
- •Imperfection
- •Chapter 4 Crystal Defects and Noncrystalline structure – Imperfection
- •4.1 The solid solution – chemical imperfection
- •4.2 Hume-Rothery rules
- •4.3 Point defects – zero- dimensional imperfections
- •4.4 Linear defects, or dislocations – one-dimensional imperfection
- •4.5 Planar defects – two-dimensional imperfections
- •4.6 Noncrystalline solids – three-dimensional imperfections
- •4.7 Quasicrystals
- •4.8 Microscopy
- •4.8.1 Electron microscope
- •4.8.2 Scanning electron microscope (sem)
- •4.35 A commercial sem. (Courtesy of Hitachi Scientific Instruments.)
CHAPTER 4
Crystal Defects and Noncrystalline Structure.
Imperfection
Chapter 4 Crystal Defects and Noncrystalline structure – Imperfection
We discussed about perfectly repetitive crystalline structures. But we understand that nothing in our world is quite perfect. Now we discuss about imperfections.
Remember:
That is no material can be prepared without some degree of chemical impurity. The impurity atoms or ions in the resulting solid solution serve to alter the structural regularity of the ideally pure material.
The simplest type of flaw is the point defect, for example, a missing atom (vacancy). This type of flaw is the inevitable result of the normal thermal vibration of atoms in any solid at a temperature above absolute zero.
Linear defects, or dislocations, follow an extended and sometimes complex path through the crystal structure.
Planar defects represent the boundary between a nearly perfect crystalline region and its surroundings.
4.1 The solid solution – chemical imperfection
It is not possible to avoid some contamination of practical materials. Even high-purity semiconductor products have some measurable level of impurity atoms. Many engineering materials contain significant amounts of several different components (commercial metal alloys are examples). As a result, all materials that the engineer deals with on a daily basis are actually solid solutions.
1.The concept of a solid solution may be difficult to grasp, (it is essentially equivalent to the more familiar liquid solution, such as the water- alcohol system (Fig.4.1). The complete solubility of alcohol in water is the result of complete molecular mixing.
2. Similar result is seen in Fig.4.2, which shows a solid solution of copper and nickel atoms sharing the fcc crystal structure. Nickel acts as a solute dissolving in the copper solvent. This configuration is referred to as a substitutional solid solution because the nickel atoms are substituting for copper atoms on fcc atom sites.
Remember:
This configuration will tend to occur when the atoms do not differ greatly in size.
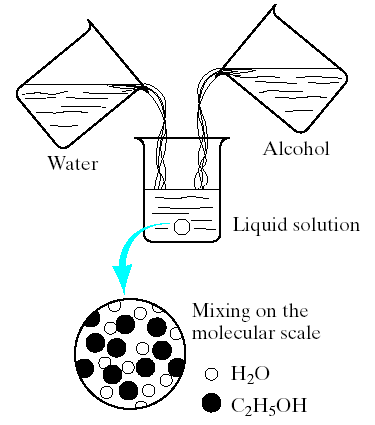
Fig.4.1. Forming a liquid solution of water and alcohol. Mixing occurs on the molecular scale.
The water-alcohol system represents 2 liquids completely soluble in each other in all proportions.
For complete miscibility in metallic solid solutions, 2 metals must be quite similar.
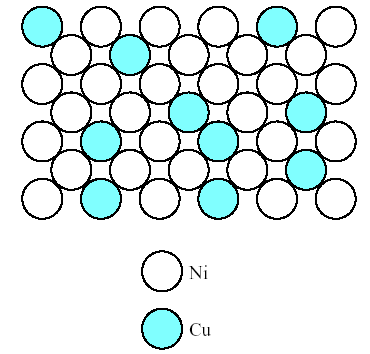
Fig.4.2. Solid solution of copper in nickel shown along a (100) plane. This is a substitution solid solution with nickel atoms substituting for copper atoms on fcc atom sites.
4.2 Hume-Rothery rules
Less than about 15 % difference in atomic radii
The same crystal structure
Similar electronegativities ( the ability of the atom to attract an electron)
The same valence
Remember: If 1 or more of the Hume-Rothery rules are violated, only partial solubility is possible. (Example: less than 2 at % (atomic percent) silicon is soluble in Al. Why? Because Al and Si violate rules 1,2,4. As regarding rule 3, electron negatives of Al and Si are quite different, despite their adjacent positions on the periodic table.
Fig. 4-2 shows a random solid solution. By contrast, some systems form ordered solid solutions (alloy AuCu3 – Fig. 4.3).
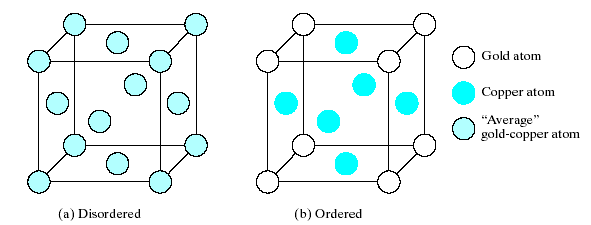
Fig.4-3. Ordering of the solid solution in the AuCu3 alloy system. (a) Above 390 0C there is a random distribution of the Au and Cu atoms among the fcc sites.(b) Below 390 0C, the Au atoms preferentially occupy the corner positions in the unit cell, giving a simple cubic Bravais lattice.
At high temperatures (above 390 0C), thermal agitation keeps a random distribution of the Au and Cu atoms among the fcc sites. Below 390 0C, the Cu atoms preferentially occupy the face-centered positions, and the Au atoms preferentially occupy corner positions in the unit cell. Ordering may produce a new crystal structure similar to some of the ceramic compound structures. For AuCu3 at low temperatures, the structure is based on the simple cubic Bravais lattice. When atom sizes differ greatly, substitution of the smaller atom on a crystal structure site may be energetically unstable. In this case, it is more stable for the smaller atom simply to fit into one of the spaces, or interstices, among adjacent atoms in the crystal structure. Fig 4.4 shows such interstitial solid solution, which carbon dissolved interstitially in –Fe. This interstitial solution is a dominant phase in steels. Although more stable than a substitutional configuration of C atoms on Fe lattice sites, the interstitial structure of Fig. 4.4 produces considerable strain locally to the –Fe crystal structure, and less than 0,1 at % C is soluble in –Fe.
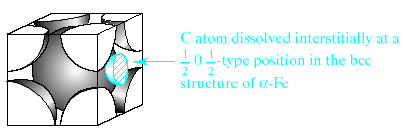
Fig.4.4. Interstitial solid solution of carbon in –iron. The carbon atom is small enough to fit with the some strain in the interstice (or opening) among adjacent Fe atoms in the structure of importance to steel industry.
To this point, we have looked at solid-solution formation in which a pure metal or semiconductor solvent dissolve some solute atoms either substitutionally or interstitially. The principles of substitutional solid-solution formation in these elemental systems also apply to compounds. Example: Fig. 4.5 shows a random, substitutional solid solution of NiO in MgO.
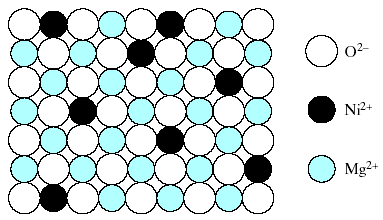
Fig.4-5. Random, substitutional solid solution of NiO in MgO. The O2- arrangement is unaffected. The substitution occurs among Ni2+ and Mg 2+ ions.
Here the O2- arrangement is unaffected. The substitution occurs between Ni2+ and Mg 2+. Fig. 4.5 example is very simple. In general, the charged state for ions in a compound affects the nature of the substitution. In other words, one could not indiscriminately replace all of the Ni2+ ions in Fig. 4.5 with Al3+ ions. This would be equivalent to forming a solid solution of Al2O3 in MgO, each having distinctly different formulas and crystal structures. The higher valence of Al3+ would give a net positive charge to the oxide compound, creating a highly unstable condition. As a result, an additional ground rule in forming compound solid solutions is the maintenance of charge neutrality.
Fig. 4.6 shows how charge neutrality is maintained in a dilute solution of Al3+ in MgO by having only 2 Al3+ ions fill every 3 Mg2+ sites.
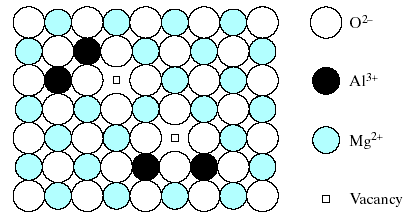
Fig.4.6. Substitutional solid solution of Al2O3 in MgO is not simple as the case of NiO in MgO (Fig.4-5). The requirement of charge neutrality in the overall compound permits only two Al+ ions to fill every three Mg2+ vacant sites, leaving one Mg 2+ vacancy.
This leaves 1 Mg2+ site vacancy for each 2 Al3+ substitutions. This type of vacancy and several other point defects will be discussed further. This example of a defect compound suggests the possibility of an even more subtle type of solid solution. Fig. 4.7 shows a nonstoichiometric compound, Fe1-xO, in which x is ~ 0.05.An ideally stoichiometric FeO would be identical to MgO with a NaCl- type crystal structure consisting of equal numbers of Fe2+ and O2- ions.
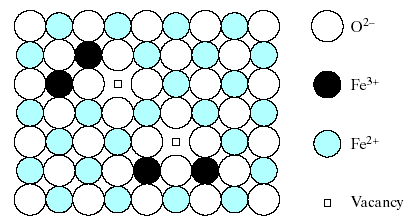
Fig. 4.7 Iron oxide, Fe1-xO with x ~ 0.05 is an example of a nonstoichiometric compound. Similar to the case of Fig.4-6, both Fe2+ and Fe3+ ions occupy the carbon sites with one Fe2+ vacancy occurring for every two Fe3+ ions present.
Remember: ideal FeO is never found in nature due to the multivalent nature of iron. Some Fe3+ ions are always present. As a result, these Fe3+ ions play the same role in the Fe1-xO structure as Al3+ in the Al2O3 in the MgO solid solution of Fig. 4.6. One Fe2+ site vacancy is required to compensate for the presence of every 2 Fe3+ ions in order to maintain charge neutrality.
