
- •1. Think of the place you live in and answer the following questions.
- •2. Find out what these abbreviations mean. Write them in words.
- •3. Read and translate the words given below. Mind the stresses.
- •4. Give Russian equivalents of the following words and phrases. Try to memorize them.
- •5. Answer the following question and read the text below to check your answer.
- •Properties of Air
- •6. Decide whether the following statements are true or false according to the text.
- •7. Answer the following questions.
- •16. Translate the following sentences paying attention to the types of Conditionals.
- •17. Translate the following sentences paying attention to the functions of the verb “to have”.
- •18. Give Russian equivalents of the following words and phrases. Try to memorize them.
- •Verbs and verbal phrases
- •19. Answer the following question and read the text below to check your answer.
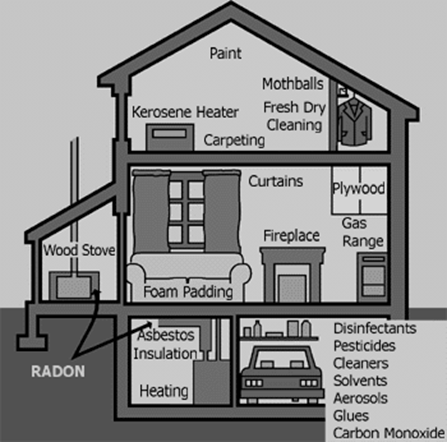
- •Indoor Environmental Quality
- •20. Correct the following statements.
- •21. Analyze the Figure 1.
- •22. Choose the best abstract for the text.
- •23. Write a summary of Text 2.
- •24. Combine the words from the column on the left with the suitable nouns from the column on the right. Translate them into Russian.
- •27. Fill in the table with the derivatives.
- •28. Translate the following sentences paying attention to the functions of the verb “to be”.
- •29. Give Russian equivalents of the following words and phrases. Try to memorize them.
- •Verbs and verbal phrases
- •30. Do the following task and read the text below to check your answers.
- •How to Avoid In-Home Air Pollution
- •31. Decide whether the following statements are true or false according to the text.
- •32. Answer the following questions.
- •33. Combine the words from the column on the left with the suitable nouns from the column on the right. Translate them into Russian.
- •40. Read the texts of Unit 1 again and make notes under the following headings. Then use your notes to talk about Indoor Environment.
- •1. Read the following text and fill in the phrases from the list below.
- •Indoor Air Pollution and Health
- •Качество воздуха внутри помещений
Unit 1__________________________________Indoor Environment
START HERE
1. Think of the place you live in and answer the following questions.
 What
factors can affect air at home?
What
factors can affect air at home?What factors from the given below affect air at your place mostly? (appreciate an influence with points from 1 to 5)
-furniture and curtains
-smells of cooking
-pets
-inadequate ventilation
-outdoor pollution
-finishing agents
-occupants’ activities
-gas stove
-inadequate humidification
-anything else
What can be possible ways to improve indoor air quality?
Is it important for you? Why?
Make an oral summary to the point.
2. Find out what these abbreviations mean. Write them in words.
Example: km = kilometre
deg C, mbar, Kg/cu metre, psia, ft, bar, RH
3. Read and translate the words given below. Mind the stresses.
Oxygen Neon |
Nitrogen Helium |
Argon Krypton |
Carbon Dioxide Xenon |
|
Hydrogen |
ACTIVE VOCABULARY
4. Give Russian equivalents of the following words and phrases. Try to memorize them.
Nouns and noun phrases
vapour |
gravity |
|
dust |
altitude |
|
microbe |
lid |
|
pollen |
pump |
|
scales |
|
|
|
|
|
Verbs and verbal phrases
to pump |
to squeeze |
|
to compress |
to qualify |
|
to stack up |
to saturate |
|
to contract |
to drip out |
|
to collapse |
|
|
to interrelate |
|
|
Adjectives Adverbs
accurate |
slightly |
|
obvious |
accurately |
|
obsolete |
|
|
READING TASK: Text 1
5. Answer the following question and read the text below to check your answer.
Why can it be important to know properties of air?
Properties of Air
Air has all sorts of properties. First of all let's consider its properties and try to get rid of some misunderstandings.
Air has weight.
Air is under pressure.
Air has temperature.
Air has a volume.
Air usually contains some water vapour.
General Properties of Air
Air is a mixture of gases, mainly nitrogen and oxygen. The typical composition of natural air is as follows.
Component. |
Mass% (dry air) |
Volume % (dry air)
|
Oxygen |
23.14
|
20.9476
|
Nitrogen |
75.52
|
78.084
|
Argon
|
1.288
|
0.934
|
Carbon Dioxide |
0.048 |
0.0314
|
Hydrogen |
0.000003 |
0.00005
|
Neon |
0.00127 |
0.001818
|
Helium |
0.000073 |
0.000524
|
Krypton |
0.00033 |
0.000114
|
Xenon
|
0.000039 |
0.0000087
|
Air also contains water vapour and hard matter such as dust, microbes and pollen. These variables depend upon climatic conditions, which vary worldwide. The table therefore reflects the European average dry gas content of air, which may vary slightly in your area. To confuse things a little further, it takes almost twice the amount of energy to heat up the water vapour in wet air, than it would take to heat up an equal number of molecules of dry air.
Weight
Air has its own weight. It's quite easy to demonstrate this by weighing an empty balloon on a set of accurate scales. Then pump the balloon up with a bicycle pump and weigh it again. The balloons got heavier. The reason it's got heavier is because the balloon is now full of compressed air. If air didn't have any weight, then the balloon with compressed air in it would weigh the same as the empty balloon. Because it’s heavier, we've demonstrated that air has weight.
Because the weight of air varies with pressure and temperature it has to be defined accurately.
Pressure
Air is under pressure; this is caused by gravity. If gravity didn't exist, then air pressure would not exist. Air would also be weightless, although it would still have mass.
Air pressure at sea level is approximately 1013 mbar, which is about the same as 14.7psia (pounds per square inch, absolute).
The reason for this pressure is because there is so much air stacked up on top of it. If you were higher up, say in an aeroplane, the air pressure outside the 'plane would be much lower.
We know that the air pressure at 18,000 ft (about 5500 metres) is approximately half that at sea level.
At 32,000 ft (about 10,000 metres) the air pressure is only a quarter of that at sea level.
The reason for the reduction in pressure is because there is less air stacked up on top at these high altitudes.
Just a quick note to explain the difference between mass and weight. Here on earth we assume weight and mass to be the same, in our normal day to day lives we don't need to appreciate the difference.
If you were up in space and floating around weightless, you would quickly appreciate the difference. Imagine launching two satellites from earth, each weighing a couple of tonnes. Once up in space these satellites would be weightless. If something went wrong and they bumped into each other and you were between them, you'd get squashed. Even though they are weightless in space, they retain their mass - and it's the mass that squashed you.
Temperature
Air has temperature, an obvious statement really. Like most things around us, air expands when it gets hot and contracts when it gets cold. Heat up an empty can and then put the lid on it. When the can cools down it collapses. The reason for this is that the air inside has cooled and it now occupies a smaller volume. This also means that the pressure inside is lower than the pressure outside, which has in turn caused the can to collapse. We have just demonstrated that Temperature has an effect on Volume, and that Volume has an effect on Pressure.
An important thing to remember is that whenever we use Temperatures and Pressures in a calculation, they are always absolute values.
Volume
Another really obvious statement is that air occupies a specific volume. This volume is inter-related with pressure and temperature. If you squeeze air into a smaller space the air gets hotter. This is easily demonstrated when you pump up a bicycle tyre. The harder you pump, the hotter the air gets and the hotter the hand pump gets.
Because the amount of air contained within a box will vary with temperature and pressure, it is necessary to qualify the temperature and pressure. For this reason we have developed Standard Volume and Normal Volume.
Standard Volume is measured at standard reference conditions, which for compressor performance testing has been defined as 20 deg C, at 1 bar absolute pressure. This is also sometimes called the Standard Temperature and Pressure condition although technically it is incorrect as Standard Reference Conditions replace the obsolete term STP.
Normal Volume is measured at normal temperature and pressure conditions which has been defined as 0 deg C, at 1 bar absolute pressure. This is also called the Normal Temperature and Pressure condition.
The amount of air contained within a Standard cubic metre (or standard cu ft) is different to the amount contained within a Normal cubic metre (or normal cu ft). Even though the volume is the same, the weight will be different because air at Normal conditions is denser than air at Standard conditions.
Air usually contains some Water Vapour
Life gets a bit more complicated. Air behaves a bit like a sponge, if there's any water around it will try to absorb it. Like a sponge it can only hold just so much water before it becomes saturated. Again like a sponge, if you squeeze it (compress it) the water will drip out.
A dry sponge doesn't have any water in it; therefore it has a relative humidity of 0%.
A soaking wet sponge can't take in any more water because it's already saturated. Therefore this sponge has a relative humidity of 100.
If you pick up the saturated sponge and squeeze it, water drips out. If you dip it in a bucket of water, keeping it squeezed, it doesn't absorb any water out of the bucket. We've demonstrated that by compressing the sponge we have reduced its ability to hold water. Although a squeezed sponge holds less water than an un-squeezed sponge, the squeezed sponge is nevertheless 100% saturated.
Air acts in the same way. Compressed air can't hold as much water vapour as atmospheric air of the same temperature.
Hot air also has the ability to hold more water than cold air, however if you cool down hot air which has a 100% RH, the water vapour condenses out into liquid.
COMPREHENSION CHECK
