
Ceramic Technology and Processing, King
.pdfSlip Preparation Procedures 93
Volume Percent Solids
It is a common practice to batch coarse slips to a much higher solids content. The coarse-grain pack determines the overall volume of the slip. Intermediate and fine sizes fill the interstices. Solids up to 85% are common, where 50-55% are typical for fine slips. Techniques for obtaining these high percentages will be discussed in the section on forming in Chapter VI.
Surfactant Concentration
The grain pack dominates slip rheology. However, the fine sizes have to be deflocculated. Since the amount of coarse material is large, less surfactant is needed in proportion to the amount of fines. Surfactants are ineffective on coarse particles as they have much less surface in proportion to their mass, gravity dominates.
Binders
Often, one uses coarse materials for refractory applications where cost is an important factor. Normally, one would not use acrylic binders, but methyl cellulose, waxes, gums, and PVA are often used. Binders do not have to impart a high strength to the green body as it will not be green machined and ordinarily is not formed into a delicate shape.
pH/Zeta Potential
Since gravity controls the rheology, these measurements are not generally made. Some knowledge of how the fines behave is relevant. One can ascertain this by viscosity measurements.
94 Ceramic Technology and Processing
Settling
Settling is an important property for coarse slips. The large particles settle out very quickly and result in segregation with the coarse grain on the bottom and fine grain on the top. During sintering, the top will shrink more than the bottom, resulting in distortions and fractures. Even before that, the slip will segregate in the drying process, requiring remixing. It is difficult to prevent segregation since it often happens in dry grain storage. In a slip, one can slow the settling process by increasing the viscosity, often with a thixotropic additive such as Hectorite, or a soluble polymer. Although not a coarse material, latex paint is an example of the use of these additives. Slip rolling is discussed a little later in this chapter. Rolling prevents settling of the coarse fraction during storage.
Check List, Coarse Particle Slips
In dealing with course particle slips, one needs to pay attention to factors as listed below.
•Particle size measurements Screen selection Contamination Sieving
•Viscosity
Dilatant
•Milling
•Volume % solids
•Binders
•Settling, can be a serious problem
5.0SOLIDS RECOVERY
Occasionally, it is necessary to recover the solids from a slip especially when the water contains dissolved impurities that have to be
Slip Preparation Procedures 95
removed or when the slip has been leached, often with an acid. There are a few commonly used ways to recover the solids.
Sedimentation
When a slip is placed in a vessel, it will settle out. The settling rate is determined by Stokes' law. High density and coarse particles settle faster. Liquid viscosity and high solids slow the settling rate. It is not practical to recover solids of a sub micrometer particle size unless they are flocced. Floccing will result in a soft cake, which makes it easy to remove. Fine grained dispersed slips will settle out as hard as a rock making it difficult to remove them from the vessel. Once the slip settles out, the supernatant liquid can be decanted or siphoned off. Please do not suck on the siphon tube to start the siphon. Fill the siphon tube with water, and with a finger at the bottom of the siphon tube, stick the tube into the vessel.
Settling will segregate the particles with coarse and higher specific gravity particles at the bottom. The very fine material and solid organic contamination will be on the top. When dried in this condition, the top slime will form hard agglomerates that are difficult to remix. Often, after settling, the material is re-blunged in clean water or in dilute acid for a second wash, and repeated as often as necessary. Following this, the material is dried or made into a slip for further processing. It will segregate unless it is remixed after drying.
Filtering
One can use a variety of filters to recover the solids. In the laboratory, only some few techniques are ordinarily available. The Buchner funnel, shown in Figure 4.21, is especially useful. The funnel is porcelain with a perforated plate that holds the filter paper. A vacuum is drawn on the lower portion creating a pressure differential that increases the filtering rate. Often, an aspirator is used to draw the vacuum. There is a technique for using a Buchner funnel (filter) to produce the best results and avoid having the slip disappear down the drain.
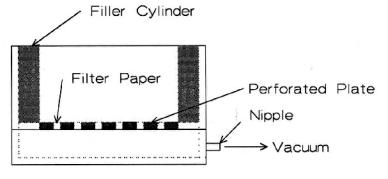
96 Ceramic Technology and Processing
Figure 4.21: Buchner Funnel. The funnel is used to recover solids by filtration.
The fineness of the filter paper determines particle retention. Use a hard paper. Often, one uses a slightly coarser paper because it filters faster. When a thin filter cake forms, the cake itself will act as the filter. With the cake in place, one can recover material that initially passed through the filter. Placing the filter paper on the perforated porcelain plate is tricky. One needs to center the filter paper, smooth it out, and then wet it with water. Following this, one then pulls a vacuum on the funnel to seat the paper without wrinkles. Wrinkles are the main cause for losing the slip. The funnel is constructed with the perforations placed a distance from the edge, resulting in a dead volume around the perimeter. This is of no concern unless the material is to be washed on the filter; which means the edge will not be washed and will contaminate the batch. There are two ways to avoid this: re-blunge the material and repeat filtering or cover the dead space around the edge with an insert as shown in Figure 4.21. A vacuum is usually obtained with an aspirator, however a vacuum pump can be used. The pump will pick up water vapor and slip unless it has adequate traps. Pour the slip onto the center of the filter. Pouring the slip down the edge can dislodge the filter paper and cause the slip to be washed down the drain. When washing on the filter, the cake will crack if left too long where it dries in the funnel. The crack will act as channels for the wash water. If
Slip Preparation Procedures 97
this happens, re-blunge the material and filter again. The best way to remove the filter cake from the funnel is to tip the funnel up so that all of the filtrate drains out of the base. One then has to clean the nipple, turn the funnel upside down over a pan, and place the lips on the nipple and blow. The cake and paper will then drop out neatly. If the cake is to be dried at this point, it is much better to leave the paper on because removing the paper when wet can cause it to tear, leaving pieces in the batch. To clean the Buchner funnel, back flush it through the nipple while still wet and tip the funnel around to flush all locations in the base. When left to dry without cleaning, the solid will cake and be hard to remove.
Another filtering technique is to use a pressure filter. This device is especially useful for filtering very fine slips that are viscous. A lab pressure filter (Millipore) is shown in Figure 4.22. The filter has a slotted base plate, a shell, and a top plate with gaskets to make the seals. A filter medium is chosen and placed on the base. Pour in the slip and pressurize the apparatus to about 60 psi. Always use a pressure relief valve that limits the pressure below the rated capacity. Turn up the pressure to just above 60 psi to assure that the valve is operating correctly. Pressurized gases are very dangerous. Dried ceramic slips on the valve port will render it useless. Also, routinely check the pressure gauge to double check safety.

98 Ceramic Technology and Processing
Figure 4.22: Pressure Filter Apparatus. The filter is used to remove extraneous material. (Courtesy of Millipore).
Slip Preparation Procedures 99
Centrifuge
Fine, particle-sized slips can be difficult to recover by other methods, and centrifuging can come in handy. There are three main types of centrifuges: solid bowl, overflow, and filtering. The solid bowl packs the solid particles against the sides or base by increasing the gravitational force and causing an increase in the settling rate. The bowl is a cylinder that rotates around its axis. After centrifuging, the liquid on top is decanted. For small quantities of material, a lab centrifuge with polymer tubes is useful. Lab centrifuges have a rotor with holes into which one inserts tubes, like test tubes. One commonly uses polymer tubes such as polyethylene as glass tubes break. Pour the slip into these tubes and spin them until the solids sediment out. These centrifuges are available up to high gravitational forces, but are limited to the amount of material they can handle. Even then, when the particles are very fine, this is a practical lab approach. At lower speeds and if the slip is flocculated, solid bowl centrifuges can recover materials in the sub micrometer range. A problem is that the cake is extremely dilatant and exceptionally hard to remove. Filtering centrifuges have a filter medium around the bowl that separates the solids from the liquid. Problems are similar to those with filters. Decanting centrifuges are essentially related to the solid bowel centrifuge, but the effluent is a liquid containing the finer particles. The decanting centrifuge is not so much for the recovery of solids as for classification. An example is shown in Figure 4.23.
One technique is to circulate the slip through the centrifuge and an ultrasonic disperser, with the effluent comprising the fraction recovered.

100 Ceramic Technology and Processing
Figure 4.23: Decanting Centrifuge. Slip enters into the bottom by a peristaltic pump. Fines are taken off up the shell and can be recirculated to remove additional coarser particles. (Courtesy of IEC).
Casting
This is an old potter's method for recovering solids. How simple! Just pour the slip out onto a plaster plate and have the plaster remove the
Slip Preparation Procedures 101
excess water. When there is a concern that the plaster may contaminate the ceramic powder, place a flat disc of filter paper on top of the plaster to act as a separating plane.
Check List, Solids Recovery
For recovering solids, observe the following methods:
•Sedimentation
•Filtering
Buchner technique
Pressure filter technique
•Centrifuge
•Casting on plaster
6.0SLIP CONDITIONING/STORAGE
Since there are differences between fine and coarse grained slips, we will address them separately.
Fine Particle Sized Slips
Surface area increases as the inverse square of the particle size. Fine slips are in more rapport with the liquid, than coarse slips that are in rapport with gravity. It can take much longer for fine slips to equilibrate with the liquid, perhaps as long as 24 hours. During this interval, the viscosity is changing (increasing). An industrial practice is to incrementally add slip allocates to a master batch that has had time to equilibrate, resulting in a consistent slip for processing. Viscosity measurements, when leveling out, will suggest when the slip is stabilized. The author has heard that in ancient China, the master lays down a bed of clay for the next generation to make beautiful ceramics.
102 Ceramic Technology and Processing
Equilibration
Particles in the slip are undergoing adsorption/desorption reactions with electrolytes and surfactants in the liquid. These reactions can take time to reach equilibrium, perhaps as long as 24 hours. During this interval, the slip is altering its viscosity and casting characteristics. Fresh out of the mill, the slip will be warm - or even hot - and can cook organic additives. Reaction rates are very temperature dependant, and equilibrium at an elevated temperature may not be equilibrium at room temperature. If this slip needs time to equilibrate, it is a good idea to age it before using it. Not all slips have this problem. The slip is stored during the equilibration period, and this is the subject of the following section.
Filtering
Filtering was discussed in the preceding section on solids recovery. However, filtering the slip to remove irregularities is a different subject. Fine slips will not pass through a filter in the usual manner since they are too viscous. It is necessary to pressure filter these slips in the apparatus previously shown in Figure 4.22, only here the filter is a cloth mesh such as that shown in Figure 4.24.
This filter cloth has an opening of 20 μm, so anything finer than that can pass right through. Meshes as fine as 10 μm are available, but slow. Filtering removes clumps of binders, agglomerates, and extraneous materials. Figure 4.25 shows some debris recovered from what was thought to be a clean slip.
