
Ceramic Technology and Processing, King
.pdf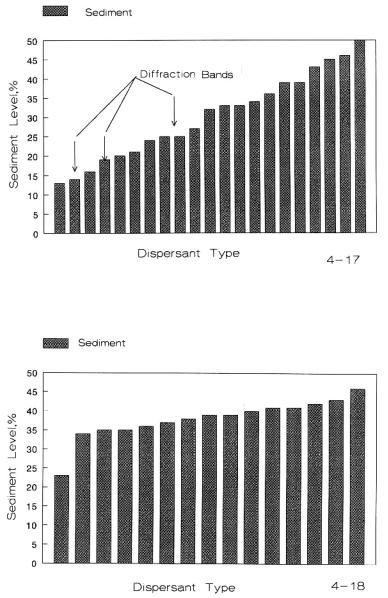
Slip Preparation Procedures 83
Figure 4.17: Sedimentation of SiC vs. Surfactants in Water.
Figure 4.18: Sedimentation of SiC vs. Surfactants in Isopropyl Alcohol.
84 Ceramic Technology and Processing
Binders
There are many binders to choose from, including waxes, alginates, starch, gums, and synthetic materials. Only a few synthetic binders will be discussed. All these binders are used for slips and include the following: polyethyleneglycol (PEG), polyvinyl alcohol (PVA), methylcellulose and its varieties, and acrylic emulsions. These four binders are manufactured by chemical processes where the composition and properties are controlled. While there can be some refinement in naturally occurring binders, they are variable in both composition and properties. Samples of binders can be obtained from suppliers in quantities suitable for lab use. Another source is from a ceramic plant.
The function of the binder in slip casting is different from its function in pressing. The density of a pressing (small pressed part) increases with the use of a binder plasticizer. In slip casting, the binder is added to increase green strength for purposes of handling or green machining. Green density is achieved by the casting process itself.
PEG. PEG is available in molecular weights from 400 to 12,000. Low molecular weights are liquids at room temperature. Useful molecular weights are from 6,000 to 12,000 with most applications at the higher end. These are waxy solids that are soluble in water, methylene chloride, and acetone. Some volatile organics require special handling, ventilation, and fire/explosion protection. Methylene chloride is toxic. Chapter Two discusses safety problems in greater detail.
PEG is a clean chemical that burns without a residue. While it is not a high-strength binder, it is adequate for most purposes. All of the binders discussed here are added as solutions or as a fluid emulsion. The reason for this is to obtain a homogeneous fluid without clumps of binder that have not completely dissolved.
The PEG solution is prepared on a hot plate using a double boiler and a stirrer. Flakes of PEG are sifted into the water bath while stirring. Ordinarily, the solution will contain between 3-5% of PEG by weight. It is a good idea to plug the stirrer into a variable voltage transformer. Stir fast enough to mix, but avoid whipping in bubbles, especially fine bubbles as
Slip Preparation Procedures 85
they are difficult to remove. It is not a good idea to center the stir rod as this helps to form a vortex that will draw in air. It also helps to tip the stir rod at a slight angle from the vertical position as this creates turbulence. When the stir rod is centered and vertical, the binder solution will not mix well. Double boilers are available at most hardware or cook ware stores. By using a double boiler, one avoids overheating at the bottom of the pot. After the solution is completed, the pot is covered, aged overnight, and is stirred again the following day. To remove solid impurities or clumps of thick binder, the binder solution can be pressure filtered as is described in a later section. Depending on the prevailing viscosity, the bubbles will arise after a day or so. Binder solutions can be vacuum de-aired as long as the viscosity is not too high. It is a better practice to stir the binder solution into the slip and then vacuum de-air. This practice will be described in a later section.
PVA. A variety of grades of PVA make solutions over a range of viscosities. For ceramic use, one generally uses a fully-hydrolyzed binder of medium viscosity. PVA is a strong binder and is suitable and widely used for handling and green machining ceramic parts. The process for making up PVA solutions is identical to that for PEG, except that it has a higher viscosity at the same concentrations and requires more care to assure that it is homogeneous. It is a clean chemical that burns without a residue.
Methylcellulose and varieties. These products are available in a variety of grades. Variability is achieved by molecular weight, molecular weight distribution, and degree of substitution of methoxyl and hydroxypropoxyl groups. One can simplify the process of selecting a binder by phoning the supplier for additional information. This binder is unusual in that its viscosity increases as the temperature increases. Unlike the solution procedure for PEG and PVA, methylcellulose is dissolved cold. This can be a useful property that has found application in extruding honeycomb structures through a heated die, resulting in a stiffening of the mix that then retains the honeycomb structure. One can obtain a wall thickness as thin as 0.010 inches.
Methylcellulose is not as clean a chemical as PEG or PVA There
86 Ceramic Technology and Processing
is a burnout residue that is a problem only when the ceramic is sensitive to foreign impurities. Unlike PEG and PVA, it is not a strong binder and is not really suitable if the part has to be green machined. Otherwise, it is strong enough to impart handling capabilities.
Acrylic Emulsions. These emulsions are strong binders and burn out cleanly. They are useful for press mixes as well for slip casting. Being emulsions, they can break or gel. This does not infer that they are difficult to use if handled properly. As a container will be used for a long time, a crust will form on the jar lid and top. Pieces of this crust can get into the slip creating voids in the part when sintered. The emulsion can also gel in its container forming clusters that float in the emulsion. These problems can be avoided by taking allocates of the emulsion of a convenient volume and sealing them in individual glass vials. A polyethylene liner in the lid is inert and will make a good seal. When needed, one can extract a measured amount of emulsion and discard the remaining emulsion and vial. This may seem wasteful but if the allocate is not too large this procedure is much less expensive than a ruined part. Other liquids in the ceramics lab can have this same problem, and one can follow a similar procedure.
Rohm and Haas introduced an acrylic binder, Duramaxtm B-1031 that has a high green strength. Due to the high green strength, this binder is recommended for green machining applications. Duramax is compared to PVA and PEG in Table 4.2.
Table 4.2: Binders and Green Strength
Binder |
Green Strength (MPa) |
|
|
Duramax |
6.5 |
|
|
PVA |
0.7 |
|
|
PEG |
0.4 |
|
|
Slip Preparation Procedures 87
pH/Zeta Potential
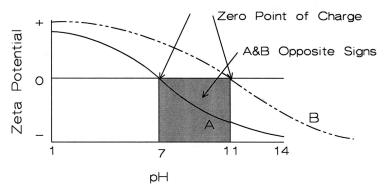
The viscosity of the slip is related to the charges on the surface of the particles. Charged surfaces, when of the same sign, repel each other and disperse the slip, reducing its viscosity. Measurements of the zeta potential with pH result in a plot such as shown in Figure 4.19.
Figure 4.19: Zeta Potential vs. pH. Two materials are shown with different ZPC. A has a zero point of charge at pH 7. B has a ZPC at pH of 11.
An S-shaped curve is typical. The point where the curve crosses the zero (0) axis is the zero point of charge (ZPC). This is where the slip flocculates. For aluminum oxide, this occurs at a pH of about seven. Add either an acid or a base and the slip will liquefy. Figure 4.19 shows curves for two different materials. Material A has a ZPC at a pH of seven. Material B has a ZPC at a pH of 11. Below a pH of seven both materials have a positive charge and will be dispersed. Above a pH of 11, both materials have a negative charge and will be dispersed. Between a pH of 7 and 11, the different materials will have opposite charges on their surfaces and will attract one to the other. This phenomenon will increase viscosity, however it will also inhibit segregation of the phases. Another complication to consider is that at a pH of 7 material A will floc, while B will still be
88 Ceramic Technology and Processing
dispersed. The parallel but opposite relation occurs at a pH of 11. These details in the surface chemistry enable the ceramist to control the structure of the slip and the final sintered ceramic.
Since there may not be a continuing demand for zeta potential measurements, purchasing the instrument may not be justified. Work can be contracted out where one will pay for such measurements.
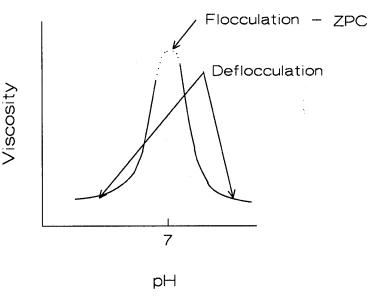
For normal laboratory work, a pH/viscosity plot can be easily created. A typical plot is shown in Figure 4.20.
Figure 4.20: Viscosity vs. pH. Viscosity increases sharply at a specific pH for each material. The slip is deflocculated on each side of the maximum as suggested by the arrows.
A peak in the curve is typical for alumina and relates back to the zeta potential curve. The viscosity curve is more easily available however.
So far, there are four factors that affect the viscosity of the slip:
Slip Preparation Procedures 89
particle size, % solids, surfactant, and pH. It is a good idea to adjust the pH and to add the surfactant to the water before adding the powder. The pH will change when the powder is added. A common practice is to add the binder toward the end of the milling or after the milling. The reason for this is that the binder can be mechanically or thermally altered during milling. Adding, the binder at this point can alter the rheology. A series of trials is often necessary to derive the optimum composition. When considering a statistically designed experiment, this is a good place to zero in on the optimum formulation.
Settling
With fine slips, settling is not too big a problem. It becomes a problem when one stores the slip over an extended period. If the viscosity is low and the casting time long, there could be settling of the particles. In this case, one may need to adjust the deflocculation and viscosity.
Check List, Fine Particle Slips
In dealing with fine particle slips, one needs to pay attention to particle size measurements and viscosity factors as listed below.
•Particle size measurement techniques Sampling and dispersion SEM, TEM
Light scattering
Linear intercept measurements Vertical measurements
•Viscosity
Rheology
Choice of viscometer type
Effect of milling time
Particle size distribution/milling time
90 Ceramic Technology and Processing
Mill critical speed
Effect of solids v/o on viscosity
Type and amount of surfactant, saturation
Settling
Choice of binder, green machine
Preparation of the binder solution
Measurement of pH, Zeta potential
4.0 COARSE PARTICLE SLIPS
There are differences in the processing of fine and coarse particle slips. To chart these differences, the same topics will be discussed as in the previous section.
Particle Size Measurements
Screening is one technique for measuring coarse particle size distributions. We will also discuss the topics of contamination and sieving.
Screen Selection
Lab screens are usually 8 inches in diameter and a full height. These screens are available in sizes from a 4-mesh screen to a 600-mesh screen. Anything below a 325-mesh screen is difficult to use because of blinding where particles become embedded in the sieve openings. Stainless steel mesh with a brass body and brass pans and lids are preferred. Stainless steel mesh is much more durable than brass because of its greater strength. When working below a 40-mesh size, a stainless steel screen is a preferred screen to use. Above this 40-mesh size, one can use coarser mesh size screens. Fine mesh brass screens are easily damaged. A 200-mesh screen with a hole is no longer a 200-mesh screen. One can repair a screen damage
Slip Preparation Procedures 91
by soldering a split back onto the brass body. A hole can be plugged with a drop of epoxy or solder.
One can damage the screens while cleaning them after use or by accidentally pushing a mix through the screen with a scraper. The body of the screen should be brass as they do not gall and are easier to handle. Stainless screen bodies will gall when stacked. Grit, that sifts into the space between screens, makes it difficult to separate the screens. With time, prying tools will be needed to separate the stack. Often, the stainless lip on the screen will become deformed.
Contamination
It is difficult to remove particles that become wedged between the wires in the mesh. Since stainless is harder and more durable than brass, it is a little easier to clean. With screens to about a 20-mesh size, an awl can be used to singly pop out embedded particles from the back side. Fine particles can be partially removed by ultrasonic cleaning with a detergent. One can also brush the back of the screens with a fine brass brush. This could ruin a fine mesh brass screen. When contamination is not permissible, a separate set of screens should be reserved for each material. This is not as bad as it sounds considering that one will be processing only a few materials and will only need a few sizes. One should mark these screens to identify the material before locking them away. Keep a general purpose set for borrowing or general use. A storage rack with each slot marked as to size is a good way to keep the set in order rather than being scattered throughout the facility.
Sieving
A sieve stack consists of coarse sieves on top with progressively finer sieve sizes below. One should not forget to include the lid and pan. One then places the stack into the shaker and tapes the edges. When the sieves get old and have stainless bodies, they will rust. Sieve shakers can
92 Ceramic Technology and Processing
shake, tap, or vibrate. Vibrating shakers have controls for time and intensity. These controls are set for standard conditions that can be determined by a test. When the size distribution starts to level out, that time and intensity can be your standard. Sieve shakers are noisy so a sound enclosure is a good idea. Some larger shakers have rubber balls that bounce against the bottom of the screen and reduce blinding, which can be a troublesome problem.
Viscosity
Viscosity is not as closely controlled for coarse slips as it is for fine slips. This is because the coarse grain pack has many grain-to-grain contacts, producing a flow characteristic not unlike that of concrete. In both cases, the large particles dominate.
Viscometers
Cup and cone viscometers are not suitable for coarse slips. One can use rotor-type viscometers for coarse slips. Unless the grain is too coarse, in which case viscosity measurements would not be especially meaningful.
Milling time
The coarse fraction is not milled down to finer sizes. Milling, instead, is done to disperse the fine fraction that will act as a bond in the sintered structure. A milling function of the coarse fraction is to act as a grinding media assisting the deagglomeration of the fines. Therefore, milling time is shorter than that used in fine slips. The size of the coarse fraction is controlled by batching rather than by milling. A coarse slip may be milled for only a few hours to disperse the fines.
