
- •Ministry of Education and Science of Ukraine National Technical University of Ukraine "kpi"
- •1. Electrostatic field
- •1.1. Electrostatic field. Electric charge and its properties
- •1.5. Connection between intensity and potential.
- •§2. Description of the vector field
- •2.6. Stokes' theorem
- •§ 3. Stress Evaluation of Field according to Gauss' theorem
- •3.1. Impossibility of stable equilibrium of charge in an electric field
- •3.3 The field of two parallel charged planes
- •§4. Dielectric in external electric field
- •§5. Conductor in external electrostatic field
- •5.4. Connecting capacitors
- •§6. Energy of the electrostatic field
- •§7. Direct electric current and its characteristics.
- •§ 8. Classical electronic theory of electrical conductivity of metals
- •8.3. Ohm's law, Joule-Lenz, Wiedemann-Franz, their consideration on the basis of the theory of Drude-Lorentz
- •§10. Electric current in liquids
- •§11. Electric current in gases
§10. Electric current in liquids
10.1. Electrolytes. Electrolytic dissociation
According to the chemical composition we distinguish one-component or pure, liquids and two- or multicomponent liquid mixtures (solrents). For the electrical conductivity of the substance in the liquid state they are divided into conductors (rare metals, some semimetals and semiconductors, after melting they have electronic conductivity); semiconductors (after melting some semiconductors retain some special characteristics: have electronic or hole conductivity; is sensitive to light and temperature changes); dielectrics (mobile charge carriers in them can be electrons and ions, but their concentration is extremely small and is caused by impurities). Clear fluids are mainly dielectrics (oil products, alcohol, etc.). This is due to the fact that pure liquids consist of neutral atoms or molecules; their charges are bound in atoms and therefore are not able to create the electric current participate in a creating of a current.

fig.10.1
Among two- or multicomponent liquid mixtures the electrolytes are defined. In the broadest sense they are substances that have ionic conductivity mechanism, they are often called conductors of the second kind. The most typical representatives of electrolytes are aqueous solvents of inorganic acids (НС1, Н25О4, НМО3), alkalies (КаОН, КОН, Са(ОН)2), salts солей (КаСІ, Сu5О4). Instead of water the solvents may be also alcohol or inorganic liquids (hexane, dioxane, benzene, etc.). These solvents of salts, acids, alkalis also have ionic conductivity, but their conductivity is much less than the electrical conductivity of aqueous electrolytes. Note that not all substances of the aqueous solutions are electrolytes. For example, a solvent of sugar in water is not an electrolyte and does not conduct electricity.
The arranged movement of ions (current) in electrolytes takes place under the effect of an electric field that is created by a current source connected to the electrodes immerse into the electrolyte.
What processes evoke the formation of charge carriers in the electrolytes (ions)? Modern physical theory of electrolytes conductivity explain it by the fact that the molecules of the kissolred substance in the solvent on components , which during dissolution acquire opposite or negative charges, so that they became positive and negative ions. The phenomenon of dissolved substances on the opposite charged ions under the effect of the solvent is called electrolytic dissociation.
The process of disintegration of molecules into individual ions can be represented as follows. Around each of the ions of the solute, such NaC1, it is oriented the polar molecules of the solvent (water). The positively charged sodium ions attract the negative pole of the dipole water molecules. However, they repel the negative chloride ions. A similar pattern will occur for chloride ions (fig. 10.1). The process of interaction of ions with dipolar molecules of the solvent is called solvation. This process weakens the bonds between the ions of sodium and chlorine. Due to the thermal motion of molecules some breaks are possible with the formation of sodium and chlorine. With the next increase in the distance between the ion strength of the Coulomb interaction decreases in ε times (ε - dielectric constant of the solvent). Solute particles interact with solvent molecules, forming complexes - solvates (for aqueous solutions - hydrates).
At the approaching of the positive and negative ions, they can connect (recombined) and form a neutral molecule. Other neutral molecules, in contrast, can dissociate into ions. As a result, it is a dynamic equilibrium of dissociation and recombination processes; and in this equilibrium the statistical fraction of dissociated molecules remains unchanged on average over time. Creator of the theory of electrolytic dissociation is a Swedish physical chemist S. Arrhenius (1859 - 1927).
When web creat an electric field lowering in the electrolyte the metal electrodes connected to the positive and negative poles of the power source then there is a moving of positive ions (cations) to the cathode and the negative ions (anions) in the opposite direction - to the anode. In this way an electric current appears. Thus there is a separation and release of ions formed (decay products of solute) on the electrodes. This is known as electrolysis.
As
mentioned, the ability of molecules of the solute to dissociate in
different solvents varies. For quantitative characteristics of the
dissociation it’s injected the coefficient of dissociation α,
which is determined by the ratio of the number of dissociated
molecules of solute to the total number of them. Suppose that for a
given volume of solution is
 solute molecules, of whichn
was dissociated. The
coefficient of dissociation
solute molecules, of whichn
was dissociated. The
coefficient of dissociation
 .
(10.1)
.
(10.1)
For
α=1
dissociation will be complete, and with α=0 it will be absent. If
α 1,such
electrolities are calledstrong
and when α
1,such
electrolities are calledstrong
and when α 0they
are weak.
0they
are weak.
The theory of strong electrolities was developed by the German scientists P. Debye (1884 - 1966) and E. Hyukkel (1896 - 1980).
Depending
on quantity of various ions which are formed in solution,
electrolytes can be
binary
(КС1, NaОН,
НN3),
ternary
(СаС12,
Н2SО4,
SrСl2)
etc.
For simplification we will consider binary electrolytes. Generally
in unit of volume of not dissociated molecules will be
 . Obviously, quantity of molecules which disintegrate in unit of time
in unit of volume
. Obviously, quantity of molecules which disintegrate in unit of time
in unit of volume
 ,
it is proportional quantity of not dissociated molecules, so
,
it is proportional quantity of not dissociated molecules, so
 ,
,
Where A- coefficient of proportionality which depends on nature of electrolyte and its temperature.
Number of
acts of recombination
 in unit of volume for the unit of time is proportional to the
quantity of the positive
in unit of volume for the unit of time is proportional to the
quantity of the positive and negative ions
and negative ions ,
containing in unit of volume of binary electrolyte,
,
containing in unit of volume of binary electrolyte,
 ,
,
Where B -
coefficient of proportionality . In a state of dynamic balance
between both processes
 ,
or
,
or
 .
(10.2)
.
(10.2)
From here we have
 (10.3)
(10.3)
Formula
(10.3) expresses the Oswald
law. Relation А / В is called a
constant of balance or a constant of dissociation.
It follows from Oswald's law that for enough diluted solutions( )
the
dissociation coefficient
)
the
dissociation coefficient
 , so that all molecules of the dissolved substance dissociate. The
Oswald’s
law is well carried out for weak electrolytes, that is such for which
degree of dissociation is small. For strong electrolytes (almost all
molecules of solvent dissociate) Oswald's law for the concentrated
solutions is badly agreed with the results of experiments, except for
strongly diluted solutions, for which valid is
, so that all molecules of the dissolved substance dissociate. The
Oswald’s
law is well carried out for weak electrolytes, that is such for which
degree of dissociation is small. For strong electrolytes (almost all
molecules of solvent dissociate) Oswald's law for the concentrated
solutions is badly agreed with the results of experiments, except for
strongly diluted solutions, for which valid is
 .According
to the modern theory of solutions is consider that for strong
electrolytes actually all molecules of solvent are dissociated, and
deviations from Oswald's law can be explained by interaction of ions
among themselves and with solution molecules.
.According
to the modern theory of solutions is consider that for strong
electrolytes actually all molecules of solvent are dissociated, and
deviations from Oswald's law can be explained by interaction of ions
among themselves and with solution molecules.
10.2. Electrolysis. Faraday laws .
Electrolysis is the phenomenon of allocation of substance on electrodes that occurs while direct electric current passes through electrolyte. Thus on the cathode there is a reaction of restoration of cations. It is connected with joining of electrons to cations. There are oxygenating reactions on the anode connected with loss of an electron by anions.
The phenomenon of electrolysis quantitatively was studied by Michael Faraday. He first made the electrolysis products division into primary and secondary. In 1833 Michael Faraday through established two laws of electrolysis. Which is named after him.
The first law of Faraday formulated as: weight t recho¬vyny allocated to each of the electrodes is directly proportional zarya¬dovi q, which passed through the electrolyte, ie
 (10.4)
(10.4)
where k - electrochemical equivalent,is not the same for different substances. It is numerically equal to the mass of substance that is released during electrolysis when passes the constant electric current I during the time t, then q = 1 C. If through the electrolyte passes the constant electric current I during the time t, then q = It and equation (10.4) is written in the form
 .
(10.5)
.
(10.5)
If the current strength changes over time, the
 .
.
The second Faraday's law indicates that the electrochemical equivalent of a substance is directly proportional to their chemical equivalents:
 (10.6)
(10.6)
where C - the proportionality, which has the same value for all substances. Chemical equivalent of x is called the ratio of atomic mass A to the valence z of the substance. Instead of constant C using inverted to it value, which is called the Faraday number and denote F = 1 / C. Then equation (10.6) is rewritten as follows:
 .
(10.7)
.
(10.7)
On the basis of formulas (10.4) and (10.7) we receive general Faraday's law
 (10.8)
(10.8)
or
 .
(10.9)
.
(10.9)
10.3. Electrical conductivity of electrolytes
If there is
no external electric field, the ions in electrolyte are in a random
thermal motion. Since all the directions of thermal motion are
equally likely, the dominant direction of transport of ions doesn’t
exist, so that there will be any current intensity. If there is an
electric field, the ions are under force of
 ,
so
they can acquire more speed. It should be noted that the ions do not
move themselves but solvates – the ions surrounded by neutral polar
solvent molecules. In this way, the moving is of complexes. Positive
ions acquire an additional speed in the direction of intensity
electric field and the negative - in the opposite direction. Directed
movement of positive and negative ions under the influence of
external electric field creates an electric current. Current density
,
so
they can acquire more speed. It should be noted that the ions do not
move themselves but solvates – the ions surrounded by neutral polar
solvent molecules. In this way, the moving is of complexes. Positive
ions acquire an additional speed in the direction of intensity
electric field and the negative - in the opposite direction. Directed
movement of positive and negative ions under the influence of
external electric field creates an electric current. Current density
 in electrolyte consists of current density
in electrolyte consists of current density
 caused
by the directed movement of positive ions and the negative current
density of mobile ions
caused
by the directed movement of positive ions and the negative current
density of mobile ions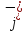 ,
so that
,
so that
 (10.10)
(10.10)
For
simplicity of considering the phenomenon of conductivity electrolytes
let’s think that the concentration of positive ions
 is
the concentration of negative ions
is
the concentration of negative ions ,
so
,
so =
= =
n. Then charge
=
n. Then charge of
positive ion is the charge q- of negative ion, so that
of
positive ion is the charge q- of negative ion, so that =
= = q. Since in electrolytes, as well as in metals, the concentration
of charge carriers don’t depend on the presence of an external
electric field, and the total charge of positive and negative charges
in any volume of electrolyte will be zero, then we can write
= q. Since in electrolytes, as well as in metals, the concentration
of charge carriers don’t depend on the presence of an external
electric field, and the total charge of positive and negative charges
in any volume of electrolyte will be zero, then we can write

 (10.11)
(10.11)
where
 and
and - velocity of the ordered movement under the influence of external
field respectively to the positive and negative ions (solvates).
- velocity of the ordered movement under the influence of external
field respectively to the positive and negative ions (solvates).
During the
ordered motion the ions in electrolytes undergo numerous impactions
with other molecules. They are under the influence of viscous
friction, which have the same origin as the motion of macroscopic
bodies in liquids or gases. From mechanics we know that the viscous
friction force experienced by the body during motion in liquids or
gases at low velocity is proportional to the velocity. Therefore, the
positive and negative ions in their directed motion shall act in
conformity viscous friction force
 and
and
 (
(

 -
friction coefficients for positive and negative ions). Then the
dynamic equations for the directed movement of ions will have such
expression
-
friction coefficients for positive and negative ions). Then the
dynamic equations for the directed movement of ions will have such
expression

 (10.12)
(10.12)
where
 ,
, - acceleration respectively to positive and negative ions.
- acceleration respectively to positive and negative ions.
The
experiments show that when there is the absence of polarization of
electrodes (low current density) electric current strenght during
electrolysis does not change. This fact proves that at certain speeds
of directional movement of ions the friction is balanced with
strength of the electric field, so that the acceleration of ions are
equal to zero ( =
= = 0). Then from equation (10.12) we obtain:
= 0). Then from equation (10.12) we obtain:
 =
=
 =
= (10.13)
(10.13)
where
the value of
 = q / k + and
= q / k + and = q /
= q / is called the mobility of positive and negative ions. The equation
(10.13) shows that the ion mobility is numerously equal to the rate
of directional movement with strength of electric field E = 1 V / m.
On the basis of formulas (10.11) and (10.13) expression (10.10) takes
the form
is called the mobility of positive and negative ions. The equation
(10.13) shows that the ion mobility is numerously equal to the rate
of directional movement with strength of electric field E = 1 V / m.
On the basis of formulas (10.11) and (10.13) expression (10.10) takes
the form
 =qn(
=qn( (10.14)
(10.14)
If the ratio of dissociation is α, then n = αn0 (n0 - concentration of solute) and expression (10.14) can be rewritten as follows:
 =qαn(
=qαn( (10.15)
(10.15)
Equation (10.15) expresses Ohm's law in differential form for electrolytes. The electrical conductivity of the electrolyte
 (10.16)
(10.16)
Let us introduce the concept of equivalent concentration η, which refers to an amount equal to the number of moles of solute that is per unit of volume (1 m3) solution. Since n0 is the number of solute molecules per unit volume, and N А is the number of molecules in a mole, then η = n0/ N А. Let us write qn0 product through N А:
 .
.
Since qN А = F, а n0 / N А =η, , then formula (10.16) will take a look like
 .
(10.17)
.
(10.17)
The ratio of conductivity σ to an equivalent concentration of solute is called the equivalent electrical conductivity:
 .
(10.18)
.
(10.18)
For
infinitely diluted solution ( )
equivalent conductivity
)
equivalent conductivity
 .
(10.19)
.
(10.19)
When from (10.18) and (10.19) we have
 .
(10.20)
.
(10.20)
By
measuring the conductivity equivalent to the formula (10.20), we can
calculate the rate of dissociation. With the help of the formulas
(10.18) and (10.19) we are able to find the sum of ion mobility
 .
.
At
low concentration of the solution the dissociation rate is constant
value. Total mobility
 under
this condition also remains approximately constant magnitude.
under
this condition also remains approximately constant magnitude.
Thus, at low concentration the electrical conductivity is proportional to the concentration.
Concentration dependence of conductivity at high concentrations of the solution is much more complicated. Here we must take into account the overdependence of the coefficient of dissociation and ion mobility from concentration. In concentrated solutions ion mobility is reduced due to the electrical interaction of ions with one another. Therefore, at great concentration of the solution the direct proportionality between electrical conductivity and concentration of the solution is not observed. With a temperature increasing the dissociation rate also increases since a more intensive movement of molecules prevents from molecule formation from ions and facilitates the dissociation of molecules. During the heat solution process its viscosity decreases, so that the mobility of ions lifts. Therefore, the conductivity of the electrolyte increases at high temperatures.
At
high electric field strength ( V
/ m) it is observed a deviation from Ohm's law and the dependence
V
/ m) it is observed a deviation from Ohm's law and the dependence is of nonlinear character.
is of nonlinear character.
10.4. Electrochemical potentials
Experiments show that there is a mutual electrification when the metals are immersed in an electrolyte. The metal and electrolyte acquire the charges of opposite signs. With that the metal will have some potential relatively to the electrolyte, which is called electrochemical.
The orogin of electrochemical potentials was explained by German physicist W. Nernst (1864-1941). For quality clarifying of this issue, consider the metal is immersed in an aqueous solution of the salt of the same metal, such as zinc in a solution of zink sulfate ZnSO4. Water molecules have a large dipole moment; they surround the positively charged ions of zinc metal surface and cleave them. Zinc ions, which will pass from the electrode into the solution, have no difference from zinc ions arising from the dissociation of ZnSO4. Along with the process of dissolution of zinc occur the reverse process in which zinc ions due to thermal motion deposit on the zinc electrode. After a certain period of time, these processes are balanced.
In this case zinc acquires a negative charge and the electrolyte – positive one. At the fringe of zinc and electrolyte a thin surface layer of charges of opposite sign is formed, called the electric double layer. In it an electric field appears which intensity is directed from the electrolyte to the metal. This field counteracts the positive transition of zinc ions in the electrolyte. The same thing happens when zinc electrode is immersed in water. When metal is immersed in its metal salt solution it is not always charged negatively. Thus, when copper stem is immersed in a solution of a copper sulfate CuSO4, the copper ions are deposited on copper. Consequently, the copper is charged positively and the electrolyte - negatively. So, when metal is immersed in water or metal aqueous solution, in which there are the ions of the same metal, on the verge of the metal solution arises an electric double layer, i.e. there is the potential difference between the metal and the electrolyte, which value depends on the concentration of the metal ions in solution and on nature of the metal.
To measure the potential difference between the metal electrode and the electrolyte, it is necessary to connect it to a voltmeter, and the second terminal of the voltmeter to connect to the electrolyte through another electrode. Then between the electrolyte and the auxiliary electrode arise also a potential difference and the voltmeter will not measure already the potential difference between the metal electrode and the electrolyte but the potential difference between two different electrode immersed in the same electrolyte. The potential difference between two different metal electrodes depends on their nature and concentration of the electrolyte solution. For ease of calculation electromotive forces of galvanic current sources in practice it is determined the potential of any metal electrode with respect to the so-called normal hydrogen electrode, which is taken as standard. This electrode is made of platinum coated by electrolysis with atomized platinic and immersed in a solution containing hydrogen ions at normal concentration. Recall that the concentration of the solution is called normal if 1 m3 of solution contains of 1 kmol of hydrogen ions.
Electrochemical potential of the electrode in a solution of its salt with a normal concentration of ions measured relatively to hydrogen electrode, called normal electrochemical potential.
Lection 1
Section 8. Electric current in gases
