are completely obscured in these models.37 In contrast, human- to-guinea-pig transmission studies provide a unique model that routinely shows clear evidence of the earliest, often transient, focal host−pathogen interactions produced by long-term (months) exposures of hundreds of immunologically naïve guinea pigs to the exhaust air from a TB ward in South Africa (Figure 6.1). Using this model, we routinely measure early innate immune responses that begin to trigger adaptive immunity (TST and T-cell stimulation responses), which are often aborted, and sometimes progress to sustained infection and disease progression. Evidence that these transient TST responses are due to viable, infectious, and not dead Mtb or environmental bacteria, is provided by the observations that they do not occur at all without exposure to exhaust air from TB patients, and are prevented if exhaust air from patients is UV irradiated.38–40 Presumably, there are other early host−pathogen interactions that resolve with microbial death, but without stimulating measurable TST responses. This leads to the question, what exactly is infection? While not presuming to answer that question here, we suggest that if latent Mtb, which often persists but does not progress to disease—manifest only by adaptive immunological response—is called “latent infection,” then specific immunological responses that do not persist, reflecting early innate host−pathogen interactions, also deserve to be called infection, albeit, transient infection. That this phenomenon is not better recognized than it is, although observed in the H-GP model almost 70 years ago, is attributable to the difficulty of measuring these reproducible events in exposed humans where serial TST or IGRA testing is rarely done, and to the absence of transient infection in experimental animal models employing unnaturally high doses of aerosolized laboratory strains of Mtb specifically intended to infect most animals and lead to disease.
Reinfection
Smith theorized that reinfection was an integral part of TB pathogenesis under endemic conditions, allowing organisms to seed the vulnerable lung apex when systemic hematogenous seeding is blocked by adaptive immunity (Figures 6.2 and 6.3).41 Apical lung seeding is the favored location for reactivation and lung cavitation, a critical part of airborne transmission (Figure 6.2). But
Endogenous reactivation (Endog. Path.)
beyond a secondary, airway route to the lung apex, reinfection can be considered another form of dose. Because individual airborne droplet nuclei can contain relatively few organisms and still be airborne, and because not all infections initiated in macrophages are successfully sustained, repeated inhalations and reinfection increase the probability that some “hits” progress even if many do not. In a study of household contacts of infectious cases in British Columbia, Grzybowski showed that, given infection, children in households with a sputum smear positive index case were more likely to progress to disease than children in households where the index case was culture positive, but sputum smear negative, indicating a lower bacterial burden in the source case.42 Children in households with a sputum smear positive index case likely had many more “hits” and a greater chance that at least one would progress to disease.
Determinants of transmission
A convenient way to discuss the determinants of transmission and potential interventions is through the Wells-Riley equation (Equation 6.1), an idealized steady-state model that has been widely used to better understand the theoretical relationship among factors.43 For this purpose, it is less important as an equation for accurately predicting risk of infection than as a single place to consider the relative importance of the major Mtb transmission factors.
Number of Infected Persons = Number of Susceptible Hosts
×Chance of Not Becoming Infected
C =S(1−e−Iqpt/Q ) |
(6.1) |
Equation 6.1 is the Wells−Riley mass balance equation for airborne equation—explained in the text.43
In this model, C represents the number of infected persons; S is the number of susceptible persons exposed to contaminated air generated by one or more infectious source or sources (I) at a certain generation rate (q) over a given time period (t). Infection rate (C/S) is directly related to the generally low probability of inhaling an infectious dose of Mtb as droplet nuclei in dilute air—hence the Poisson distribution (infrequent events) is used, represented
|
|
|
|
|
Primary complex |
|
|
|
|
|
|||
|
CMI response |
is eventually |
|
|
1013 cfu |
|
|
|
|
||||
|
|
activated |
sterilized |
|
|
Cavitary |
|
|
|
|
|||
|
|
|
|
|
Bacillary population |
|
|
|
|
||||
|
|
|
|
|
pulmonary |
|
|
|
|
||||
|
|
|
|
|
drops to dormant level |
|
|
|
|
||||
|
|
|
105 cfu |
tuberculosis |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
103 |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
cfu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|||
Infection* |
|
Bacillemia and seeding |
Immunosuppressive event |
Sputum positive for |
|||||||||
via airway |
|
|
of reactivatable site |
leads to drop in the CMI |
|
tubercle bacilli |
|||||||
|
|
|
|
in the lung apex |
|
|
response |
|
|
|
Transmission |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
to contact |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 6.2 Smith figure showing Stead unitary theory, that active disease in low burden settings where active disease almost always represents reactivation of infection early in life. (From Smith D et al. Rev Infect Dis. 1989;11: S385–S393.)
Книга в списке рекомендаций к покупке и прочтению сайта https://meduniver.com/
Introduction 101
|
CMI response |
|
|
|
|
|
|
|
Bacillary population |
|
|
|
|
|||||
|
|
activated |
|
|
|
|
|
|
|
drops to dormant level |
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cavitary |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
pulmonary |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
104 cfu |
|
103 cfu |
|
103 cfu |
103 cfu |
|
|
|
103 cfu |
tuberculosis |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Immunosuppressive |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
1st infection** |
2nd infection |
3rd infection |
4th infection |
|
|
|
Nth infection |
|||||||||||
via airway |
via airway |
via airway |
via airway |
|
via airway leads to |
event |
|
CMI |
|
|
||||||||
|
|
|
||||||||||||||||
|
|
|
||||||||||||||||
|
|
|
|
|
|
|||||||||||||
apical implant
Aerosol implants to mid-to lower lung
Figure 6.3 Smith concept of reinfection as an alternative pathway to cavitary disease, especially in persons with enhanced immunity from prior immunization or mycobacterial infection. (From Smith D et al. Rev Infect Dis. 1989; 11:S385-S393.)
by the natural logarithm (e). C/S is directly related to the generation rate of infectious doses (I × q). t represents the duration of exposure. p represents the host pulmonary ventilation, which is a species-specific constant for the purposes of this relationship. C/S is inversely related to only one factor under steady-state condi- tions—building or room outdoor ventilation rate (Q).
Under non-steady state, non-well-mixed conditions, outside, for example, dilution is infinite and changing as infectious droplet nuclei disperse, so the equation does not apply. The relationship is simpler than the equation would suggest. For example, doubling source strength doubles infection rate and doubling ventilation rate halves it. Importantly, doubling ventilation again, reduces the risk again by half. Like all first-order kinetic processes (target theory), where the magnitude of the effect depends on the target
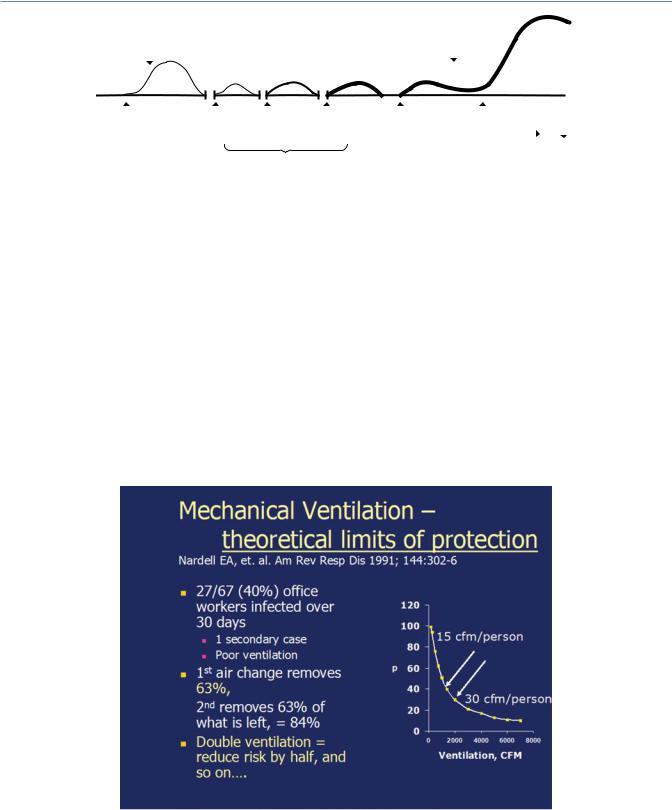
concentration, it becomes increasingly difficult and expensive to decontaminate air as the concentration of contaminant (Mtb) falls. Put another way, a first air change removes approximately 63% of contaminated air under well-mixed conditions, and a second air change removes approximately 63% of what remains, and so on, with decreasing increments in protection from each step increase in ventilation (Figure 6.4). As discussed later, upper room air disinfection also works on first-order kinetics, allowing comparison of protection expressed as equivalent air changes (1 Eq air changes per hour (ACH) = a 63% reduction in risk) achieved by GUV with that achieved by ventilation (Figure 6.5).
From Figure 6.6 it is evident that there are other transmission factors not included in the Wells−Riley equation, such as those determining source strength, as detailed below. For the
Figure 6.4 Example of using the Well−Riley equation to estimate source strength and the effect of changing room ventilation on infection rate. The graph illustrates of probability of infection as a function of ventilation, in cubic feet per minute. See ventilation discussion below. In a TB exposure in an office building, a worker exposed 67 known TST negative co-workers for 1 month, resulting in 27 documented TST conversions and one secondary case. The Well−Riley equation estimated that the source case produced approximately 13 infectious quanta (doses) per hour. It further estimated that increasing the estimated ventilation rate of 15 cfm per person to 30 cfm per person would reduce the infection rate from 40% to 20%, an example of the general rule that doubling outside ventilation reduces the transmission rate by half. (From Nardell EA et al. Am Rev Respir Dis. 1991;144:302–6. Reprinted with permission of the American Thoracic Society. Copyright © 2020 American Thoracic Society.)

102 Tuberculosis Transmission Control
|
120 |
|
|
|
|
|
|
|
100 |
|
|
|
|
|
|
infected |
|
|
|
|
250 qph |
|
|
80 |
|
|
|
|
|
|
|
occupants |
|
|
|
|
|
|
|
60 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60 qph |
|
|
Percent |
40 |
|
|
|
|
|
|
|
|
|
13 qph |
|
|
|
|
|
20 |
|
|
|
|
|
|
|
|
1.25 qph |
|
|
|
|
|
|
0 |
|
40 |
60 |
80 |
100 |
120 |
|
0 |
20 |
|||||
|
|
|
Fresh air ventilation (CFM per occupant) |
|
|||
Figure 6.5 Impact of ventilation on transmission—results from a modeling exercise based on the office building exposure cited earlier. Note that while increasing ventilation always reduces transmission, protection is a function of source strength, that is, the number of quanta of infection generated per hour (qph). At very high generation rates room occupants are not protected fully even at high ventilation rates.4
organism, the model assumes uniform virulence and viability, and for host resistance, it assumes uniform susceptibility despite possible immunization or prior mycobacterial exposure, and it assumes no interventions, such as respiratory protection. Given several unrealistic assumptions and unknowns, the basic Wells−Riley is useful primarily for understanding general relationships among factors, but not for precise predictions, and more sophisticated mathematical enhancements are likely not warranted.44
Source factors
Human-to-human Mtb transmission requires the presence of an infectious source (or sources, I) and susceptible hosts sharing the same breathing space (shared air). Infectious droplet nuclei are generated at a certain rate (q) and removed from the air at a certain rate (or inhaled by susceptible hosts). Among factors known to increase source strength are: (1) the number of untreated (often unsuspected) source cases, (2) the presence of lung cavities in non-immunocompromised persons (HIV patients are less likely to cavitate but may harbor large numbers of organisms), (3) the ability to cough and generate aerosol (also related to sputum viscosity), (4) the absence of effective treatment, (5) the presence of drug resistance (often unsuspected), (6) the use of cough hygiene or surgical masks, and (7) separation or isolation of the infectious source case.
Organism factors
Mtb strains are well known to vary in virulence (ability to infect and cause disease) with the most dramatic examples being the vaccine strain of bovine TB, M. bovis and the laboratory strain, H37Ra, which have mutations leading to a loss of virulence for immunocompetent hosts, producing at most a transient infection in the case of BCG.45 Circulating clinical strains also appear to vary in virulence with the Beijing family of strains being notoriously virulent as well. Other strains, for example CDC 1551, were suspected as being hyper-virulent because of the amount of skin test hypersensitivity produced among contacts in large outbreaks, but that appeared to represent just the opposite, a strain stimulating a greater immune response (despite the appearance of wider transmission) but perhaps, resulting in less disease as a result of a vigorous immune response.46
Figure 6.6 Schematic of key factors in airborne transmission and major intervention sites as discussed in the text.
Книга в списке рекомендаций к покупке и прочтению сайта https://meduniver.com/

Interventions 103
Environmental factors
How specific environmental conditions impact Mtb viability is poorly understood. Person-to-person Mtb transmission is successful in the heat and humidity of the tropical climates as well as in dry, cold climates, although crowded, poorly ventilated indoor conditions where transmission occurs may be humid, despite dry, cold outside conditions. The primary indoor factor associated with transmission is crowding and lack of ventilation, leading to the propensity to re-breathe contaminated air. Rudnick and Milton have argued that indoor CO2 levels may be a good surrogate for rebreathed air fraction, an important transmission risk determinant, given infectious sources in the room.47 Ambient CO2 is influenced by both crowding, dilution, and outdoor (non-recir- culated) ventilation. High levels could result from overcrowding, underventilation, or a combination of both factors. Wood and colleagues have monitored individual ambient CO2 levels encountered during the day to estimate where students, for example, experience the greatest rebreathed air fraction. They concluded that schools and minibuses could be important sites of TB transmission in South Africa.32,48 We have used the same methodology to monitor the rebreathed air experience of nurses working in two somewhat differently constructed hospitals in the Cape Town, South Africa, area, finding that both were reasonably well ventilated relative to occupancy in that breezy climate, the highest CO2 levels being experienced in the crowded tea break room where an infectious TB case is unlikely—but where influenza among staff could more easily spread from person to person.
With global warming and soaring temperatures in megacities in India and other hot climates, air conditioner use is escalating dramatically, immediately increasing the risk of airborne infection as windows are closed for efficient operation. Similar conditions have long existed in cold climates with tightly sealed windows and radiant heating, leading to high rates of transmission, especially of drug-resistant TB, compounded by prolonged hospitalization and delayed diagnosis of drug resistance. Both administrative and environmental control strategies are needed.
Host factors
Humans vary greatly in resistance to Mtb infection, reinfection, and disease progression, based on both inherited innate immunity, epigenetic modification, and adaptive immunity, further complicated by age, medication, and risk factors such as smoking, air pollution, diabetes and HIV co-infection. Previous exposure to mycobacteria and related organisms such as BCG vaccination, environmental mycobacteria, Mtb, and M. leprae are associated with greater resistance to Mtb infection, reinfection, and/or disease progression. Long attributed to adaptive immunity, there is growing evidence of innate learned immunity derived from epigenetic exposure, mediated at the level of bone marrow.49–52 In addition, populations vary greatly in the extent to which they have co-evolved with TB infection, reflected in selective pressure for resistance in populations. For example, Stead and Bates have argued that central African populations were spared exposure to the TB epidemic raging in Western Europe and the Americas until recent centuries, resulting in less inherited resistance to infection
among black residents of Arkansas nursing homes compared to Caucasian residents. Once infected, however, the risk of progression to disease was similar, but disease clinical manifestations differed in the two populations.53 Disease in blacks tends to be more acute whereas disease in whites more indolent and chronic. These differences they argued are not inherently racial, but strictly based on evolutionary population exposure to Mtb. Aboriginal populations in North America and the Pacific Islands similarly had little inherited resistance to Mtb, with devastating results when these naïve populations encountered European settlers.53
INTERVENTIONS
Source strength interventions: The rapid impact on transmission of effective treatment
There has long been evidence that the principle source of Mtb transmission in congregate settings is persons with unsuspected TB not on therapy, or patients with known TB, but unsuspected drug resistance, not on effective therapy. Nonetheless, most TB transmission control interventions focus almost exclusively on known or suspected cases who are likely to be on effective treatment. Hospitals focus on TB wards, on the isolation or separation of known or suspected cases. Healthcare workers focus on respiratory protection when caring for known or suspected patients even after treatment has started. Part of the problem is lack of familiarity with the evidence showing how promptly transmission stops once effective treatment starts. If asked, many healthcare workers will quote the “two-week rule”—that transmission can be assumed to cease after two weeks of effective treatment (Figure 6.5). Where does the two-week rule come from and is it valid?
Following the introduction of effective chemotherapy in the early 1950s, long-term hospitalization for TB became unnecessary, but the safety of ambulatory treatment regarding household and community transmission became an issue. The Madras study, showing the efficacy of ambulatory treatment through a controlled clinical trial (one of the first of any intervention), also demonstrated no difference in household conversion rates when patients were treated at home vs. hospital.54 Based on several household transmission studies, Rouillion and colleagues concluded in 1976 that chemotherapy at that time (only INH, streptomycin, and PAS) was likely to render patients non-infectious quickly, probably in less than two weeks. This was more consensus than science.7 That conclusion did not incorporate the human-to-guinea-pig studies published by Riley and colleagues over a decade earlier, below, showing that patients admitted at exactly the same time that treatment was started (not two weeks earlier) were 98% less infectious for guinea pigs (Table 6.1).39 Guinea pigs are believed to be much more susceptible to human Mtb than are normal humans, so these stunning results strongly suggest that effective treatment, even treatment less effective than current regimens, rapidly shuts down human−human transmission.
As Riley stated, “The treated patients were admitted to the ward at the time treatment was initiated and were generally

104 Tuberculosis Transmission Control
Table 6.1 Outcome of human-to-guinea-pig Mtb transmission studies, showing the rapid impact of effective treatment on transmission among DS and DR patients
Patients with |
Numbers of |
Relative |
susceptible Mtb |
GPs infected |
infectiousnessa% |
Untreated |
29 |
100 |
Treated |
1 |
2 |
Patients with DR Mtb |
|
|
Untreated |
14 |
28 |
Treated |
6 |
5 |
Source: From Riley et al. Am Rev Resp Dis 1962;85:511–525. Reprinted with permission of the American Thoracic Society. Copyright © 2020 American Thoracic Society.
Note: The only available drugs at the time were isoniazid, streptomycin, and PAS, so DR patients, usually INH resistant at least, had very few treatment options.
a All smear positive patients, relative to the amount of time spent on the ward.
removed before the sputum became completely negative. Hence the decrease in infectiousness preceded the elimination of the organisms from the sputum, indicating that the effect was prompt as well as striking.”39 This is the clearest demonstration to date that effective treatment has an almost immediate effect on the ability of organisms, still viable on culture, to infect a new host. Based on our own human-to-guinea-pig studies in South Africa, we recently published evidence suggesting that even MDR-TB is rapidly rendered non-infectious for guinea pigs by effective chemotherapy, but unsuspected XDR-TB, inadvertently treated (ineffectively) as MDR-TB, continued to transmit.6 Moreover, the addition of bedaquiline and linezolid to failed MDR treatment had not reduced transmission to guinea pigs over an 11-day period, but these drugs both require prolonged loading time to reach full therapeutic levels. However, the NIX regimen, containing the more rapidly acting pretomamid (a delamamid-like drug), promptly stopped XDR transmission (Prof. Anton Stoltz, 2018, personal communication).
FAST: FIND CASES ACTIVELY, SEPARATE, AND TREAT EFFECTIVELY
BASED ON RAPID MOLECULAR DIAGNOSTIC TESTING
Renewed awareness of the rapid impact of effective treatment on transmission, combined with the broad implementation of rapid molecular diagnostic testing for TB, has led to a refocused administrative control strategy that we call “F-A-S-T.”8 The active case finding component (A) acknowledges the long-held belief that transmission most often occurs from persons with unsuspected TB or unsuspected drug resistance. How common is that? There are few studies. Willingham and colleagues screened 250 patients for TB admitted to a large female medical ward in a busy general hospital in Lima, Peru over a period of one year.5 They found 40 patients who were sputum Mtb culture positive, including 26 (65%) smear positive and 13 (33%) unsuspected TB patients. Of the 40 culture-positive cases, 8 had MDR-TB (6 unsuspected, including 3 smear-positive cases). Without prompt identification of Mtb and drug resistance, followed by effective treatment, transmission
from such patients continues. Other screening efforts, in Zambia for example, have had similar results.11 Such surveys are important as they would suggest futility of the FAST approach in lowburden settings like the US. There, depending on the population served, most cough and upper lobe chest x-ray findings would not likely be TB-related.
The FAST strategy is an attempt to prioritize TB surveillance and effective treatment as the single most important way to reduce institutional TB transmission. Thus far, FAST has been selectively implemented in Bangladesh, Vietnam, South Africa, and Russia to name a few early adopters.55,56 Recently published results in Russia are dramatic, although limited to the profound effect of preventing delayed diagnosis of MDR-TB.57 Before presenting those results, the unusual Mtb transmission situation in Eastern Europe and Central Asia deserves discussion.
Hyper-transmission of MDR-TB in
Eastern Europe and central Asia
Kendall and colleagues recently published a model to estimate the percentage of MDR-TB transmitted compared to that acquired by poor treatment globally and in selected high and low MDR-TB risk countries.58 Overall, their model showed that WHO global estimates of 3.5% MDR-TB among new cases, and 20.5% among retreated cases translated to a median of 96% transmitted among all incident cases and 61% among retreated cases. Thus, of the countries studied, apart from low-MDR rate Bangladesh, most MDR-TB results from transmission, not poor treatment. In the 2016, WHO report, MDR-TB percentages of new cases for Belarus, Kazakhstan, Kyrgyzstan, Moldova, Russia, Tajikistan, and Ukraine, were 38, 26, 27, 26, 27, 22, and 27, respectively.59 Clearly, these distinctly high MDR-TB rates represent hyper-transmis- sion—resulting, we propose, from a combination of prolonged hospitalization, delayed diagnosis of DR-TB, and poor ventilation in generally cold climates in Eastern Europe and Central Asia. With the introduction of universal rapid molecular drug susceptibly testing on admission, however, can change dramatically as discussed below.57 The following scenario from Tomsk, Siberia, provides insight into why hyper-transmission has been happening in Eastern Europe and Central Asia.
ROLE OF HOSPITALIZATION
A retrospective study of risk factors for MDR-TB in the Russian oblast of Tomsk found the unanticipated result that hospitalization among adherent patients during an initial course of treatment for drug susceptible TB was the major risk factor (OR >6) for development of MDR-TB compared to adherent patients treated in an ambulatory setting.60 Having previously been treated, these cases would have been routinely classified as acquired drug resistance rather than primary MDR-TB, but that is clearly not the case. In many former Soviet Union (FSU) countries, patients are admitted to hospital and treated for presumed drug susceptible (DS TB) based on smear or CXR with culture pending. Not until months of clinically failing treatment are drug susceptibility tests done, and still months later when MDR-TB is diagnosed and effectively treated. In the meantime, other patients are admitted to the same
Книга в списке рекомендаций к покупке и прочтению сайта https://meduniver.com/

Environmental control interventions 105
poorly ventilated congregate rooms, and many of those with DS TB become re-infected with drug-resistant (DR TB). Prompt diagnosis and effective treatment based on rapid molecular testing can stop hyper-transmission and reinfection, as demonstrated, below.
SUCCESSFUL INTERVENTION
In Voronezh and Petrozavodsk, Russia, for example, investigators implemented a targeted form of F-A-S-T in two TB hospitals to reduce transmission from patients with unsuspected MDR-TB. Hospitalization was prolonged before and after implementation— an average of 20.7 weeks before, and 20.0 weeks after implementation. Universal Xpert testing was initiated on all admissions to both the 800-bed and 120-bed facilities, followed by prompt (<48 h) Xpert-directed treatment of DS and Rifampin-resistant TB. Before implementation of universal Xpert testing it took an average of 76.5 days before MDR-TB was diagnosed and patients started on effective treatment.57 They compared the subsequent rate of MDR-TB generation associated with hospitalization to the baseline rate, pre-FAST. Of a total of 450 patients that were HR sensitive on admission before implementation, 12.2% were diagnosed with MDR-TB within 12 months of finishing treatment. Of 259 patients that were HR sensitive after implementation of universal molecular testing and prompt, effective treatment, only 3.1% were subsequently diagnosed with MDR-TB within 12 months of finishing treatment—a 78% odds reduction in MDR acquisition through the interruption of transmission by prompt, effective treatment of MDR-TB cases in hospital.57
ENVIRONMENTAL FACTORS
Climate change
Transmission and reinfection are driving the epidemic in warm as well as cold climates. Without comparing molecular fingerprints of original and relapse isolates (infrequently available), there is no specific test for reinfection, and it is rarely discussed. As noted, reinfection is an important pathogenesis distinction because it usually implies recent transmission, whereas reactivation does not.61,62 In rural South Africa, for example, the widely publicized report of rapidly fatal XDR-TB cases called the world’s attention to the potential for rapid spread from one or more unsuspected XDR-TB cases to HIV-infected patients in multi-bed wards common throughout resource-limited regions.63–65 Fifty-five percent of the 53 cases initially reported had not been treated previously, but two-thirds had been hospitalized, and 85% of cases had isolates with the same genotypes, strongly suggesting transmission and presumably mostly reinfection among previously infected adults. Moreover, with climate change and soaring temperatures and humidity, ductless (split system) air conditioning is being introduced widely, in India for example, resulting in windows being closed and an increased risk of transmission.66 Although rapid diagnosis and effective treatment is essential for stopping the spread of DS and MDR-TB, stopping XDR transmission may depend more on isolation and air disinfection since evidence of a rapid effect of treatment is limited. This is also true for situations where active case finding, drug susceptibility testing, and
prompt effective treatment are not yet available or feasible in the near future.
ENVIRONMENTAL CONTROL
INTERVENTIONS
Even if active case finding efforts were widely implemented, some indoor situations will continue to defy prompt diagnosis and effective treatment as an administrative approach to preventing transmission. Around the world, crowded, poorly ventilated corridors continue to serve as waiting rooms in ambulatory centers (Figure 6.7). Patients and family members typically wait for hours to be seen, potentially exposing others—or being exposed—to undiagnosed infectious TB. However, the logistics of effective triage based on symptoms or screening tests, the resources and time required, and the absence of a truly simple, rapid point of care “rule in” or “rule out” test all make the “FAST” approach impractical as the main approach to transmission control in these settings. Other administrative approaches to lessen indoor crowding using outdoor waiting areas (where climate permits), creative scheduling of patients, and appointment systems, are important, but rarely implemented due to tradition, economic factors, and simple inertia.
For example, in a new MDR-TB Clinic in Karachi, Pakistan, the architect designed natural ventilation into the infrastructure in that windy location, but also planned to have patients wait outside under sun cover (see Figure 6.8, right) with small groups of patients called in according to their appointment in order to reduce indoor crowding. In this way patients are exposed to
Figure 6.7 Typical corridor serving as an overcrowded waiting area. At least in the setting pictured, windows exist on both sides. Often both sides are lined by doors to clinic consultation rooms.

106 Tuberculosis Transmission Control
Figure 6.8 MDR-TB Clinic, Karachi, Pakistan, Tarique Alexander Qaiser, architect. Note wind scoop design in the main building and a covered outdoor waiting area to decrease crowding.
unsuspected TB cases either outside, with optimal ventilation, or inside in smaller groups to reduce transmission.
Importance and limitations of natural ventilation
The Karachi example illustrates two of the optimal uses of natural ventilation: outdoor waiting areas and buildings designed to assure good airflow patterns. However, many buildings are not optimally designed, positioned, located, or operated for optimal natural ventilation, all day, every day, and in all seasons. Even in generally warm climates, windows are often closed at night to keep out cool air, for security or superstition, and to keep out vermin. Outdoor conditions change between day and night, by season, and wind velocity and direction can change constantly, favoring cross-contamination of rooms one second, preventing it the next. That said, in favorable climates such as Lima, Peru; Karachi, Pakistan; and Cape Town, South Africa, conditions are so consistent that generally good air disinfection can be achieved most of the time by simply keeping windows open.9 But in high altitude Johannesburg, South Africa, for example, closed windows at night virtually assure air stagnation. In the very cold climates of Eastern Europe, Russia, and Central Asia, for example, natural ventilation is uncommonly a reliable means of air disinfection. Moreover, for heating efficiency by radiators and space heaters, windows are often made leak proof, reducing even natural infiltration of outside air.
Mechanical ventilation
Some buildings in TB high-burden settings have mechanical ventilation systems, but maintenance is an ongoing problem unless
maintenance contracts are in place. Even if functioning properly, mechanical systems often mostly recirculate air with few outdoor air changes per hour (ACH). Recirculating air within a building contributes to dilution and redistribution, but not removal of infectious droplet nuclei. The risk near an infectious source may be reduced, but at the expense of a higher risk elsewhere in the building served by the heating/ventilation system for as long as droplet nuclei remain infectious.
Depending on occupancy, low outside air ventilation may be measurable as relatively high indoor CO2 levels (above outside levels), indicating increased shared air breathing.47 As noted above, personal monitoring of ambient CO2 levels is being used to assess where during a typical day vulnerable persons experience the highest percentage of rebreathed air—at home, in school, on the bus, or in other congregate settings.32, 48 As mentioned, as a research tool, we are using the same approach to assess buildings and their use from an airborne infection risk perspective. For example, do nurses working in a poorly ventilated emergency department experience a higher average rebreathed air fraction compared to nurses doing similar work in another building? Ambient CO2 reflects building ventilation and occupancy, and personal average ambient CO2 experience for occupants also reflects building usage by individuals or groups of individuals.
As mentioned, reducing indoor CO2 levels by half by doubling ventilation results in half the risk of transmission. But also as noted, reducing the CO2 levels by half again, requiring another doubling of ventilation, becomes increasingly impractical and expensive to achieve. The theoretical limits of protection by mechanical ventilation has been discussed in the literature.4 High-level natural ventilation can overcome these limits, but as noted, is inherently inconsistent, and requires attention to building design, location, and operation. As also noted, the reduced ventilation often found in cold climates is increasingly found in
Книга в списке рекомендаций к покупке и прочтению сайта https://meduniver.com/

Environmental control interventions 107
hot climates where ductless cooling system are commonly being used in response to climate change. What alternative strategies are available when both natural and mechanical ventilation are not entirely reliable or protective?
Upper room GUV air disinfection: The only practical alternative to natural ventilation
The above claim may strike some as extreme, but consider the alternatives. Both natural and mechanical ventilation require outside air exchange for effective air disinfection, with a resulting energy cost associated with heating and cooling outdoor air for comfort. Upper room GUV air disinfection with air mixing works by inactivating airborne pathogens without energy losses due to exhausting air outside, and the need to heat, cool, and dehumidify outdoor air. With upper room GUV, outside air is required for comfort only—for odor control, for example. Here, we briefly discuss the general theory and application of upper room GUV, referring readers to published guidelines and reviews for details.67,68
GUV refers to short-wavelength UV (UV-C, 254−270 nm wavelength). For more than 70 years, upper room GUV lamps have been used in rooms, often with air mixing fans, to rapidly disinfect room air of airborne pathogens in the occupied space. Most commonly, GUV fixtures are mounted on walls as shown in Figure 6.9.
Figure 6.9 Upper room germicidal fixture in use in Russia.
Current upper room GUV utilizes mercury-based fluorescent lamps nearly identical to lamps commonly used for fluorescent room lighting. GUV differs in two ways: (1) utilize UV-transparent quartz or Vicor® glass to permit UV to escape the lamp, and (2) absent coating on the inner surface of GUV lamps that, in fluorescent lamps, produces visible light in response to UV. Good quality mercury lamps produce a narrow spike of 254 nm UV, close to the peak germicidal action spectrum of 265–270 nm, but less penetrating of skin and outer eye structures.69–71 Newer LED GUV may be closer to 265–270 nm, more germicidal but also slightly more penetrating and potentially irritating. For safety reasons, upper room GUV fixtures are designed to confine UV irradiance to the space above peoples’ heads. Although excessive direct or reflected exposure of eyes and skin to 254–270 nm UV will cause temporary irritation, properly applied germicidal UV does not cause skin cancer or cataracts because it is much less penetrating than longer wavelength UV A and B found in sunlight. A full discussion of GUV safety has been published.72
Despite repeated demonstrations of safety and efficacy, upper room GUV has not become a routine intervention like mechanical ventilation when air disinfection beyond natural ventilation is needed, even though it is demonstrably more effective, less expensive, and more easily adapted to existing buildings without major installation costs. Fear of UV overexposure and difficulty demonstrating efficacy are likely part of the reason for the limited adoption, as is lack of accessible expertise to plan and maintain GUV systems. Engineers trained in GUV air disinfection are available, but maintenance remains a problem for GUV and many other devices in hospitals. A maintenance manual on upper room GUV has been published by the Stop TB initiative in English, Spanish, and Russian (http://www.stoptb.org/wg/ett/assets/documents/ MaintenanceManual.pdf).
The air disinfecting efficacy of upper room GUV with air mixing has been quantified three times in two very different hospital settings: an HIV ward in Peru with mostly DS TB, and an MDR-TB hospital in South Africa where the majority of patients are also HIV infected.10,31 The most recent published study showed approximately 80% efficacy, or the equivalent of adding approximately 24 equivalent air changes to the existing 6 mechanical air changes on the ward. The effective dose was a total GUV fixture output of 17 mW/m3 of total room volume. Because air is wellmixed in the room, relevant dosing volume is the entire room, not just the irradiated volume. In that study, the average calculated UV flux (irradiance from multiple sources) was 6 µW/cm2. A repeat study, not yet published, used less GUV flux (12 mW/ m3) and also had excellent air disinfection. Based on these studies, we recommend the lower GUV dose of 12 mW/m3 total room volume.
After determining where upper room GUV is likely to be most useful, a knowledgeable GUV consultant needs to identify quality GUV fixtures, the output of which has been professionally measured by gonioradiometry or integrating sphere—two lighting laboratory methods of measuring total fixture output. It is impossible to rationally dose upper room GUV without knowing total fixture output, and ideally, total directional output, that is, gonioradiometry.67,73 Many of the best GUV fixture manufacturers have

108 Tuberculosis Transmission Control
had their fixture’s output measured by established lighting laboratories. Knowing the risk areas, their room volumes and configurations, the consultant identifies locations for specific fixtures.
As important as providing sufficient upper room GUV energy (flux), adequate room air mixing is required. We recommend air turnover rates from low-velocity ceiling fans of 25 per hour. The direction of airflow, up or down, makes little difference to GUV efficacy since both directions produce airflow through the upper room. Fan speed is also not usually a critical factor. This is because more frequent, shorter transits of airborne infectious droplet nuclei through the upper room at higher fan speed, or fewer, longer exposures at low speeds result in approximately the same germicidal dose. Ideally, organisms reaching the upper, irradiated zone would be 100% inactivated on one pass, but residual exposure in the lower room still represents dramatic risk reduction for occupants.
Like new construction, before GUV systems are fully activated, they need to be commissioned, that is, tested for safety and efficacy under real-life conditions before occupants are exposed. Testing GUV systems requires a high-quality photometer sensitive at both high (upper room) and low (lower room) detection ranges. GUV systems should be re-checked every 3–6 months for lamp output and safety. The major causes of output deterioration are dust accumulation, lamp failure, or ballast failure. Although hospital staff can be trained to use a photometer properly, a better option, if available, is to hire a consultant, using frequently calibrated meters, with the correct specifications, trained to make accurate, reproducible measurements. Details on meter selection and use are available in a recently published GUV maintenance manual, referenced above.
Room air cleaners: Plausible in theory, but rarely effective in practice
Faced with unreliable natural ventilation, day and night, and season to season, and in cold climates, some facility managers purchase room air cleaners, promoted by companies as a simple, relatively inexpensive solution to air disinfection. They are generally not. As proof of efficacy, sales representatives for these devices often present data showing contaminated air entering the device, and nearly 100% decontaminated air leaving the device. What they rarely reveal is the clean air delivery rate, or CADR, essentially the flow rate of air through the machine. While it is not difficult to decontaminate air in a device using air filtration, irradiation, or even more exotic electrostatic or plasma technology, it is difficult to move enough air through the device to result in adequate numbers of equivalent air changes per hour (6–12 Eq ACH) to meet air disinfection recommendation.74 To make matters worse, many room air cleaners by necessity have their air intake and outlet located close to one another, resulting in recapture of decontaminated air (short-circuiting), and an even lower effective air disinfection rate than suggested by the equivalent air changes per hour, calculated from the CADR. Disregarding the negative effects of short-circuiting of air, if a room air cleaner promoted in South Africa has a 28.3 lps CADR, and is placed in a small hospital isolation room with a 48 m3 (4 × 4 m, 3 m high ceilings) volume, an equivalent ACH of only 2.1 will result—less if any substantial
Comparison:
Room air cleaner vs. upper room GUV
(Pretoria meeting, July, 2016)
|
48 m3 |
|
4 m |
1 ACH = 48 m3/h |
|
1 ACH = 13.3 L/s |
||
|
4 m |
Room air cleaner (RSA) = 60 cfm CADR |
|
|
|
|
|
= 28.3 L/s |
|
|
= 2.1 ACH (assuming no re-capture |
and good air mixing)
Upper room UVGI – avg 30 uW/cm2 for TB, Z = 41
with good air mixing, = approx 20 ACH!
Figure 6.10 Comparision of room air cleaner vs. upper room GUV. In a hypothetical small room with a volume of 48 m3 one air cleaner with the clear air delivery rate specified would produce just 2.1 Eq ACH compared to more than 20 Eq ACH easily produced by GUV.
recapture (short-circuiting) occurs (Figure 6.10). In contrast, just one upper room GUV fixture providing 0.5 W total fixture output, with a ceiling fan, could provide approximately 20 EqACH based on the studies previously discussed. In other words, without inherent short-circuiting of air, 10 of these room air cleaners would be necessary to approach the air disinfecting effect of just one upper room GUV fixture and ceiling fan. Of course, larger room air cleaning devices are available, with much higher CADRs. Five such units, selected for optimal performance produced about 16 EqACH, and were tested twice in the South African Airborne Infection Research Facility in exactly the same way as upper room GUV had also been tested twice. The same ceiling fans were used to make conditions comparable. The results (unpublished) were surprisingly disappointing—approximately 20% protection compared to 80% with upper room GUV. The possible reasons for these poor results were thoroughly and independently investigated by the South African Council for Scientific and Industrial Research (CSIR). The CADR was re-checked and found to be correct. Some leakage around HEPA filters was found, but not enough to explain the poor results. Computerized fluid dynamic (CFD) studies failed to suggest competing interactions between the room air cleaners and the mechanical ventilation system of the AIR facility. As noted, a repeat study confirmed the poor results. Long experience with a variety of room air cleaners of many designs has convinced air disinfection experts that they are not a practical solution for most hospital and clinic applications. They may be occasionally useful in small rooms with ceilings too low (<8 m) for upper room UVGI. Where used, CADR should produce at least 6–12 Eq ACH, depending on their application.
RESPIRATORY PROTECTION,
RESPIRATORS AND SURGICAL MASKS
By recent convention in the medical literature, face masks refer to surgical masks designed to protect the surgical field. Similarlooking face coverings to protect healthcare workers are called
Книга в списке рекомендаций к покупке и прочтению сайта https://meduniver.com/

References 109
respirators. Surgical face masks stop large particles that exit the mouth or nose and can be loose fitting. For TB control they are often worn by potentially infectious patients to reduce the generation of airborne infectious droplet nuclei. This application of surgical masks is a form cough hygiene, like covering the nose or mouth with a hand or tissue. We tested the effectiveness of simple surgical masks for reducing Mtb transmission using the human- to-guinea-pig transmission test facility and found 56% efficacy under study conditions.33 We did not test tighter fitting respirators for the purpose because they are too expensive for simple barrier use and are unlikely to be substantially more effective. Leakage around surgical masks with coughing is the major limitation and respirators are unlikely to resist the force of a cough. Surgical masks are also sometimes used to protect wearers from predominantly droplet spread infections, such as influenza—serving as a simple reminder not to touch your mouth with contaminated fingers, but they offer the wearer little protection from truly airborne infections such as Mtb.
For protecting healthcare workers from Mtb and other airborne infections, respirators rather than masks are used.73–76 Respirators can be disposable, intended for limited re-use, or reusable “elastomeric” respirators with replaceable filters, primarily used in industry—although they can be economical compared to disposable masks for healthcare applications. Respirators differ from surgical masks by having a tight fit, usually designed with two elastic straps rather than one, and a design intended to create a seal to the face without gaps. Unlike surgical masks, the face-seal is critical in protecting the wearer from infection. People have a variety of face shapes that cannot be adequately fitted with any one style or size of respirator. Because face-seal leak is the major cause of respiratory protection failure, fit testing of respirators prior to selection for each wearer is required—using a distinctive volatile test substance (e.g., bitter tasting material, or banana oil) within a hood. Commercial respirator fit-testing kits are available, and a respirator fit-testing program can easily be implemented in high burden settings.75 Given the high cost of disposable respirators and the danger of TB infection, an effective respiratory program must include fit testing and the availability of a variety of respirator models and sizes to assure adequate protection.
The major limitation of respiratory protection, apart from face-seal leak, is that they cannot be worn all day due to discomfort and may not be used when sharing air with a patient with unsuspected TB. They are essential for high-risk procedures such as bronchoscopy, autopsy, sputum induction, and other cough aerosol-generating procedures. They are far less important in caring for patients already started on effective therapy and rendered rapidly non-infectious, as noted above. Because of these limitations, respiratory protection is considered a third priority in the traditional hierarchy of airborne transmission controls—admin- istrative, environmental (engineering), and respiratory protection. In this chapter we have prioritized FAST as a refocused, intensified approach to administrative TB transmission control, and natural ventilation as well as upper room GUV as environmental controls. There are many more comprehensive guidelines with additional details, as listed below.
MORE COMPREHENSIVE, EVIDENCE-
BASED GUIDELINES
A revised WHO TB transmission control policy has recently been issued, and an accompanying implementation guide is being developed.76 The policy document is limited by recommendations based on (scarce) hard evidence supplemented by expert opinion, whereas the implementation guide is less constrained, based on experience, with additional practical application advice.
We have presented some of experimental evidence favoring FAST and GUV, for example, but demonstrating the efficacy of most accepted interventions for preventing Mtb infection or disease in high burden settings has proven difficult. Sources of Mtb infection are common in the community; tuberculin reactivity is common among healthcare workers and in the community and confounded by BCG immunization. Newer gamma interferon release assays were designed to circumvent BCG confounding, but are expensive, complex tests, with limited routine application thus far in high burden settings.77 Mtb contamination of air cannot be routinely measured, and quantifying infectiousness is even harder. Unlike drug trials, randomized-controlled clinical trials for TB prevention strategies are rare. In short, most interventions to prevent TB transmission in healthcare facilities are not supported by the highest level of data showing reduced infection or disease.
TREATMENT OF LATENT TB
IN HEALTHCARE WORKERS
The treatment of latent infection is discussed elsewhere in this book. Healthcare workers are at increased risk of transmission and disease progression and should be considered for chemopro- phylaxis—although this is uncommonly done in high burden settings as of this writing. It is done in low burden settings, ideally in response to true test conversions reflecting recent infection. Currently, treatment is sometimes offered in response to small changes in tuberculin skin test responses and IGRA results that may not reflect true, sustained infection.
REFERENCES
1.Behr MA, Edelstein PH, and Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ. 2018;362:k2738.
2.Yates TA et al. The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect Dis. 2016;16:227–38.
3.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49:1–51.
4.Nardell EA, Keegan J, Cheney SA, and Etkind SC. Airborne infection. Theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144:302–6.
5.Willingham FF et al. Hospital control and multidrug-resistant pulmonary tuberculosis in female patients, Lima, Peru. Emerg Infect Dis. 2001;7:123–7.
