
- •Contents
- •1.1 Introduction
- •1.2 Selection of dental materials
- •1.3 Evaluation of materials
- •2.1 Introduction
- •2.2 Mechanical properties
- •2.3 Rheological properties
- •2.4 Thermal properties
- •2.5 Adhesion
- •2.6 Miscellaneous physical properties
- •2.7 Chemical properties
- •2.8 Biological properties
- •2.9 Suggested further reading
- •3.1 Introduction
- •3.2 Requirements of dental cast materials
- •3.3 Composition
- •3.4 Manipulation and setting characteristics
- •3.5 Properties of the set material
- •3.6 Applications
- •3.7 Advantages and disadvantages
- •3.8 Suggested further reading
- •4.1 Introduction
- •4.2 Requirements of wax-pattern materials
- •4.3 Composition of waxes
- •4.4 Properties of dental waxes
- •4.5 Applications
- •4.6 Suggested further reading
- •5.1 Introduction
- •5.2 Requirements of investments for alloy casting procedures
- •5.3 Available materials
- •5.4 Properties of investment materials
- •5.5 Applications
- •5.6 Suggested further reading
- •6.1 Introduction
- •6.2 Structure and properties of metals
- •6.3 Structure and properties of alloys
- •6.4 Cooling curves
- •6.5 Phase diagrams
- •6.6 Suggested further reading
- •7.1 Introduction
- •7.2 Pure gold fillings (cohesive gold)
- •7.3 Traditional casting gold alloys
- •7.4 Hardening heat treatments (theoretical considerations)
- •7.5 Heat treatments (practical considerations)
- •7.6 Alloys with noble metal content of at least 25% but less than 75%
- •7.7 Soldering and brazing materials for noble metals
- •7.8 Noble alloys for metal-bonded ceramic restorations
- •7.9 Biocompatibility
- •7.10 Suggested further reading
- •8.1 Introduction
- •8.2 Composition
- •8.3 Manipulation of base metal casting alloys
- •8.4 Properties
- •8.5 Comparison with casting gold alloys
- •8.6 Biocompatibility
- •8.7 Metals and alloys for implants
- •8.8 Suggested further reading
- •9.1 Introduction
- •9.2 Investment mould
- •9.3 Casting machines
- •9.4 Faults in castings
- •9.5 Suggested further reading
- •10.1 Introduction
- •10.2 Steel
- •10.3 Stainless steel
- •10.4 Stainless steel denture bases
- •10.5 Wires
- •10.6 Suggested further reading
- •11.1 Introduction
- •11.2 Composition of traditional dental porcelain
- •11.3 Compaction and firing
- •11.4 Properties of porcelain
- •11.5 Alumina inserts and aluminous porcelain
- •11.6 Sintered alumina core ceramics
- •11.7 Injection moulded and pressed ceramics
- •11.8 Cast glass and polycrystalline ceramics
- •11.9 CAD–CAM restorations
- •11.10 Porcelain veneers
- •11.11 Porcelain fused to metal (PFM)
- •11.12 Capillary technology
- •11.13 Bonded platinum foil
- •11.14 Suggested further reading
- •12.1 Introduction
- •12.2 Polymerisation
- •12.3 Physical changes occurring during polymerisation
- •12.4 Structure and properties
- •12.5 Methods of fabricating polymers
- •12.6 Suggested further reading
- •13.1 Introduction
- •13.2 Requirements of denture base polymers
- •13.3 Acrylic denture base materials
- •13.4 Modified acrylic materials
- •13.5 Alternative polymers
- •13.6 Suggested further reading
- •14.1 Introduction
- •14.2 Hard reline materials
- •14.3 Tissue conditioners
- •14.4 Temporary soft lining materials
- •14.5 Permanent soft lining materials
- •14.6 Self-administered relining materials
- •14.7 Suggested further reading
- •15.1 Introduction
- •15.2 Requirements
- •15.3 Available materials
- •15.4 Properties
- •15.5 Suggested further reading
- •16.1 Introduction
- •16.2 Classification of impression materials
- •16.3 Requirements
- •16.4 Clinical considerations
- •16.5 Suggested further reading
- •17.1 Introduction
- •17.2 Impression plaster
- •17.3 Impression compound
- •17.4 Impression waxes
- •18.1 Introduction
- •18.2 Reversible hydrocolloids (agar)
- •18.3 Irreversible hydrocolloids (alginates)
- •18.5 Modified alginates
- •18.6 Suggested further reading
- •19.1 Introduction
- •19.2 Polysulphides
- •19.3 Silicone rubbers (condensation curing)
- •19.4 Silicone rubbers (addition curing)
- •19.5 Polyethers
- •19.6 Comparison of the properties of elastomers
- •19.7 Suggested further reading
- •20.1 Introduction
- •20.2 Appearance
- •20.3 Rheological properties and setting characteristics
- •20.4 Chemical properties
- •20.5 Thermal properties
- •20.6 Mechanical properties
- •20.7 Adhesion
- •20.8 Biological properties
- •20.9 Historical
- •21.1 Introduction
- •21.2 Composition
- •21.3 Setting reactions
- •21.4 Properties
- •21.6 Manipulative variables
- •21.7 Suggested further reading
- •22.1 Introduction
- •22.2 Acrylic resins
- •22.3 Composite materials – introduction
- •22.4 Classification and composition of composites
- •22.5 Properties of composites
- •22.6 Fibre reinforcement of composite structures
- •22.7 Clinical handling notes for composites
- •22.8 Applications of composites
- •22.9 Suggested further reading
- •23.1 Introduction
- •23.2 Acid-etch systems for bonding to enamel
- •23.3 Applications of the acid-etch technique
- •23.4 Bonding to dentine – background
- •23.5 Dentine conditioning – the smear layer
- •23.6 Priming and bonding
- •23.7 Current concepts in dentine bonding – the hybrid layer
- •23.8 Classification of dentine bonding systems
- •23.9 Bonding to alloys, amalgam and ceramics
- •23.10 Bond strength and leakage measurements
- •23.11 Polymerizable luting agents
- •23.12 Suggested further reading
- •24.1 Introduction
- •24.2 Composition
- •24.3 Setting reaction
- •24.4 Properties
- •24.5 Cermets
- •24.6 Applications and clinical handling notes
- •24.7 Suggested further reading
- •25.1 Introduction
- •25.2 Composition and classification
- •25.3 Setting characteristics
- •25.4 Dimensional change and dimensional stability
- •25.5 Mechanical properties
- •25.6 Adhesive characteristics
- •25.7 Fluoride release
- •25.8 Clinical handling notes
- •25.9 Suggested further reading
- •26.1 Introduction
- •26.2 Requirements
- •26.3 Available materials
- •26.4 Properties
- •27.1 Introduction
- •27.2 Requirements of cavity lining materials
- •27.3 Requirements of Iuting materials
- •27.4 Requirements of endodontic cements
- •27.5 Requirements of orthodontic cements
- •27.6 Suggested further reading
- •28.1 Introduction
- •28.2 Zinc phosphate cements
- •28.3 Silicophosphate cements
- •28.4 Copper cements
- •28.5 Suggested further reading
- •29.1 Introduction
- •29.2 Zinc oxide/eugenol cements
- •29.3 Ortho-ethoxybenzoic acid (EBA) cements
- •29.4 Calcium hydroxide cements
- •29.5 Suggested further reading
- •30.1 Introduction
- •30.2 Polycarboxylate cements
- •30.3 Glass ionomer cements
- •30.4 Resin-modified glass ionomers and compomers
- •30.5 Suggested further reading
- •31.1 Introduction
- •31.2 Irrigants and lubricants
- •31.3 Intra-canal medicaments
- •31.4 Endodontic obturation materials
- •31.5 Historical materials
- •31.6 Contemporary materials
- •31.7 Clinical handling
- •31.8 Suggested further reading
- •Appendix 1
- •Index

242 Chapter 23
tend to be greater than those measured using more conventional methods.
Problems with the test can be a concern over stress development during sectioning of specimens which can lead to premature failures and uncertainties over the interpretation of results since several test specimens may be derived from a relatively small number of teeth.
Leakage studies are designed to give an indication of the ability of a material to form an effective seal against fluids and bacteria at the tooth/adhesive interface. Tests are normally performed by placing restorations in restored teeth and then subjecting them to storage, normally with an element of thermal cycling, in a solution of dyestuff or other marker (e.g. radioisotope). At the end of the prescribed period of testing the restoration and tooth are sectioned and the effectiveness of the seal is judged by how far the dye (or other marker) has penetrated down the margin towards the cavity floor. Guidelines for leakage testing are also included in ISO TR 11405.
23.11 Polymerizable luting agents
Resin-based luting agents are being used increasingly in association with adhesive dentine bonding agents to enhance the retention of prostheses to tooth tissue. These lutes tend to be chemically setting unless they are being used with porcelain veneers, as light penetration through either all ceramic crowns or ceramic or resin inlays is poor and for obvious reasons light activated materials cannot be used with metallic restorations. They can be used for all forms of restoration but to achieve maximum benefits there must be mechanisms for attachment between the fitting surface of the restoration and the surface of the preparation on the tooth. The latter is relatively straightforward when the preparation is wholly on tooth tissue (either dentine or enamel) when contemporary dentine bonding agents can be used. The lightly-filled diacrylate resin luting agents must be used with total-etch dentine bonding agents rather than self-etching systems. The residual acidity of the latter interferes with the polymerisation of chemically curing resin systems. However when the surface of the ‘tooth’ preparation is predominantly on some form of core material there are greater problems achieving a bond between a resin lute and the surface of the prepared tooth. Amalgam is difficult to bond to with anything
other than the chemically active lute. A gold post and core is even more difficult in terms of adhesion, whereas a composite resin is more receptive to bonding with a resin lute. However all of the difficulties that are discussed in Section 22.7 concerning bonding between ‘old’ prepared composite and newly polymerised resin remain.
Polymerisable lutes are available based on both composite resins and resin-modified glass ionomer cement (RMGIC) technology (see Chapter 25). Obviously the latter offer the theoretical benefit of fluoride release at the margin but there is no clinical evidence to substantiate any benefit for their use. The RMGIC lutes tend to be used with relatively retentive tooth preparations unlike the composite-based materials which are now often used to attach onlays and overlays with minimal if any retentive capacity, as well as ceramic veneers, all ceramic crowns and adhesive bridgework.
The composite based lutes come in two forms; those that are essentially dilute or lightly filled composite resins and those that have some form of intrinsic adhesive capacity due to the resin molecules in the lute itself (see also section 23.9). The technology required to retain restorations with these differing materials is separate and distinct.
Lightly-filled diacrylate resins
These materials can be regarded as lightly filled composite resins with both low filler loading and small particle size fillers to facilitate the formation of thin films of lute. They are available in both chemical and light initiated versions, but the most common form are so-called ‘dual-cure’ materials. These products can either be activated by light alone or when the light-activated paste is mixed with a second paste containing a chemical catalyst where there is a dual cure mechanism. This approach is intended to give the benefits of command set at the periphery of translucent restorations (for example a porcelain veneer) whilst ensuring that some degree of cure occurs when a translucent restoration is sufficiently thick that it attenuates the light from a curing source sufficiently to prevent light initiated activation of the setting reaction. This approach is necessary, for example, when luting a ceramic or composite resin inlay or onlay, when either the thickness of the inlay/onlay is sufficiently great or there may be shadowing effects from the residual tooth
Adhesive Restorative Materials: Bonding of Resin-based Materials |
243 |
|
|
structure or from adjacent teeth to prevent effective light activation. Such resins have a limited ability to form adhesive bonds to restorations.
Attachment strengths to prefabricated composite resin structures (for example a composite resin inlay, see section 22.8) are surprisingly low. This has been attributed to the relatively high conversion rate that can be achieved in resin materials in a laboratory setting where curing can be undertaken either at increased temperature or under pressure, or both.
They have no intrinsic capacity to bond to ceramics. The fitting surface of the ceramic has to be roughened, usually by etching with hydrofluoric acid and then coated with a silane coupling agent before an adequate bond strength can be achieved. There is some controversy in the literature whether the silane is best applied in the production laboratory or at the chairside. In practical terms it is easiest to apply the silane at the chairside having ensured that the ceramic restoration has an appropriate quality of fit to the underlying prepared tooth. This approach can only be used when the fitting surface of the ceramic is susceptible to etching with hydrofluoric acid. Some of the high strength ceramics used for crowns cannot
be treated in this way, particularly the glass infused material InCeram® (section 11.6).
These resins have no intrinsic capacity to bond to metals, but once again a coupling agent can be used or the metal surface roughened in some way to provide mechanical interlocking of the resin to metal (see also section 23.9).
Chemically active resins (see also section 23.9)
Chemically active resin lutes contain organic groups which have intrinsic adhesive activity. This chemical activity principally works with metal substrates and application of some form of ceramic primer is required for use with porcelain. There are two types of chemically active materials:
(1)Phosphorylated materials: (see Fig. 23.5 and Fig. 23.11) these materials contain phosphorylated resins, for example phenyl P (2-meth- acryloxyethyl phenyl hydrogen phosphate) and MDP (10-methacryloxydecyl dihydrogen phosphate), which are intrinsically acidic and have the potential to provide some chemical interaction with both tooth substance and with metal surfaces, particularly those
|
|
Fig. 23.22 Adhesive techniques for |
|
|
|
the management of fractured and |
|
(b) |
(c) |
worn teeth. A metal ceramic |
|
addition (a) bonded to a non- |
|||
|
|
||
|
|
retentive cavity (b) using an |
|
|
|
adhesive luting agent to restore the |
|
|
|
tooth (c). From Walls, A.W.G. |
|
|
|
(2001) in Advances in Operative |
|
|
|
Dentistry, eds Roulet, J.-F., Wilson, |
|
(a) |
|
N.H.F. & Fuzzi, M., pp. 229–239. |
|
|
Quintessence Publishing. |

244 Chapter 23
with a stable oxide layer on the surface (for example stainless steel and non-precious metal casting alloys). Bonding to metals may be enhanced by roughening the surface by sandblasting and by applying proprietary priming agents. The Panavia range of materials is particularly susceptible to oxygen inhibition and is provided with a gel barrier to facilitate full curing of the material. These materials are available as chemical set alone or in dual cured forms and some also require the use of a dentine bonding system to optimize attachment to tooth tissue. However they also have some activity as self-etching primers to enamel and dentine.
(2)4-META products: The resin 4-methacrylo- loxyethyl trimellitate anhydride (4-META) also exhibits chemical reactivity with tooth and with metal oxide layers. Its incorporation into a resin lute again facilitates bonding but there are some concerns about the chemical reactivity of this agent (see Fig. 23.10).
It is undoubted that these resin lutes can result in substantial bonds being developed between the lute and both tooth surface and a restoration being bonded in place. Their development has allowed a new era of restorative dentistry where mechanical retention is not necessarily a pre-req- uisite for long-term success (Fig. 23.22) and conservation of tooth tissue becomes the overriding feature of preparation design and clinical practice. They are not however a panacea for all problems.
There must be surfaces that can be bonded on both the ‘tooth’ and the crown/restoration and it must be possible to achieve an adequate standard of moisture control for adhesively retained restorations to be a predictable part of clinical care. Contamination of an etched tooth surface with saliva or crevicular fluid will prevent bond formation and facilitate marginal leakage as a consequence. Furthermore they are methacrylate-based products and undergo substantial shrinkage during setting. Whilst the lute space should be relatively small in most circumstances the C factor is very large as the only free surface is at the margin of the restoration. This may not be a problem for extra-coronal restorations and those with limited mechanical retention. It is however a problem with inlay type restorations where considerable strain continues to be applied in tensile mode to the remaining tooth structure.
23.12 Suggested further reading
Eick, J.D., Gwinnett, A.J. Pashley, D.H. & Robinson, S.J. (1997) Current concepts in adhesion to dentine.
Grit. Rev. Oral Biol. Med. 8, 306.
ISO TR 11405 Dental Materials – Guidance on Testing of Adhesion to Tooth Structure.
Jordan, R.E. (ed.) (1991) Eight articles on bonding to various substrates. J. Esthet. Dent. 3, 117.
Swift, E.J., Perdigao, J. & Heymann, H.O. (1995). Bonding to enamel and dentine: a brief history and state of the art. Quint. Int. 26, 95.
Van Meerbeck, B. et al. (1998) The clinical performance of adhesives. J. Dent. 26, 1.

Chapter 24
Glass Ionomer Restorative
Materials (Polyalkenoates)
24.1 Introduction
Glass ionomer restorative materials have been available since the early 1970s and were derived from the silicate cements (Section 20.8) and polycarboxylate cements (Chapter 30). Polycarboxylates were developed several years earlier and were the first dental cements for which an inherent adhesion to tooth substance could be demonstrated. They quickly gained popularity as luting cements but could not be used as restoratives because of high solubility, poor mechanical properties and unacceptable appearance caused by the residual opaque zinc oxide. It was soon discovered that when the zinc oxide of the polycarboxylate material was replaced with a reactive ionleachable glass similar to that used previously in silicate cements a stronger, less soluble and more translucent cement could be produced.
24.2 Composition
These materials may be supplied as a powder and liquid or as a powder mixed with water (Fig. 24.1). The composition is outlined in Table 24.1. For powder/liquid materials the powder consists of a sodium alumino-silicate glass of similar composition to that used in silicate materials. The ratio of alumina to silica in the glass is increased compared to that used in silicates. This increases the reactivity of the glass to a level where it reacts rapidly with polyacrylic acid, which is a weaker acid than the phosphoric acid used in silicate materials. As for the silicates, the glasses contain significant levels of fluoride which, although not directly involved in the setting reaction, may have an effect on the caries susceptibility of the surrounding tooth substance.
In the original glass ionomer material the liquid component was a 50% aqueous solution of poly-
acrylic acid. Unfortunately, gelation of the polyacid occurred after only a few months, probably by intermolecular hydrogen bonding. Gelation can be reduced or eliminated by using copolymers instead of homopolymers. This creates sufficient steric hindrance to prevent hydrogen bonding.
Nowadays the liquid component may consist of an aqueous solution of acrylic acid or of a maleic acid/acrylic acid copolymer. Tartaric acid, which is used to control setting characteristics, is also included in the liquid component by many manufacturers. Other products are supplied as a powder/water preparation. The powder/water materials are of two types; both consist of a powder which contains vacuum dried polyacid, in addition to the glass powder. For some materials this is mixed with water and the manufacturers supply a dropper bottle to aid proportioning. With other products, the manufacturer supplies a dilute aqueous solution of tartaric acid. For products in which the polyacid forms part of the powder component, the manufacturers are able to revert to the use of homopolymers of acrylic or maleic acid or copolymers of these two acids as there is no problem of gelation in this solid form. Cements formed from these homopolymers tend to have improved physical characteristics when compared to those formed from acid copolymers.
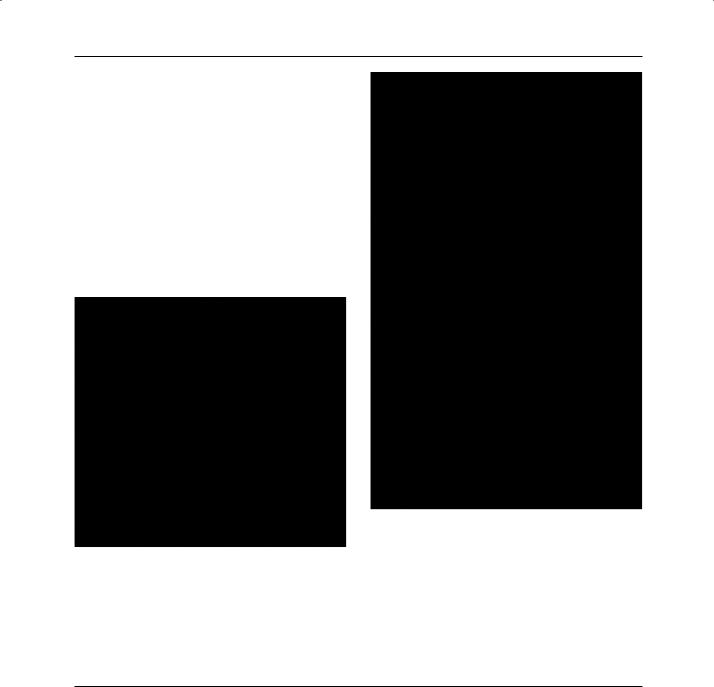
Most glass ionomers for restorative use are now available in encapsulated form (Fig. 24.2). This offers a great advantage – for these materials probably more than any others. The proportions are set and controlled by the manufacturer and mixing is a quick and clean process.
Another factor which is related to the method of mixing is porosity within the mixed and set cement. As indicated in Section 24.4 the properties of a material, and particularly a brittle material such as a dental cement, are sensitive to levels
245
246 Chapter 24
of porosity. Traditional wisdom teaches that mixing a powder and liquid by hand produces greater levels of porosity than are incorporated through mechanical mixing of a powder and liquid in a capsule. However, detailed study using micro CT scanning has revealed that the relationship between mixing and porosity is a complex one. Fluid cements mixed by shaking in a capsule using a device such as that shown in Fig. 24.3 may develop a ‘frothy’ structure during mixing and this is likely to produce a high level of porosity in the set material. Alternative mixing devices involve
Fig. 24.1 A glass ionomer cement restorative material supplied in the form of a powder and liquid. Proportioning of the powder is achieved by using a scoop and the liquid is proportioned according to the number of drops. Powder and liquid are mixed on a mixing pad.
Fig. 24.2 A glass ionomer cement filling material provided in encapsulated form. The powder and liquid used are similar to those used in the material shown in Fig. 24.1 but now the components are mixed together using a device such as that shown in Fig. 24.3. The powder and liquid are brought into contact and mixed for about 10 seconds before extruding the mixed material through the nozzle directly into the cavity.
Table 24.1 Composition of glass ionomer cements.
Powder/liquid materials |
|
Powder |
Sodium aluminosilicate glass with about 20% CaF and other minor additives |
Liquid |
Aqueous solution of acrylic acid/itaconic acid copolymer |
|
or |
|
Aqueous solution of maleic acid polymer or maleic/acrylic copolymer |
|
and |
|
Tartaric acid in some products to control setting characteristics |
Powder/water materials |
Glass (as above) + vacuum-dried polyacid (acrylic, maleic or copolymers) |
Powder |
|
Liquid |
Manufacturers supply a dropper bottle which the operator fills with water |
|
or |
|
The manufacturer supplies a dilute aqueous solution of tartaric acid |
|
|
