
Sulfuric acid
Sulfuric acid is probably the most important chemical substance which is not found in Nature. The total world consumption is about 25 000 000 tons per year.
Production in industry:
1. Contact method. Catalytic oxidation of SO2 by air oxygen (440 oC, in the presence of V2O5 catalyst):
2SO2 + O2 2SO3; Н0298 =-184.4 kJ/mol
SO3 is absorbed by concentrated H2SO4. Usage of water is not efficient because gaseous SO3 reacts first of all with water steam and then predominately H2SO4. Fog is the product of this interaction. The solution of SO3 in H2SO4 has a technical name oleum that emphasizes its high viscosity (oleum - Lat. Oil).
H2SO4 dissolves SO3 in any proportions, so the composition of oleum is H2SO4·nSO3. Oleum contains several polysulfuric acids:

The content of SO3 in oleum is 20-65%. To produce acid, oleum is mixed with H2SO4 having the necessary amount of water. Under the influence of water S—O—S bonds of polysulfuric acid are destroyed and converted into H2SO4 acid of required concentration.
H2SO4 produced by this method has high purity and can be of any concentration.
2. Lead Chamber process. It was called so because the chamber is lined with lead, which resists the action of cold sulfuric acid; the homogeneous catalyst is nitrogen oxide. The method was applied for the first time in the middle of 18th century.
It was the most important method of industrial production of H2SO4 before the modern contact method was developed. Its essence is SO2 oxidation by nitrogen oxide, NO2, in the presence of water:
SO2 + NO2 + H2O = Н2SO4 + NO
Nitrogen(II) oxide (NO) is oxidised again into the initial NO2 and returned to the reaction: 2NO + О2 = 2NO2.
The process is carried out in special towers (see scheme below). This method gives about 76% H2SO4 used in the manufacture of fertilizers:

Structure. Н2SO4 and НSO4- -ion are the distorted tetrahedrons, with sulfur atom in the centre of a polyhedron. SO42--ion has the shape of regular tetrahedron where all distances and angles are equivalent. Orbitals of sulfur have the state of sp3-hybridisation:

S—O bonds are very strong because of the additional -bonding: displacement of unshared electron pairs of O atoms to the vacant 3d-orbital of S. The equal S—O bonds lengths is a consequence of delocalisation of -electron density and the negative ion charge.
Properties. Anhydrous (100%) Н2SO4 under the normal conditions is a colourless oily liquid (m.p. = 10.4 oC). Н2SO4 molecules have intermolecular H-bonds with one another in the liquid and solid state either.

Н2SO4 azeotropei contains Н2SO4 98.3 % wt. and 1.7% by weight of water, b.p. = 338.8 oC.
Н2SO4 is a strong dibasic acid in aqueous solutions:
Н2SO4 Н+ + НSO4-, K1= 1·103
НSO4- Н+ + SO42-, K2= 1,2·10-2
Due to the large differences in dissociation constants, Н2SO4 forms sulfates and hydrogen sulfates. The scheme of their mutual conversion is shown below:
H2SO4
K2SO4 KHSO4
KOH
Being dissolved Н2SO4 in water, a lot of heat liberates owing to hydrates formation. Therefore, concentrated Н2SO4 and water must be mixed with caution. To prevent spray of liquid, pour Н2SO4 (as a heavier component) into water, not vice versa. Formation of very stable hydrates explains Н2SO4 water absorption properties.
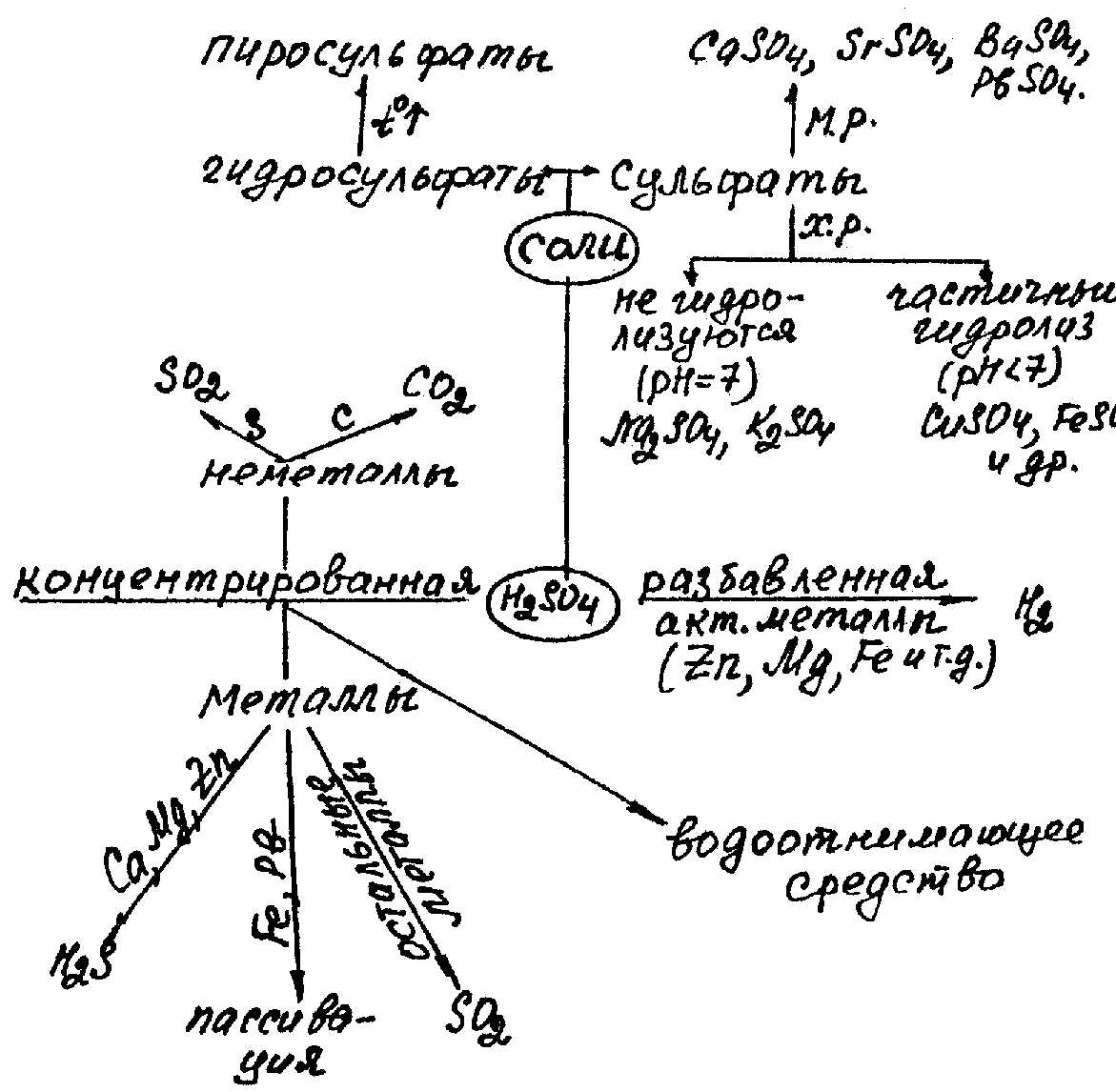
Sulfuric acid is a strong oxidising agent. Its reaction with metals depends on the concentration and activity of metal:
Diluted acid (oxidant is hydrogen ion H+). Therefore, it dissolves (oxidises) only metals that are situated before H in the electromotive series of metals:
Zn0 + Н2SO4(dil) = Zn+2SO4 + Н20
Zn + 2H+ = Zn2+ + H2
Concentrated Н2SO4 (oxidant is sulfur(VI)):
Sulfur is reduced to SO2 at the interaction with nonactive metals (Cu, Ag, Hg):
Cu0 + 2Н2SO4(conc.) = CuSO4 + SO2 + 2H2O
Sulfur is reduced to the elemental S or H2S (a mixture of reduction products often appears)at the interaction with active metals:
3Zn + 4Н2SO4(conc.) = 3ZnSO4 + S + 4H2O
4Zn + 5Н2SO4(conc.) = 4ZnSO4 + H2S + 2H2O
It can oxidise other simple substances and compounds:
2HBr + Н2SO4(conc.) = Br2 + SO2 + 2H2O
8HI + Н2SO4(conc.) = 4I2 + H2S + 4H2O
C + 2Н2SO4(conc.) = CO2 + 2SO2 + 2H2O
S + 2Н2SO4(conc.) = 3SO2 + 2H2O
Н2SO4 is a very acidic solvent. CH3COOH and HNO3 behave as bases in Н2SO4. HClO4 is one of the strongest acids, but it is a weak one in Н2SO4 medium:
HClO4 + Н2SO4 = Н3SO4+ + ClO4-
The only acid that is stronger than Н2SO4 in its medium is hydrogen tetra(hydrogen sulfato) borate, H[B(HSO4)4]. It has not been obtained in the free state, but can be isolated from its solutions in Н2SO4:
B(OH)3 + 6Н2SO4 = [B(HSO4)4]- + 3H3O+ + 2HSO4-

Н2SO4 salts. Sulfates are very abundant. Most of them are well-soluble in water. The low soluble sulfates are CaSO4, SrSO4, BaSO4, PbSO4.
Solid hydrogen sulfates are obtained only for the most active metals (NaHSO4, KHSO4 etc.). Disulfates (pyrosulfates) are salts of disulfuric (pyrosulfuric) acid obtained at heating of hydrogen sulfates:
2NaНSO4 = Na2S2O7 + Н2O,
in turn, when heated stronger, the following reaction proceeds:
Na2S2O7 = Na2SO4+ SO3
Under the influence of water, disulfates are transformed again into hydrogen sulfates.
Chemical properties of sulfuric acid can be summarised in the scheme:
Uses
The production of 'superphosphate' (calcium hydrogen phosphate + calcium sulfate) for fertilisers is the biggest use of sulfuric acid. Second to this is the manufacture of ammonium sulfate from ammonia (by the Haber process). This is also a fertiliser. Other uses are: conversion of viscose to cellulose in the manufacture of artificial silk, and so on; manufacture of explosives, pigments and dyestuffs, as well as many other chemicals, for example hydrochloric acid; refining of petroleum and sulfonation of oils to make detergents; and in accumulators.
PEROXOACIDS. There are the following peroxoacids of sulfur: Н2S2O8 is called peroxidisulfuric acid (supersulfuric acid), and Н2SO5 is peroxomonosulfuric acid (Caro acid).
Production. Н2S2O8 is obtained electrolytically at the anode in 50% Н2SO4 aqueous solution:
2HSO4- = Н2S2O8 + 2e
Н2S2O8 can form peroxomonosulfuric acid (Caro acid) under the influence of concentrated Н2O2 (Heinrich Caro was a German organic chemist, who obtained this acid in 1898):
Н2S2O8 + Н2O2 = 2Н2SO5
Structure. These acids are regarded as Н2O2 derivatives because groups —O—O— are present in their structure

Properties. Н2S2O8 and Н2SO5 are crystalline substances. Their melting points make up 65o and 47o, respectively.
Н2S2O8 is a strong dibasic acid. The O—H bond of the Caro acid dissociates strongly. At the same time, —O—O—H group (like hydrogen peroxide, K = 4∙10-10) dissociates weakly. Therefore, it is a monobasic acid that is stable also in the free state. Salts exist only in solutions.
Peroxoacids and their salts are very strong oxidising agents due to the presence of —O—O— bonds:
2Mn+2SO4 + 5K2S2O8 + 8Н2O = 2KMn+7O4 + 4K2SO4 + 8Н2SO4
When heated with water Н2S2O8 and Н2SO5 hydrolyse:
Н2S2O8 + H2O = Н2SO4 + H2SO5
H2SO5 + H2O = Н2SO4 + H2O2 (industrial method of H2O2 production)
THIOSULFURIC ACID AND ITS SALTS
Production in industry: 2Na2S2 + 3O2 = 2Na2S2O3.
In the laboratory: 1. boiling of sulfites aqueous solution with sulfur powder:
Na2SO3 + S = Na2S2O3

Structure. Thiosulfate, salt of thiosulfuric acid, is a distorted tetrahedron as S—S bond is longer than S—O one. Thiosulfuric acid has two tautomeric forms:

Properties. The strength of thiosulfuric acid is close to H2SO4 (K2 = 2∙10-2), although it is unstable under normal conditions. Therefore, thiosulfates decompose in acidic medium by the reaction:
Na2S2O3 + H2SO4 = Na2SO4 + Na2S2O3 = S + Н2SO3 = SO2 + H2O.
The presence of sulfur (-2) predetermines the reducing properties of thiosulfate. Strong oxidising agents oxidise thiosulfates to elementary S and S (VI) compounds:
Na2SO3S-2 + 4Cl20 + 5H2O = 2H2+6SO4 + 2NaCl-1 + 6HCl.
Na2SO3S-2 + Br20 + H2O = S0 + 2NaBr-1 + H2SO4.
"Soft" oxidants (such as I2) form tetrathionates:
2Na2SO3S + I2 = Na2S4O6 + 2NaI.
This reaction is widely used in iodometric methods of analysis in analytical chemistry.
PolythioNIC acids. Na2S4O6 is salt of tetrathionic acid.
Preparation. When gaseous Н2S passes through dilute solution of SО2 Backenroder liquid is obtained. Its composition includes colloidal sulfur and polythionic acids Н2SnO6, where n = 3-20.
Structure. A chain of elemental sulfur atoms between sp3-hybridized sulfur is a structure feature of polythionic acids. For example, pentathionic acid:
 .
.
Properties. Unknown in the free state, polythionic acids are strong acids. Relatively stable are their salts with alkali metals. Acid salts are unidentified.
Important. Н2S2O6 is not polythionic acid, since two pyramidal [SO3]-groups are bound directly through the sulfur atoms. Manganese dithionate can be prepared by passing gaseous SO2 through MnO2 suspension:
2SO2 + MnO2 = MnS2O6
HALIDES AND OXOHALIDES OF sulfur
Sulfur reacts directly with all halogens except for iodine.
Thus:
S2F2 (b.p. -380)
SF2 (-350)
SF4 (-400)
SF6 (-640, sublimates)
S2Cl2 (13.70)
SCl2 (590, decomposes)
SСl4 (-300, decomposes)
S2Br2 (540 at 0.2 mm Hg)
In the series of compounds of sulfur with halogens from fluorine to bromine stability of these compounds sharply drops. The most stable halide of fluorine is SF6, of chlorine is S2Cl2. The latter compound is decomposed by water:
2S2Cl2 + 2H2O = SO2 + 3S + 4HCl
Sulfur forms oxohalides:
SOCl2 is thionyl chloride
SO2Cl2 is sulfuryl chloride
SOCl2 is an extraordinarily active compound.

Preparation:
PCl5 + SO2 = SOCl2 + POCl3
It is hydrolysed readily:
SOCl2 + H2O = SO2 + 2HCl
SO2Cl2 is an extremely active liquid.
Preparation:
SO2 + Cl2 = SO2Cl2
CuCl2 + 2SO3 = CuSO4 + SO2Cl2
Hydrolysis of SO2Cl2 takes place:
SO2Cl2 + 2H2O = H2SO4 + 2HCl
Therefore, SO2Cl2 is a mixed anhydride or halogenanhydride.
Oxygen compounds of Se(VI), Te(VI), and Po(VI). The most important of them are SeO3 and TeO3 oxides, relevant acids and their salts.
OXIDES. EO3 are produced indirectly, whereas EO2 are the products of direct synthesis from simple substances.
SeO3 is separated during boiling of selenates with liquid SO3:
K2SeO4 + SO3 = K2SO4 + SeO3,
or at dehydration H2SeO4 by P2O5.
Molecules of SeO3 exist only in the gaseous state. Tetramers (SeO3)4 are condensed when cooling. The solid tetramer (SeO3)4 has two polymorphic modifications: vitreous and asbestos-like. It is difficult to obtain (SeO3)4 without impurities (i.e. to use recrystallisation) because it explodes while interacting with any solvent. SeO3 is a crystalline substance (m.p. = 121 C) under normal conditions, which decomposes at t > 180 C as follows:
2SeO3 = 2SeO2 + O2,
but sublimes in vacuum without decomposition. SeO3 is an acid oxide (acid anhydride of selenic acid):
SeO3 + H2O = H2SeO4.
In concentrated solutions, it forms a mixture of polyselenic acids similar to polysulfuric acids (H2Se2O7, m.p. = 19 C, H2Se3O10, m.p. = 39 C). When diluting, polyselenic acids solutions are hydrolysed to final product H2SeO4.
TeO3 is obtained by thermal dehydration of orthotelluric acid:
Н6ТeO6
![]() ТeO3
+ 3Н2O
ТeO3
+ 3Н2O
TeO3 forms two polymorphs in the solid state. The reaction above leads to the -TeO3, amorphous phase of yellow colour (density 5.1 g/cm3), which is low soluble in cold water (0.5 g /L), and is gradually transforming again into orthotelluric acid in hot water. The crystalline - TeO3 phase of gray colour (density 6.2 g/cm3), which no longer reacts with acids and alkali solutions even when heated, can be prepared at a long-term heating of -TeO3 in a sealed tube (300 C).
In general, SeO3 and TeO3 oxides are soluble in alkalis forming the corresponding selenates and tellurates:
SeO3 + NaOH = Na2SeO4
SeO3 oxide’s oxidising ability is so high that oxidises HCl to Cl2 even at cooling. TeO3 oxidises HCl only when heated. Therefore, oxidising properties are weakened at the transition from SeO3 to TeO3.
ACIDS. Preparation. The highest acids of chalcogens are mostly produced by the action of strong oxidising agents:
H2Se+4O3 + H2O-12 = H2Se+6O-24 + H2O-2
Te + 3H2O2 = H6TeO6
Te + HClO3 + 3H2O = H6TeO6 + HCl.
H2SeO4 is also prepared by electrochemical oxidation of selenious acid.
H2SeO4. A convenient laboratory method of preparation.
H2SeO4 is obtained by processing Ag2SeO3 suspension with bromine water:
Ag2SeO3 + Br2 + H2O = 2AgBr + H2SeO4
Selenic acid exists in the form of meta-acid, H2SeO4, telluric is the ortho-acid, H6TeO6. Both acids are colourless crystalline substances.
Structure. H2SeO4 (m.p. = 62.4 C) can be mixed with water at any proportion; its concentrated solutions are viscous and similar to H2SO4. It has strong affinity to water and will remove 'combined' water in sugars and other organic compounds through the formation of solid hydrates H2SeO4∙H2O (m.p. = 26 C), H2SeO4∙2H2O (m.p. = -24 C), H2SeO4∙4H2O (m.p. = -52 C) like H2SO4. When heated over 260 C, selenic acid is transformed into SeO2:
2H2SeO4 = 2SeO2 + O2 + 2H2O.
The H2SeO4 molecule structure (sp3-hybridisation) and the acid strength are similar to H2SO4 (H2SeO4, K2 = 1∙10-2; H2SO4, K2 = 1,2∙10-2; both acids have K1 103). The significant strength is due to the presence of two very electronegative atoms of oxygen in acid residues. They shift electron density causing the growth of H—O bonds polarity and their ability to dissociate. Moreover, EO42- anions (the bond length Se—O is 0.161 nm), which appear after dissociation, are stabilised in a solution due to the negative charge uniform delocalisation between four oxygen atoms:

H6TeO6. Orthotelluric acid molecule has an octahedral structure (sp3d2-hybridisation of tellurium orbitals):

Unlike H2SO4 and H2SeO4, covalent bonds Te=O are absent in H6TeO6, which would be favourable to H—O bonds polarisation and facilitate their dissociation. Therefore, H6TeO6 is a very weak acid (K1 = 2∙10-8; K2 = 9∙10-12; K3 = 3∙10-15), which is well soluble in hot water, but its solubility is limited under normal conditions (25% at 20 C).
If heated, H6TeO6 is transformed into metatelluric acid, H2TeO4. It is much stronger than ortho-H6TeO6 form and H2TeO4 gradually turns again into orthoacid in a solution. H6TeO6 is an illustration of the well-known rule: the more a p-element atom forming an acid the more the OH-groups number associated with it, i.e. the ability of more hydrated forms formation. Tellurium (VI), in addition to other p-elements of the fifth period, has the stable coordination number 6.
When neutralising H6TeO6 by alkalis, acid salts are formed. The most abundant among them are the low soluble Na2H4TeO6 and well soluble K2H4TeO6∙3H2O. Orthotelluric acid H6TeO6 can be replaced by metals all six hydrogen atoms. For instance, Na6TeO6 neutral salt can be obtained at H6TeO6 fusion with NaOH. It gradually transforms into Na2H4TeO6∙3H2O in the humid atmosphere. There are also salts Ag6TeO6 and Hg3TeO6.
Properties. Selenic and telluric acids are powerful oxidising agents. In aqueous solutions, they are considerably stronger than H2SO4, as evidenced by E comparison:
SeO42-+ 4H+ + 2e-= H2SeO3 + H2O; E = 1,15 V
H6TeO6 + 2H+ + 2e-= TeO2 + 4H2O; E = 1,02 V
SO42-+ 4H+ + 2e-= H2SO3 + H2O; E = 0,17 V
Concentrated H2SeO4 and H6TeO6 unlike H2SO4 are able to oxidise not only I‑ and Br-, but also Cl-, turning into the more stable oxidation state of these elements (+4):
2HCl + H2SeO4 = Cl2 + H2SeO3 + H2O.
Especially strong is the oxidising ability of H2SeO4. The hot anhydrous H2SeO4 dissolves well not only Ag (like H2SO4) but Au either:
2Au + 6H2SeO4 = Au2(SeO4)3 + 3SeO2 + 6H2O.
Like aqua regia (HCl + HNO3) the mixture of H2SeO4 + HCl oxidises even Pt:
Pt + 4HCl + 2H2SeO4 = PtCl4 + 2SeO2 + 4H2O
Halides. They can be obtained by direct synthesis reaction from simple substances.
-
Composition, colour, state of halides of
Se
Te
SeF6 colourless gas
TeF6 colourless gas
SeF4 colourless
TeF4 colourless solid
SeCl4 colourless solid
TeCl4 colourless solid
SeCl2 liquid brown
TeCl2 solid green
SeBr4 solid yellow
TeBr4 solid orange
Se2Br2 liquid red
TeBr2 solid brown
TeI4 gray-black
Molecules SeF6 (m.p. = -46.6 C) and TeF6 (m.p. = -38.6 ), like SF6, have octahedral structure, which shows sp3d2-hybridisation (Se—F and Te—F bond lengths are 0.170 nm and 0.184 nm, respectively). The most abundant are SeX4 and TeX4. By its nature, selenium halides are close to the corresponding derivatives of sulfur. For instance, Se2Cl2 and Se2Br2 are decomposed even at careful heating:
2Se2Cl2 = 3Se + Se2Cl4
Tellurium halides are significantly different from those of sulfur compounds. They have salt-like behaviour. Unlike hexafluorides of S and Se, TeF6 hydrolyses by water easily:
TeF6 + 6H2O = H6TeO6 + 6HF (it is a halogenanhydride).
Thus, in the series S—Se—Te—Po nonmetallic properties are weakened, metallic ones are increased, and ionicity in molecules grow. The chemical nature of halides changes starting with covalent (Se halides) through ionic-covalent (saltlike TeXn) to the ionic (salts PoXn).
TESTS FOR SULFUR
Oxidation of a sulfur compound with concentrated nitric acid yields sulfuric acid or a sulfate, which can be tested with barium chloride.

Oxidising agent
Dehydration agent
CH2=CH2
or C2H5OC2H5
NO2
Brr
I2
CuSO4
Zn SO4
SO2
CO2
HCl
HF
CO
C
CO+CO2
For drying of gases
C6H12O6
Zn
Cu
HI
HBr
The reaction of replacement
Drying agent
Nitration
Sulfurization
Addition to CH3CH=CH2
with subsequent hydrolysis
C2H5OH
(CO2H)2
HCO2H
C
S
NaCl
CaF2


















(CH3)2CHOH
SO3H
SELENIUM. STANDARD ELECTRODE POTENTIALS, EO, V
VI |
|
IV |
|
0 |
|
-II |
Acid medium |
||||||
SeO |
1 |
H |
0,74 |
Se |
-0,11 |
H Se |
Alkaline medium |
||||||
SeO |
0,03 |
SeO |
-0,36 |
Se |
-0,67 |
Se |
i An azeotrope (pronounced /əˈzi.ətroʊp/ ə-ZEE-ə-trope) is a mixture of two or more liquids in such a ratio that its composition cannot be changed by simple distillation. This occurs because, when an azeotrope is boiled, the resulting vapor has the same ratio of constituents as the original mixture.
Because their composition is unchanged by distillation, azeotropes are also called (especially in older texts) constant boiling mixtures. The word azeotrope is derived from the Greek words ζέειν (boil) and τρόπος (change) combined with the prefix α- (no) to give the overall meaning, “no change on boiling.”
Over 9,000 azeotropic mixtures of pairs of compounds have been documented. Many azeotropes of three or more compounds are also known

 ,1
,1