
Perthes(Current concept 2012)
.pdf
659
COPYRIGHT 2012 BY THE JOURNAL OF BONE AND JOINT SURGERY, INCORPORATED
Current Concepts Review
Pathophysiology and New Strategies for the Treatment of Legg-Calve´-Perthes Disease
Harry K.W. Kim, MD, MS, FRCSC
Investigation performed at Texas Scottish Rite Hospital for Children, Dallas, Texas
Legg-Calve´-Perthes disease is a juvenile form of idiopathic osteonecrosis of the femoral head that can lead to permanent femoral head deformity and premature osteoarthritis.
According to two recent multicenter, prospective cohort studies, current nonoperative and operative treatments have modest success rates of producing a good outcome with a spherical femoral head in older children with Legg- Calve´-Perthes disease.
Experimental studies have revealed that the immature femoral head is mechanically weakened following ischemic necrosis.
Increased bone resorption and delayed new bone formation, in combination with continued mechanical loading of the hip, contribute to the pathogenesis of the femoral head deformity.
Biological treatment strategies to improve the healing process by decreasing bone resorption and stimulating bone formation appear promising in nonhuman preclinical studies.
Legg-Calve´-Perthes disease is a juvenile form of idiopathic osteonecrosis of the femoral head that affects children between the ages of two and fourteen years. It is considered one of the most common forms of pediatric femoral head osteonecrosis, with the prevalence ranging from 5.1 to 16.9 per 100,000 in various regions of the world1-4. Since the initial reports of this unique condition approximately 100 years ago by Legg5, Calve´6, and Perthes7, a number of studies have been published regarding its etiology8-15, epidemiology1,3,4, natural history16-20, radiographic classifications16,21-23, treatments24-29, and outcomes30-32. Despite the increase in knowledge, Legg-Calve´-Perthes disease remains one of the most controversial conditions in pediatric orthopaedics. Many aspects of the disease remain unknown or unclear, including the etiology and pathophysiology of the disease and the best methods to treat the patients in different age groups affected with the disease. The pur-
poses of this review are threefold: to provide an update on the outcomes of current treatments according to the results of recent prospective studies, to provide an update on the pathophysiology of Legg-Calve´-Perthes disease on the basis of the knowledge gained from recent experimental investigations, and to summarize the rationale, results, and concerns of new biological therapeutic strategies that are being explored as possible treatments for the disease.
Current Treatments and Outcomes
Current treatments for Legg-Calve´-Perthes disease are largely based on the principles of obtaining and maintaining good hip range of motion, and obtaining and maintaining containment of the femoral head in the acetabulum33,34. It is believed that, if these principles are followed, a soft femoral head will be molded into a spherical shape by the acetabular socket. Over the years,
Disclosure: The author did not receive payments or services, either directly or indirectly (i.e., via his institution), from a third party in support of any aspect of this work. Neither the author nor his institution has had any financial relationship, in the thirty-six months prior to submission of this work, with any entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. The author has had a relationship, or has engaged in another activity, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
J Bone Joint Surg Am. 2012;94:659-69 d http://dx.doi.org/10.2106/JBJS.J.01834
660
TH E JO U R N A L O F BO N E & JO I N T SU R G E RY d J B J S .O R G VO LU M E 94-A d NU M B E R 7 d AP R I L 4, 2012
TABLE I Stulberg Radiographic Outcome of Three Treatments in the Study by Herring et al.*
|
|
Radiographic Outcome |
||
|
|
According to Stulberg |
||
|
|
|
Class |
|
|
|
|
|
|
|
|
I or II |
III, IV, or V |
|
|
|
|
|
|
Age of 6 to 8 years at disease |
|
|
|
|
onset (n = 204) |
|
|
|
|
No treatment (n = 11) |
27% (3) |
73% (8) |
|
|
Range of motion (n = 44) |
48% (21) |
52% (23) |
|
|
Brace (n = 79) |
62% (49) |
38% (30) |
|
|
Innominate osteotomy (n = 39) |
69% (27) |
31% (12) |
|
|
Femoral osteotomy (n = 31) |
68% (21) |
32% (10) |
|
|
Age of ‡8 to 12 years at disease |
|
|
|
|
onset (n = 141) |
|
|
|
|
No treatment (n = 8) |
25% (2) |
75% (6) |
|
|
Range of motion (n = 33) |
30% (10) |
70% (23) |
|
|
Brace (n = 50) |
36% (18) |
64% (32) |
|
|
Innominate osteotomy (n = 29) |
41% (12) |
59% (17) |
|
|
Femoral osteotomy (n = 21) |
62% (13) |
38% (8) |
|
|
|
|
|
|
|
*The data are from Herring JA, Kim HT, Browne R. Legg-Calve´- Perthes disease. Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004;86:2121-34.
various nonoperative28,35-39 and operative treatments24,27,29,40,41 were introduced on the basis of this concept. One of the criticisms of this concept has been the inability to accurately quantify femoral head containment and to establish a direct relationship between the amount of containment of the femoral head and the ultimate outcome. According to hip modeling studies42,43, a clinical impression of containment based on radiographic assessment of the femoral head coverage may not accurately represent the degree of containment of the femoral head.
The effectiveness of current treatments based on the concept of containment has been investigated in two multicenter prospective investigations (level-II prospective cohort studies)30,44. In the study by Herring et al.30 (Table I), the success rate of achieving a spherical femoral head (Stulberg class-I or II hips)20 after one of the five treatments (no treatment, physiotherapy, Scottish Rite orthosis, femoral osteotomy, or Salter innominate osteotomy29) ranged from 27% to 69% in the 204 patients with the onset of the disease between the ages of six and eight years and from 25% to 62% in the 141 patients with the onset of the disease between the ages of eight and twelve years. The operative treatments, as a treatment group, produced better results than the nonoperative treatments for the older age group; however, the effectiveness of these treatments in achieving a spherical femoral head was modest. In the study by Wiig et al.44 (Table II), the success rate of achieving a spherical femoral head after one of the three treatments (physiotherapy, Scottish Rite orthosis, or femoral varus osteotomy) ranged from 46% to 53% in the 168 pa-
PAT H O P H Y S I O LO G Y A N D NE W ST R AT E G I E S F O R T H E
TR E AT M E N T O F LE G G -CA LV ´ -PE RT H E S DI S E A S E
E
tients with the onset of the disease before the age of six years and from 20% to 43% in the 146 patients with the onset of the disease after the age of six years. While that study found that a femoral osteotomy was beneficial in the patients who were more than six years old at the onset of the disease, the effectiveness of a femoral osteotomy in achieving a spherical femoral head was also modest.
The results of these studies raise a question regarding why a femoral or Salter innominate osteotomy produces good results in some patients and not in the others. One theory is that while these osteotomies do provide some load-relieving effects on the necrotic femoral head42,45, they do not directly or specifically address the pathobiology of the disease and the impaired healing observed in the older children with Legg- Calve´-Perthes disease. These results also clearly indicate a need to develop more effective treatments for the disease that prevent the femoral head deformity. It is generally agreed that a better understanding of the pathophysiology of the femoral head deformity in Legg-Calve´-Perthes disease is essential for the development of more effective treatments.
Pathophysiology of Legg-Calve´-Perthes Disease
Various theories on the etiology of Legg-Calve´-Perthes disease have been proposed. These include trauma, an inflammatory process, vascular occlusion, thrombophilia, insulin-like growth factor-1 pathway abnormality, maternal smoking, second-hand smoke exposure13,46-49, and, most recently, a subtle type-II collagen mutation12,14,50. Most of these theories remain unsubstantiated. Thrombophilia as a cause of Legg-Calve´-Perthes disease remains controversial, with some studies having shown an association between the disease and various coagulation factor abnormalities8,51-55, while other studies have shown no association
TABLE II Stulberg Radiographic Outcome of Three Treatments Studied by Wiig et al.*
|
|
Radiographic Outcome |
||
|
|
According to Stulberg Class |
||
|
|
|
|
|
|
|
I or II |
III, IV, or V |
|
|
|
|
|
|
Age of <6 years at diagnosis |
|
|
|
|
(n = 168) |
|
|
|
|
Physiotherapy (n = 123) |
53% (65) |
47% (58) |
|
|
Scottish Rite orthosis (n = 22) |
45.5% (10) |
54.5% (12) |
||
Femoral osteotomy (n = 23) |
52% (12) |
48% (11) |
|
|
Age of ‡6 years at diagnosis |
|
|
|
|
(n = 146) |
|
|
|
|
Physiotherapy (n = 51) |
33% (17) |
67% (34) |
|
|
Scottish Rite orthosis (n = 25) |
20% (5) |
80% (20) |
|
|
Femoral osteotomy (n = 70) |
43% (30) |
57% (40) |
|
|
|
|
|
|
|
*Data are from Wiig O, Terjesen T, Svenningsen S. Prognostic factors and outcome of treatment in Perthes’ disease. A prospective study of 368 patients with five-year follow-up. J Bone Joint Surg Br. 2008;90:1364-71.

661
TH E JO U R N A L O F BO N E & JO I N T SU R G E RY d J B J S .O R G |
PAT H O P H Y S I O LO G Y A N D NE W ST R AT E G I E S F O R T H E |
||||
VO LU M E 94-A |
d |
NU M B E R 7 |
d |
AP R I L 4, 2012 |
´ |
|
|
TR E AT M E N T O F LE G G -CA LV E-PE RT H E S DI S E A S E |
|||
at all11,56-60. The prevailing opinion is that Legg-Calve´-Perthes disease is a multifactorial disease with genetic and environmental factors playing a role. There is also the possibility that the disease is caused by several etiological factors that share a common pathological and clinical presentation.
Regardless of the cause, a disruption of blood supply to the femoral head, producing ischemic necrosis, appears to be a key pathogenic event, leading to the pathological and subsequent structural changes to the growing femoral head. Diagnostic imaging studies, such as selective angiography61-63, bone scintigraphy64, and gadolinium-enhanced magnetic resonance imaging65, provide evidence of absent blood flow to the affected femoral head. Although histological studies of the femoral head and biopsy specimens from the patients with Legg-Calve´-Perthes disease are limited in number, they show changes consistent with ischemic necrosis of the bone and the deep layer of the articular cartilage66,67. Animal studies also have shown that a disruption of the blood supply to the femoral head can produce radiographic and histological changes resembling Legg-Calve´-Perthes disease68,69.
The question of whether Legg-Calve´-Perthes disease is due to a single episode of infarction or multiple episodes of infarction remains controversial. The evidence for the single infarction theory comes from studies of immature pigs in which one episode of ischemia induction surgery produced radiographic and histological changes resembling Legg-Calve´- Perthes disease. The evidence for the multiple infarction theory comes from studies on immature dogs in which a single episode of infarction did not produce femoral head necrosis or deformity, while consecutive interruptions of the blood supply produced changes resembling Legg-Calve´-Perthes disease in some femoral heads70,71. In a subsequent clinical study, 51% of fifty-seven biopsy specimens from the femoral heads of patients with the disease revealed dead woven bone superimposed on dead lamellar bone with the marrow space occupied by dead granulation tissue, suggesting two episodes of infarction72. Catterall et al. also observed thickened trabeculae with many cement lines in the central area of two femoral heads with total head involvement (Group 4 in the Catterall classification)66. However, in the periphery of the specimens with less femoral head involvement (Group 1 in the Catterall classification), the authors observed osseous trabeculae showing only one episode of infarction. One interpretation of these findings is that multiple episodes of infarction are necessary to produce Legg- Calve´-Perthes disease. This interpretation suggests that, if the cause of the infarction episodes can be identified, and if intervention can be initiated early in the course of the disease process, then a full-blown disease may be prevented or the severity of the disease can be reduced. Another interpretation of these findings is that the disease is due to one infarction episode, but subsequent mechanical overloading may injure the vessels in the healing areas of the femoral head or produce intermittent compression of the blood vessels traversing the cartilage in the area of high loading, producing secondary episodes of infarction. This interpretation suggests that the prevention of mechanical overloading and a femoral head deformity would be beneficial to the healing process.
Pathogenesis of Femoral Head Deformity in Legg-Calve´-Perthes Disease
Development of the femoral head deformity is the most important sequela of Legg-Calve´-Perthes disease since the extent of the deformity correlates with the long-term outcome18-20. A serial radiographic examination of patients with the disease demonstrates that the development of the deformity begins in the initial stage of the disease (the stage of increased radiodensity) and progresses during the resorptive stage (the stage of fragmentation). From the limited number of biopsy and necropsy studies of Legg-Calve´-Perthes disease, various pathological changes from the proximal part of the femur that include the articular cartilage, osseous epiphysis, physis, and metaphysis have been reported66,67,73-76. The temporal sequence of the pathological changes and the functional importance of the changes are difficult to appreciate from these few studies as they only examined a limited number of specimens and in various stages of the disease.
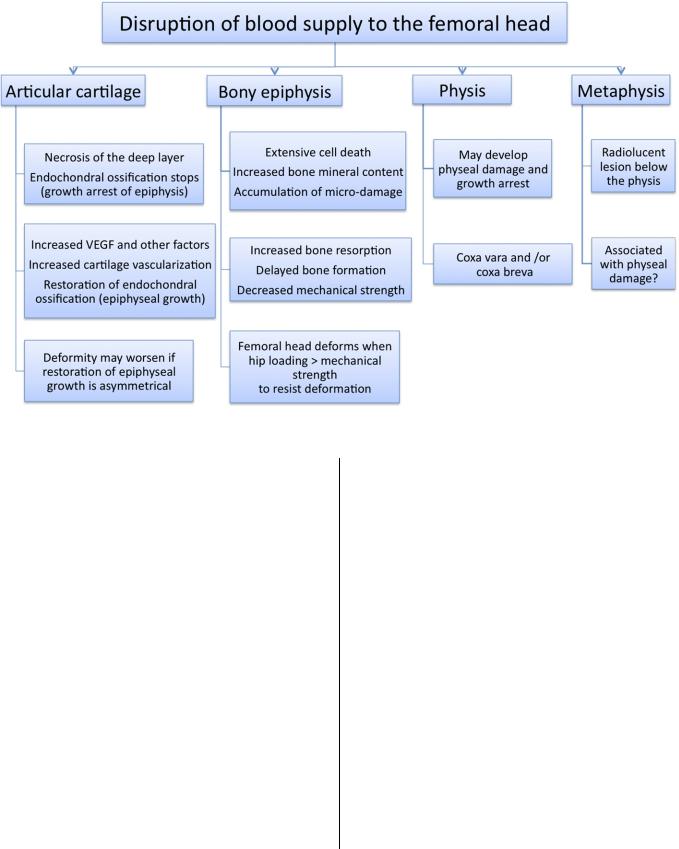
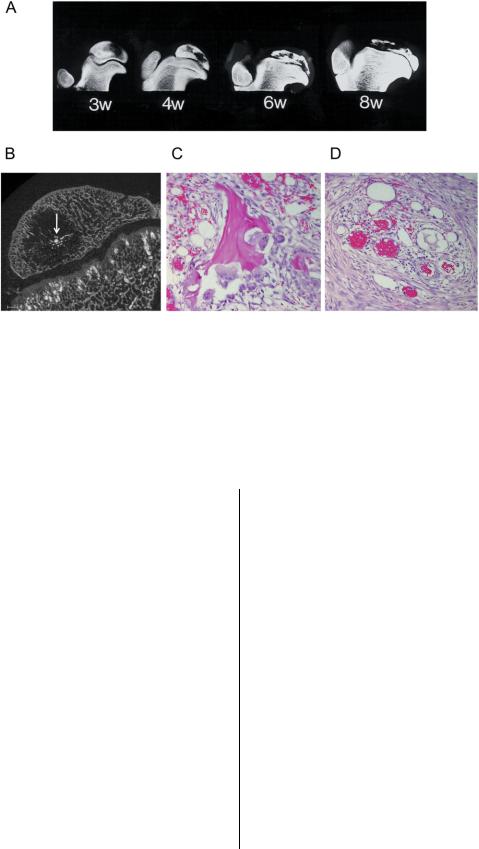
The lack of availability of clinical samples for research has prompted an alternative approach, the use of experimental models, to investigate the pathogenesis of the femoral head deformity. In particular, a piglet model has allowed more systematic and in-depth investigation. This model of ischemic necrosis of the immature femoral head is created by applying a ligature (resorbable suture) around the femoral neck to disrupt the blood flow to the femoral head. The femoral head becomes necrotic and remains avascular in the first two weeks (the avascular stage). Revascularization and resorption of the necrotic head are initiated by three to four weeks after ischemia induction (the vascular repair stage), and moderate to severe femoral head deformity is observed at eight weeks after ischemia induction68. The studies of this model have revealed that the pathogenesis of the femoral head deformity following ischemic necrosis is complex, and multiple factors contribute to the development of the deformity68,77-80 (Fig. 1). Mechanical testing of the normal and the infarcted femoral heads from immature pigs has revealed a significant and persistent decrease in the mechanical properties of the infarcted head from the early avascular stage to the later vascular repair stage in this model80. Further studies have revealed that the mechanical properties of the articular cartilage and the bone from the infarcted heads were decreased79. Proposed explanations for the early compromise of the mechanical properties have included the necrosis of the deep layer of the articular cartilage as a result of the ischemic injury, the inability of the necrotic bone to repair the microdamage incurred during normal loading of the hip, and the changes in the material properties of the calcified cartilage and the subchondral bone associated with the ischemic damage. A recent study on the mineral content of the necrotic trabecular bone, with use of a technique called quantitative backscatter electron imaging, showed a significant increase in the calcium content of the calcified cartilage and the subchondral bone (p < 0.05), making the bone more homogeneous in the calcium content and, likely, more brittle77. It is postulated that since brittle bone is more prone to microdamage and since there are no osteoclasts and osteoblasts in
662
TH E JO U R N A L O F BO N E & JO I N T SU R G E RY d J B J S .O R G |
PAT H O P H Y S I O LO G Y A N D NE W ST R AT E G I E S F O R T H E |
||||
VO LU M E 94-A |
d |
NU M B E R 7 |
d |
AP R I L 4, 2012 |
´ |
|
|
TR E AT M E N T O F LE G G -CA LV E-PE RT H E S DI S E A S E |
|||
Fig. 1
A flowchart depicting the pathogenesis of the femoral head deformity in Legg-Calve´-Perthes disease. VEGF = vascular endothelial growth factor.
the necrotic regions of bone to repair the microdamage incurred with the normal activities, this microdamage accumulates and results in a subchondral fracture or a compaction fracture with continued loading of the hip in the early stages of Legg-Calve´-Perthes disease.
In the vascular repair stage of the piglet model, a pathological repair process marked by a predominance of osteoclastic resorption and delayed bone formation contributes to the pathogenesis of the deformity68 (Fig. 2). The uncoupling of bone resorption and formation, and the replacement of the necrotic bone by a fibrovascular granulation tissue, impart further weakening to the femoral head. The repair process is clearly not ‘‘creeping substitution,’’ as defined by Phemister, in which dead bone is substituted by new bone in the infarcted segment of adult femoral heads81. The uncoupling of bone resorption and formation observed in the piglet model is also observable in the patients with Legg-Calve´-Perthes disease. The resorptive stage or the stage of fragmentation in the disease demonstrates increased bone resorption seen as radiolucent areas on serial radiographs prior to the femoral head entering the stage of reossification several months or more after the appearance of the radiolucent areas. Femoral head specimens obtained from the patients at the stage of fragmentation indeed show an increased presence of osteoclasts in the areas of repair and replacement of the bone with a fibrovascular tissue66,67. The pathological repair process (imbalance of resorption and for-
mation) has been recognized by several investigators as a potential therapeutic target to improve the remodeling of the necrotic bone and to prevent the development of the deformity in the immature femoral head82-86.
In addition to these mechanisms, ischemic necrosis of the immature femoral head produces a growth arrest of the spherical growth plate surrounding the osseous epiphysis, which can potentially worsen the femoral head deformity (Fig. 2). Histological studies of specimens from patients with Legg- Calve´-Perthes disease66,74,76 and the experimental models of ischemic necrosis78,87,88 have shown necrosis of the deep layer of the articular cartilage, where endochondral ossification of the osseous epiphysis occurs. To obtain normal spherical growth of the epiphysis, endochondral ossification of the osseous epiphysis must be restored in a symmetric, circumferential fashion as distorted, asymmetric growth may further contribute to the head deformity. At the present time, the mechanisms involved with the restoration of the epiphyseal growth are poorly understood; however, vascular endothelial growth factor appears to be involved in this process as it is increased in the cartilage overlying the necrosis in the experimental studies89,90. It is also known to play an important role in angiogenesis and endochondral ossification91. A recent experimental study has suggested that exogenous bone morphogenetic protein (BMP)-2 administration may hasten the restorative process92.
663
TH E JO U R N A L O F BO N E & JO I N T SU R G E RY d J B J S .O R G |
PAT H O P H Y S I O LO G Y A N D NE W ST R AT E G I E S F O R T H E |
||||||||||||||||
VO LU M E 94-A |
d |
NU M B E R 7 |
d |
AP R I L 4, 2012 |
´ |
|
|
|
|||||||||
|
|
TR E AT M E N T O F LE G G -CA LV E-PE RT H E S DI S E A S E |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
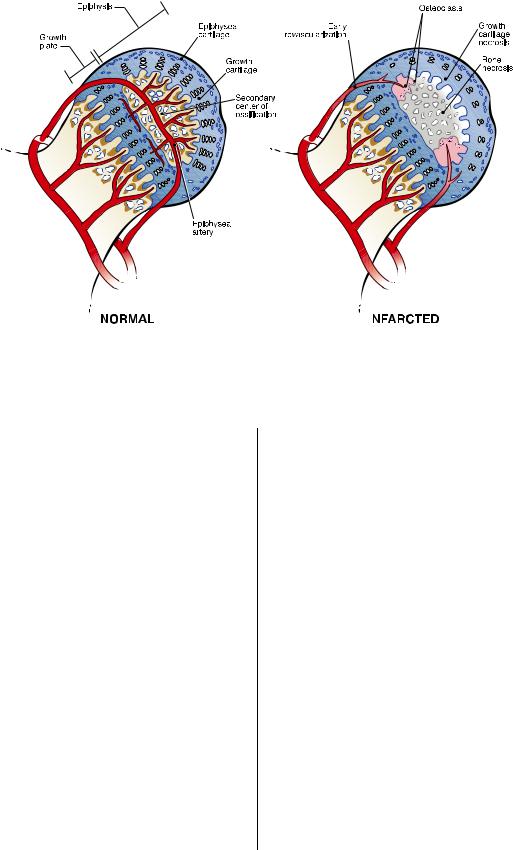
Fig. 2
A drawing representing a normal femoral head and an infarcted femoral head in an early stage of revascularization. Ischemic necrosis produces extensive cell death in the deep layer of the articular cartilage. This is the growth cartilage responsible for the circumferential growth of the secondary center of ossification. The ischemic damage produces a growth arrest of the secondary center, which may not be restored symmetrically during the healing process and produces growth disturbance of the secondary center. Revascularization of the infarcted femoral head is associated with a predominance of resorptive activity, as shown in the drawing. (Reproduced, with permission, from: the Texas Scottish Rite Hospital for Children, Dallas, Texas.)
Role of Hip Loading on the Pathogenesis of Femoral Head Deformity
Since the hip is a major load-bearing joint, the contribution of mechanical loading to the pathogenesis of the deformity in Legg-Calve´-Perthes disease must be considered. Unfortunately, very little is known about the hip forces associated with various activities of daily living in children. In adults, however, the hip contact pressures have been measured in a limited number of patients who had implantation of a strain gauge-instrumented hip replacement with a telemetric capability to transmit hip contact forces in real time93,94. These studies have shown that substantial loading of the hip occurs with normal daily activities. For instance, walking at a normal rate can produce hip forces of approximately 2.5 times the body weight with each step, while running can produce hip contact pressure of approximately five times the body weight with each stride. Along with the magnitude of loading, the frequency of loading may be important as an active child can take >7500 steps per day on average95. While it is reasonable to postulate that loading of the necrotic head contributes to the worsening of the deformity, the efficacy of restricting activities to prevent progression of the deformity is not well studied and it remains controversial.
Antiresorptive Therapy To Inhibit Pathological Resorption of the Necrotic Bone
Recognition of Legg-Calve´-Perthes disease as having an important resorptive component contributing to the femoral
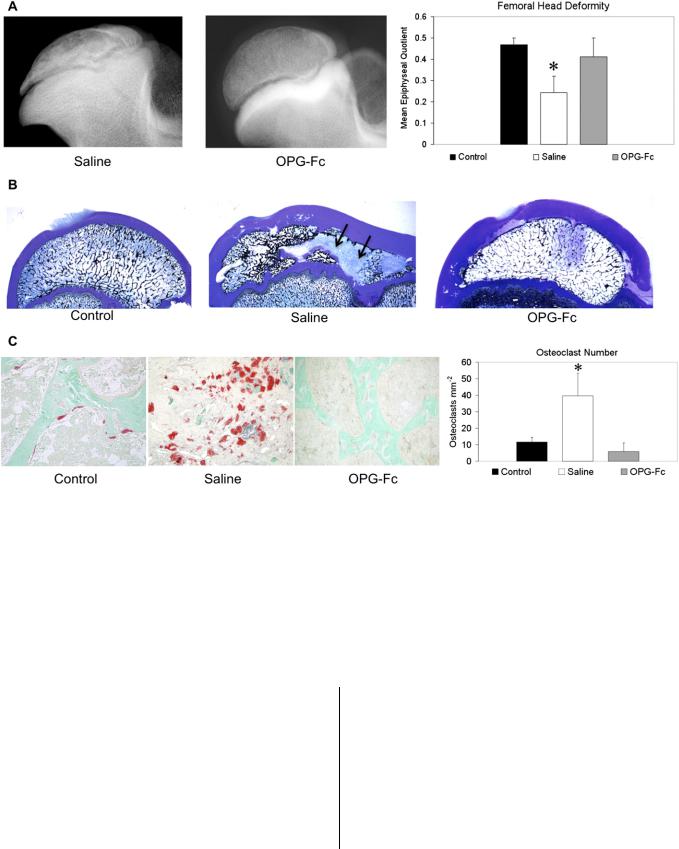
head deformity has led investigators to study the effects of inhibiting osteoclast-mediated bone resorption on preventing the deformity. The most direct evidence that osteoclastic resorption plays an important role in the development of the deformity comes from a large-animal study that used exogenous osteoprotegerin (OPG), a natural soluble decoy receptor of the receptor activator of nuclear factor (NF)-kß ligand (RANKL)83. It is well established that the interaction of the receptor activator of NF-kß (RANK) and its ligand, RANKL, is essential for osteoclast formation, function, and activation96. The binding of OPG to RANKL prevents the RANK-RANKL interaction and effectively inhibits osteoclast formation, activation, and survival. In a piglet model of ischemic necrosis, exogenous OPG therapy significantly decreased osteoclast number (p < 0.001), bone resorption (p < 0.001), and femoral head deformity (p < 0.001), providing evidence that specific targeting of osteoclastogenesis and osteoclastic function can positively modulate the outcome in this model (Fig. 3)68. Recently, a RANKL inhibitor called denosumab, a monoclonal antibody to RANKL, has become clinically available for the treatment of postmenopausal osteoporosis97. Its effect on femoral head osteonecrosis, however, has not been investigated.
More extensive studies have been performed with use of bisphosphonates, which are well-known inhibitors of osteoclastic resorption82,84-86,98,99. The mechanism of action of the aminobisphosphonates (the newer bisphosphonates) is to inhibit farnesyl pyrophosphatase, an enzyme in the HMG-CoA
664
TH E JO U R N A L O F BO N E & JO I N T SU R G E RY d J B J S .O R G |
PAT H O P H Y S I O LO G Y A N D NE W ST R AT E G I E S F O R T H E |
||||
VO LU M E 94-A |
d |
NU M B E R 7 |
d |
AP R I L 4, 2012 |
´ |
|
|
TR E AT M E N T O F LE G G -CA LV E-PE RT H E S DI S E A S E |
|||
Fig. 3
Fig. 3-A Radiographs of the central region of immature femoral heads showing a progression of the femoral head deformity in the piglet model. w = weeks following the induction of ischemic necrosis. Fig. 3-B A microcomputed tomographic image of a central region of the femoral head obtained at three weeks after the induction of ischemia, showing a circular area of bone resorption in the necrotic epiphysis. A radiodense, intravascular contrast material (Microfil; Flow Tech, Carver, Massachusetts) was infused into the distal aorta before imaging to detect revascularization in the necrotic epiphysis. The arrow is pointing to a vessel with multiple small branches within the area of resorption. Fig. 3-C A photomicrograph of a peripheral area of revascularization showing the increased presence of multinucleated cells (osteoclasts) and resorption of the trabecular bone (hematoxylin and eosin staining, ·20). Fig. 3-D A photomicrograph of a central area of revascularization showing fibrovascular tissue. The resorbed bone was not replaced by new bone (hematoxylin and eosin staining, ·10). (Figs. 3-A and 3-C are reproduced from Kim HK, Su PH. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg Am. 2002;84:1329-34.)
(3-hydroxy-3-methylglutaryl-coenyzyme A) reductase pathway, which interferes with prenylation of small GTPase proteins98. The uptake of these drugs by osteoclasts inhibits their resorptive activity and accelerates apoptosis. Unlike RANKL inhibitors, bisphosphonates do not inhibit osteoclast formation. Experimental studies in immature rat85,86 and pig models of ischemic necrosis84 have shown that systemically administered bisphosphonates can decrease bone resorption and femoral head deformity. In those studies, multiple dosing regimens were used as only a small portion of each dose was thought to access the necrotic femoral head. A study with use of 14C-labeled-ibandronate in immature pigs showed that the local bioavailability and distribution of this bisphosphonate in the necrotic head depended on the vascular status of the head99. In the early avascular stage of the piglet model, very little radioactivity was detected in the necrotic head following an intravenous dose of 14C-labeled ibandronate. A significant increase in the radioactivity was observed when the dose was administered at a later stage when revascularization of the femoral head had occurred (p < 0.05). These findings imply that oral or intravenous administration of a bisphosphonate has very limited access to the necrotic bone in the early stages of the disease. Because of this limitation, a multiple-dosing regimen is thought to be required to allow accumulation of the
drug in the necrotic region with each dose100. The repeated dosing over time is also thought to provide a greater opportunity for the drug to access the necrotic bone since the vascular status of the necrotic head improves over time.
A concern for a wide distribution of bisphosphonate with its long half-life on the immature skeleton and the limited accessibility of the systemically administered bisphosphonate on the infarcted head have led to an experimental investigation on the retention, distribution, and effects of a local, intraosseous administration of bisphosphonate for the treatment of femoral head osteonecrosis82,99. A single, local administration was shown to be effective and substantially decreased the total amount of bisphosphonate required to decrease the deformity in immature pigs82. Only 5% of the systemic dose was required in the local administration study compared with the systemic administration study. While antiresorptive drugs were found to be effective in preserving the necrotic bone in these studies, their effect on bone remodeling and new bone formation has been mixed. In rat models of osteonecrosis, new bone formation has been shown to occur on the necrotic bone surface85,86. This has not been demonstrated in the piglet model. The osteoblast surface was significantly lower in the OPG (88%; p < 0.01) and the ibandronate treatment (93%; p < 0.05) groups compared with their respective normal control groups83,84 (Fig.
665
TH E JO U R N A L O F BO N E & JO I N T SU R G E RY d J B J S .O R G |
PAT H O P H Y S I O LO G Y A N D NE W ST R AT E G I E S F O R T H E |
||||
VO LU M E 94-A |
d |
NU M B E R 7 |
d |
AP R I L 4, 2012 |
´ |
|
|
TR E AT M E N T O F LE G G -CA LV E-PE RT H E S DI S E A S E |
|||
Fig. 4
Fig. 4-A Radiographs of infarcted femoral heads in the animals treated with subcutaneous saline solution or osteoprotegerin (OPG-Fc), which inhibits osteoclast formation, function, and activation. The treatments were initiated two weeks after the induction of ischemic necrosis. The OPG group had a significantly better preservation of the femoral head, as indicated by the bar graph representing the mean epiphyseal quotient (ratio of femoral head height to its diameter) and the standard deviation. *p < 0.001 compared with the other groups. Fig. 4-B Low-magnification (·0.5) photomicrographs of the femoral heads from the control, saline solution, and OPG groups. The femoral heads from the saline solution group had areas of bone resorption (arrows) and femoral head deformity. The femoral heads from the OPG group had significantly less bone resorption and better preservation of the femoral head shape (McNeal tetrachromium staining). Fig. 4-C Higher-magnification (·10) photomicrographs of the femoral heads from the control, saline solution, and OPG groups stained for osteoclasts (red stain) (tartrate-resistant acid phosphatase staining). The mean osteoclast number (and standard deviation) was significantly lower in the OPG group, as shown on the bar graph. *p < 0.0001 compared with the other groups. (Reproduced, with permission, from: Kim HK, Morgan-Bagley S, Kostenuik P. RANKL inhibition: a novel strategy to decrease femoral head deformity after ischemic osteonecrosis. J Bone Miner Res. 2006;21:1946-54. 2006 American Society for Bone and Mineral Research.)
4). At this point, it is unclear whether this is an issue of the duration of follow-up or an issue of the antiresorptive agents having an inhibitory effect on new bone formation due to a coupling of the two processes.
Currently, the use of bisphosphonate therapy to treat Legg-Calve´-Perthes disease is investigational. A randomized clinical trial comparing intravenous administration of zoledronic acid and standard care (weight-bearing restriction and current treatments) for Legg-Calve´-Perthes disease (clinical trial registration ACTRN12610000407099) is under way in Australia.
Bone Anabolic Therapy To Stimulate New Bone Formation
The effects of BMP administration on bone formation in the context of femoral head osteonecrosis are being investigated since BMP-2 and BMP-7 are potent osteoinductive agents shown to promote bone healing under difficult clinical cir- cumstances101-103. The use of BMP-2 to stimulate healing as an adjunct to core decompression or a strut graft has been investigated in adult animal models of osteonecrosis104,105 and femoral head defect106. The animal studies have described improved bone healing and increased new bone formation with
666
TH E JO U R N A L O F BO N E & JO I N T SU R G E RY d J B J S .O R G |
PAT H O P H Y S I O LO G Y A N D NE W ST R AT E G I E S F O R T H E |
||||
VO LU M E 94-A |
d |
NU M B E R 7 |
d |
AP R I L 4, 2012 |
´ |
|
|
TR E AT M E N T O F LE G G -CA LV E-PE RT H E S DI S E A S E |
|||
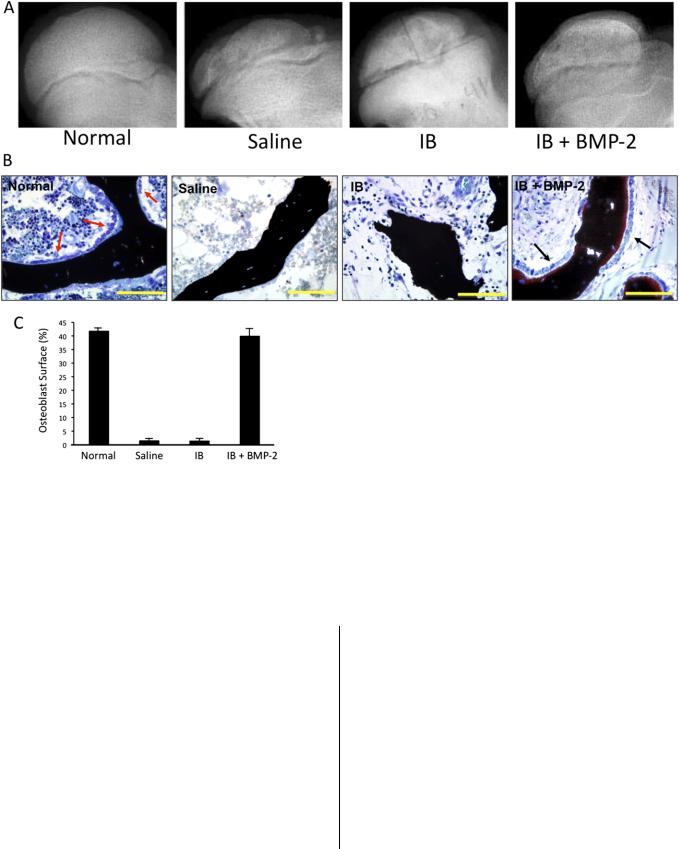
Fig. 5
Fig. 5-A Radiographs of the femoral head of animals treated with intraosseous saline solution, ibandronate (IB), or IB and bone morphogenetic protein (BMP)-2. A single injection of the respective agent(s) was rendered one week following the induction of ischemic necrosis. Fig. 5-B Photomicrographs showing osteoblasts (black arrows) on the surface of the trabecular bone in the IB 1 BMP-2 group and the normal, control group (red arrows). The femoral heads from the saline and the IB groups had a lack of osteoblasts on the trabecular surfaces. Bars = 100 mm. Fig. 5-C A bar graph showing the mean percentage (and standard deviation) of trabecular bone surface on which osteoblasts were attached, with a significantly greater osteoblast surface, an indicator of bone formation, in the IB 1 BMP-2 group than in the saline solution and the IB groups. (Reproduced from: Vandermeer JS, Kamiya N, Aya-ay J, Garces A, Browne R, Kim HKW. Local administration of ibandronate and bone morphogenetic protein-2 stimulates bone formation and decreases femoral head deformity following ischemic osteonecrosis of the immature femoral head. J Bone Joint Surg Am. 2011;93:905-13.)
the BMP treatment. BMP-2 has also been used clinically to treat femoral head osteonecrosis in adults as an adjunct to core decompression107. In a case series of seventeen hips (sixteen Ficat Stage-II hips and one Ficat Stage-III hip), three hips progressed and required total hip replacement, while fourteen hips had good results at an average follow-up duration of fiftythree months107. Since the study was retrospective in nature without a control group, the true efficacy of BMP-2 treatment could not be determined.
The use of BMPs to treat femoral head osteonecrosis in a pediatric population has not been reported, as far as we know. However, a combined treatment of bisphosphonate (ibandronate) and BMP-2 with use of a local intraosseous injection technique has been reported in a large-animal study92 (Fig. 5). The rationale for using bisphosphonate and BMP-2 together in
the study was that, along with osteoinductive properties, BMP-2 is known to transiently stimulate osteoclastogenesis and bone resorption108-111. In comparison with the group that had local administration of ibandronate alone, the group that had local administration of ibandronate and BMP-2 showed a significantly greater percent of osteoblast surface on the trabecular bone (p < 0.0001), greater bone volume (p < 0.0001), and remodeling of the necrotic femoral head92. Furthermore, in three of the six femoral heads treated with ibandronate and BMP-2, a restoration of growth of the epiphysis was observed. Along with improved bone healing, the round shape of the femoral heads was better preserved in the ibandronate and BMP-2 treatment group compared with the saline solution group. In that study, however, heterotopic ossification was observed in the hip joint capsule of the animals treated with the
667
TH E JO U R N A L O F BO N E & JO I N T SU R G E RY d J B J S .O R G |
PAT H O P H Y S I O LO G Y A N D NE W ST R AT E G I E S F O R T H E |
||||
VO LU M E 94-A |
d |
NU M B E R 7 |
d |
AP R I L 4, 2012 |
´ |
|
|
TR E AT M E N T O F LE G G -CA LV E-PE RT H E S DI S E A S E |
|||
ibandronate and BMP-2. It is postulated that a leakage of BMP- 2 into the joint on removal of the injection needle and injection of BMP-2 shortly after the surgical trauma to create the femoral head ischemia may have predisposed the soft tissues around the hip to heterotopic ossification. The use of BMPs to treat Legg- Calve´-Perthes disease is still experimental at this time, and further studies are warranted prior to the use of BMPs to treat children with femoral head osteonecrosis.
Clinical Studies on Bisphosphonate Therapy for Femoral Head Osteonecrosis
To date, only a small number of studies have investigated the effects of bisphosphonate therapy for the treatment of femoral head osteonecrosis112-117. Most of those studies assessed shortterm outcomes in adults with nontraumatic femoral head osteonecrosis. Four clinical studies on nontraumatic osteonecrosis in adults have shown some beneficial effects of bisphosphonate therapy on pain, function, and preservation of the femoral head112-115. It is of note that, in the randomized clinical trial reported by Lai et al., only two of twenty-nine hips required total hip replacement following oral alendronate therapy for six months compared with nineteen of twenty-five hips in the nontreatment group at the minimum follow-up period of two years114. A study by Agarwala et al. showed that oral bisphosphonate therapy produced the best results when initiated in the early stage of osteonecrosis (stage-1 disease). At the mean follow-up interval of four years, 56% (seventy-two) of 129 patients with stage-2 disease had a radiographic evidence of collapse, whereas only 13% (twenty-seven) of 215 patients with stage-1 disease had a collapse113. Limitations of that study were that the extent of the head involvement was not assessed and that there were no controls.
A prospective case series of adolescent patients who had traumatic osteonecrosis because of unstable slipped capital femoral epiphysis, hip fracture, or dislocation showed that those who had a cold bone scan and who were treated with intermittent intravenous bisphosphonate therapy over an average period of twenty months did reasonably well at the minimum follow-up of two years116. Nine of seventeen patients had a spherical femoral head, and fourteen of seventeen pa-
tients were pain-free. That study, however, did not have a control group. A small study of seventeen patients with osteonecrosis as a complication of chemotherapy for childhood leukemia has also been reported117. In general, improvements in the pain scores, analgesic requirement, and function were observed in the nine patients who received bisphosphonate therapy; however, a radiographic benefit of the therapy could not be demonstrated. In a small number of patients with Legg- Calve´-Perthes disease, the systemic effects of intravenous bisphosphonate have been reported recently100; however, its effect on the preservation of the femoral head has yet to be reported.
Overview
Multicenter prospective cohort studies (level-II evidence) have shown that the benefits of treatment for older children affected with Legg-Calve´-Perthes disease are modest, even with operative treatments. A better understanding of the pathogenesis of the femoral head deformity is required to develop more effective treatments. Experimental studies have revealed that an imbalance of bone resorption and bone formation plays an important role in the development of the deformity. Further studies are needed to address the questions about why there is uncoupling of bone resorption and formation during the remodeling of the necrotic femoral head, what molecular mechanisms are involved, and how we can therapeutically target them more effectively. While recent experimental studies have shown that antiresorptive and anabolic agents can effectively modulate the pathological repair process, further studies are needed to address the clinical efficacy and safety of these agents for the treatment of Legg-Calve´-Perthes disease. n
Harry K.W. Kim, MD, MS, FRCSC Center for Excellence in Hip Disorders, Department of Orthopaedic Surgery, Texas Scottish Rite Hospital for Children,
UT Southwestern Medical Center, 2222 Welborn Street, Dallas, TX 75218. E-mail address: Harry.kim@tsrh.org
References
1.Gray IM, Lowry RB, Renwick DH. Incidence and genetics of Legg-Perthes disease (osteochondritis deformans) in British Columbia: evidence of polygenic determination. J Med Genet. 1972;9:197-202.
2.Margetts BM, Perry CA, Taylor JF, Dangerfield PH. The incidence and distribution of Legg-Calve´-Perthes’ disease in Liverpool, 1982-95. Arch Dis Child. 2001;84:
351-4.
3.Molloy MK, MacMahon B. Incidence of Legg-Perthes disease (osteochondritis deformans). N Engl J Med. 1966;275:988-90.
4.Wiig O, Terjesen T, Svenningsen S, Lie SA. The epidemiology and aetiology of Perthes’ disease in Norway. A nationwide study of 425 patients. J Bone Joint Surg Br. 2006;88:1217-23.
5.Legg A. An obscure affection of the hip joint. Boston Med Surg J. 1910;162: 202-4.
6.Calve´ J. [On a particular form of pseudo-coxalgia associated with a characteristic deformity of the upper end of the femur]. Rev Chir. 1910;42:54-84. French.
7.Perthes G. [Concerning arthritis deformans juvenilis]. Deutsche Zeitschr Chir. 1910;107:111-59. German.
8.Balasa VV, Gruppo RA, Glueck CJ, Wang P, Roy DR, Wall EJ, Mehlman CT, Crawford AH. Legg-Calve-Perthes disease and thrombophilia. J Bone Joint Surg Am. 2004;86:2642-7.
9.Glueck CJ, Glueck HI, Greenfield D, Freiberg R, Kahn A, Hamer T, Stroop D, Tracy T. Protein C and S deficiency, thrombophilia, and hypofibrinolysis: pathophysiologic causes of Legg-Perthes disease. Pediatr Res. 1994;35:383-8.
10.Hall DJ. Genetic aspects of Perthes’ disease. A critical review. Clin Orthop Relat Res. 1986;209:100-14.
11.Hresko MT, McDougall PA, Gorlin JB, Vamvakas EC, Kasser JR, Neufeld EJ. Prospective reevaluation of the association between thrombotic diathesis and leggperthes disease. J Bone Joint Surg Am. 2002;84:1613-8.
12.Miyamoto Y, Matsuda T, Kitoh H, Haga N, Ohashi H, Nishimura G, Ikegawa S. A recurrent mutation in type II collagen gene causes Legg-Calve´-Perthes disease in
a Japanese family. Hum Genet. 2007;121:625-9.
13. Neidel J, Zander D, Hackenbroch MH. Low plasma levels of insulin-like growth factor I in Perthes’ disease. A controlled study of 59 consecutive children. Acta Orthop Scand. 1992;63:393-8.
668
TH E JO U R N A L O F BO N E & JO I N T SU R G E RY d J B J S .O R G VO LU M E 94-A d NU M B E R 7 d AP R I L 4, 2012
14. Su P, Li R, Liu S, Zhou Y, Wang X, Patil N, Mow CS, Mason JC, Huang D, Wang Y. Age at onset-dependent presentations of premature hip osteoarthritis, avascular necrosis of the femoral head, or Legg-Calve´-Perthes disease in a single family, consequent upon a p.Gly1170Ser mutation of COL2A1. Arthritis Rheum. 2008;58:1701-6.
15.Wynne-Davies R, Gormley J. The aetiology of Perthes’ disease. Genetic, epidemiological and growth factors in 310 Edinburgh and Glasgow patients. J Bone Joint Surg Br. 1978;60:6-14.
16.Catterall A. The natural history of Perthes’ disease. J Bone Joint Surg Br. 1971;53:37-53.
17.Gower WE, Johnston RC. Legg-Perthes disease. Long-term follow-up of thirty-six patients. J Bone Joint Surg Am. 1971;53:759-68.
18.McAndrew MP, Weinstein SL. A long-term follow-up of Legg-Calve´-Perthes disease. J Bone Joint Surg Am. 1984;66:860-9.
19.Mose K. Methods of measuring in Legg-Calve´-Perthes disease with special regard to the prognosis. Clin Orthop Relat Res. 1980;150:103-9.
20.Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calve´- Perthes disease. J Bone Joint Surg Am. 1981;63:1095-108.
21.Waldenstrom H. The definitive forms of coxa plana. Acta Radiol. 1922;1:384.
22.Herring JA, Neustadt JB, Williams JJ, Early JS, Browne RH. The lateral pillar classification of Legg-Calve´-Perthes disease. J Pediatr Orthop. 1992;12:143-50.
23.Salter RB, Thompson GH. Legg-Calve´-Perthes disease. The prognostic significance of the subchondral fracture and a two-group classification of the femoral head involvement. J Bone Joint Surg Am. 1984;66:479-89.
24.Axer A. Subtrochanteric osteotomy in the treatment of Perthes’ disease: a preliminary report. J Bone Joint Surg Br. 1965;47:489-99.
25.Brotherton BJ, McKibbin B. Perthes’ disease treated by prolonged recumbency and femoral head containment: a long-term appraisal. J Bone Joint Surg Br. 1977;59:8-14.
26.Joseph B, Rao N, Mulpuri K, Varghese G, Nair S. How does a femoral varus osteotomy alter the natural evolution of Perthes’ disease? J Pediatr Orthop B. 2005;14:10-5.
27.Kruse RW, Guille JT, Bowen JR. Shelf arthroplasty in patients who have Legg- Calve´-Perthes disease. A study of long-term results. J Bone Joint Surg Am.
1991;73:1338-47.
28.Petrie JG, Bitenc I. The abduction weight-bearing treatment in Legg-Perthes’ disease. J Bone Joint Surg Br. 1971;53:54-62.
29.Salter RB. The present status of surgical treatment for Legg-Perthes disease. J Bone Joint Surg Am. 1984;66:961-6.
30.Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004;86:2121-34.
31.Rosenfeld SB, Herring JA, Chao JC. Legg-Calve-Perthes disease: a review of cases with onset before six years of age. J Bone Joint Surg Am. 2007;89:2712-22.
32.Wiig O. Perthes’ disease in Norway. A prospective study on 425 patients. Acta Orthop Suppl. 2009;80:1-44.
33.Eyre-Brook A. Osteochondritis deformans coxae juvenilis or Perthes’ disease: the results of treatment by traction in recumbency. Br J Surg. 1936;24:166.
34.Harrison MH, Menon MP. Legg-Calve´-Perthes disease. The value of roentgenographic measurement in clinical practice with special reference to the broomstick plaster method. J Bone Joint Surg Am. 1966;48:1301-18.
35.Bobechko WP. The Toronto brace for Legg-Perthes disease. Clin Orthop Relat Res. 1974;102:115-7.
36.Curtis BH, Gunther SF, Gossling HR, Paul SW. Treatment for Legg-Perthes disease with the Newington ambulation-abduction brace. J Bone Joint Surg Am. 1974;56:1135-46.
37.Harrison MH, Turner MH, Nicholson FJ. Coxa plana. Results of a new form of splinting. J Bone Joint Surg Am. 1969;51:1057-69.
38.King EW, Fisher RL, Gage JR, Gossling HR. Ambulation-abduction treatment in Legg-Calve´-Perthes disease (LCPD). Clin Orthop Relat Res. 1980;150:43-8.
39.Purvis JM, Dimon JH 3rd, Meehan PL, Lovell WW. Preliminary experience with the Scottish Rite Hospital abduction orthosis for Legg-Perthes disease. Clin Orthop Relat Res. 1980;150:49-53.
40.Vukasinovic Z, Spasovski D, Vucetic C, Cobeljic G, Zivkovic Z, Matanovic D. Triple pelvic osteotomy in the treatment of Legg-Calve-Perthes disease. Int Orthop. 2009;33:1377-83.
41.Javid M, Wedge JH. Radiographic results of combined Salter innominate and femoral osteotomy in Legg-Calve´-Perthes disease in older children. J Child Orthop.
2009;3:229-34.
42.Rab GT. Containment of the hip: a theoretical comparison of osteotomies. Clin Orthop Relat Res. 1981;154:191-6.
43.Rab GT. Theoretical study of subluxation in early Legg-Calve´-Perthes disease. J Pediatr Orthop. 2005;25:728-33.
44.Wiig O, Terjesen T, Svenningsen S. Prognostic factors and outcome of treatment in Perthes’ disease: a prospective study of 368 patients with five-year follow-up.
J Bone Joint Surg Br. 2008;90:1364-71.
PAT H O P H Y S I O LO G Y A N D NE W ST R AT E G I E S F O R T H E
TR E AT M E N T O F LE G G -CA LV ´ -PE RT H E S DI S E A S E
E
45.Heikkinen E, Puranen J. Evaluation of femoral osteotomy in the treatment of Legg-Calve´-Perthes disease. Clin Orthop Relat Res. 1980;150:60-8.
46.Bahmanyar S, Montgomery SM, Weiss RJ, Ekbom A. Maternal smoking during pregnancy, other prenatal and perinatal factors, and the risk of Legg-Calve´-Perthes
disease. Pediatrics. 2008;122:e459-64.
47. Gordon JE, Schoenecker PL, Osland JD, Dobbs MB, Szymanski DA, Luhmann SJ. Smoking and socio-economic status in the etiology and severity of Legg-Calve´- Perthes’ disease. J Pediatr Orthop B. 2004;13:367-70.
48.Matsumoto T, Enomoto H, Takahashi K, Motokawa S. Decreased levels of IGF binding protein-3 in serum from children with Perthes’ disease. Acta Orthop Scand. 1998;69:125-8.
49.Neidel J, Sch¨onau E, Zander D, Rutt¨ J, Hackenbroch MH. Normal plasma levels of IGF binding protein in Perthes’ disease. Follow-up of previous report. Acta Orthop Scand. 1993;64:540-2.
50.Liu YF, Chen WM, Lin YF, Yang RC, Lin MW, Li LH, Chang YH, Jou YS, Lin PY, Su JS, Huang SF, Hsiao KJ, Fann CS, Hwang HW, Chen YT, Tsai SF. Type II collagen gene variants and inherited osteonecrosis of the femoral head. N Engl J Med. 2005;352: 2294-301.
51.Arruda VR, Belangero WD, Ozelo MC, Oliveira GB, Pagnano RG, Volpon JB, Annichino-Bizzacchi JM. Inherited risk factors for thrombophilia among children with Legg-Calve´-Perthes disease. J Pediatr Orthop. 1999;19:84-7.
52.Eldridge J, Dilley A, Austin H, EL-Jamil M, Wolstein L, Doris J, Hooper WC, Meehan PL, Evatt B. The role of protein C, protein S, and resistance to activated protein C in Legg-Perthes disease. Pediatrics. 2001;107:1329-34.
53.Gruppo R, Glueck CJ, Wall E, Roy D, Wang P. Legg-Perthes disease in three siblings, two heterozygous and one homozygous for the factor V Leiden mutation. J Pediatr. 1998;132:885-8.
54.Szepesi K, Pos´an´ E, Harsfalvi´ J, Ajzner E, Szucs¨ G, Gasp´ar´ L, Csernatony´ Z, Udvardy M. The most severe forms of Perthes’ disease associated with the homozygous Factor V Leiden mutation. J Bone Joint Surg Br. 2004;86:426-9.
55.Vosmaer A, Pereira RR, Koenderman JS, Rosendaal FR, Cannegieter SC. Coagulation abnormalities in Legg-Calve´-Perthes disease. J Bone Joint Surg Am.
2010;92:121-8.
56. Hayek S, Kenet G, Lubetsky A, Rosenberg N, Gitel S, Wientroub S. Does thrombophilia play an aetiological role in Legg-Calve´-Perthes disease? J Bone Joint Surg Br. 1999;81:686-90.
57.Kealey WD, Mayne EE, McDonald W, Murray P, Cosgrove AP. The role of coagulation abnormalities in the development of Perthes’ disease. J Bone Joint Surg Br. 2000;82:744-6.
58.Kenet G, Ezra E, Wientroub S, Steinberg DM, Rosenberg N, Waldman D, Hayek S. Perthes’ disease and the search for genetic associations: collagen mutations, Gaucher’s disease and thrombophilia. J Bone Joint Surg Br. 2008;90:1507-11.
59.Lopez´-Franco M, Gonzalez´-Moran´ G, De Lucas JC Jr, Llamas P, de Velasco JF, Vivancos JC, Epeldegui-Torre T. Legg-perthes disease and heritable thrombophilia. J Pediatr Orthop. 2005;25:456-9.
60.Sirvent N, Fisher F, el Hayek T, Appert A, Giudicelli H, Griffet J. Absence of congenital prethrombotic disorders in children with Legg-Perthes disease. J Pediatr Orthop B. 2000;9:24-7.
61.Atsumi T, Yamano K, Muraki M, Yoshihara S, Kajihara T. The blood supply of the lateral epiphyseal arteries in Perthes’ disease. J Bone Joint Surg Br. 2000;82:392-8.
62.de Camargo FP, de Godoy RM Jr, Tovo R. Angiography in Perthes’ disease. Clin Orthop Relat Res. 1984;191:216-20.
63.Theron´ J. Angiography in Legg-Calve´-Perthes disease. Radiology. 1980;135: 81-92.
64.Conway JJ. A scintigraphic classification of Legg-Calve´-Perthes disease. Semin Nucl Med. 1993;23:274-95.
65.Lamer S, Dorgeret S, Khairouni A, Mazda K, Brillet PY, Bacheville E, Bloch J, Pennecxot GF, Hassan M, Sebag GH. Femoral head vascularisation in Legg-Calve´-Perthes
disease: comparison of dynamic gadolinium-enhanced subtraction MRI with bone scintigraphy. Pediatr Radiol. 2002;32:580-5.
66. Catterall A, Pringle J, Byers PD, Fulford GE, Kemp HB, Dolman CL, Bell HM, McKibbin B, Ralis´ Z, Jensen OM, Lauritzen J, Ponseti IV, Ogden J. A review of the morphology of Perthes’ disease. J Bone Joint Surg Br. 1982;64:269-75.
67.Jonsater S. Coxa plana; a histo-pathologic and arthrografic study. Acta Orthop Scand Suppl. 1953;12:5-98.
68.Kim HK, Su PH. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg Am. 2002;84:1329-34.
69.Shapiro F, Connolly S, Zurakowski D, Menezes N, Olear E, Jimenez M, Flynn E, Jaramillo D. Femoral head deformation and repair following induction of ischemic necrosis: a histologic and magnetic resonance imaging study in the piglet. J Bone Joint Surg Am. 2009;91:2903-14.
70.Sanchis M, Zahir A, Freeman MA. The experimental simulation of Perthes disease by consecutive interruptions of the blood supply to the capital femoral epiphysis in the puppy. J Bone Joint Surg Am. 1973;55:335-42.
71.Zahir A, Freeman AR. Cartilage changes following a single episode of infarction of the capital femoral epiphysis in the dog. J Bone Joint Surg Am. 1972;54:125-36.
