
H2S
![]() H+
+ HS-; K1
= 8.7.10-8
H+
+ HS-; K1
= 8.7.10-8
HS- H+ + S2-; K2 = 10-14
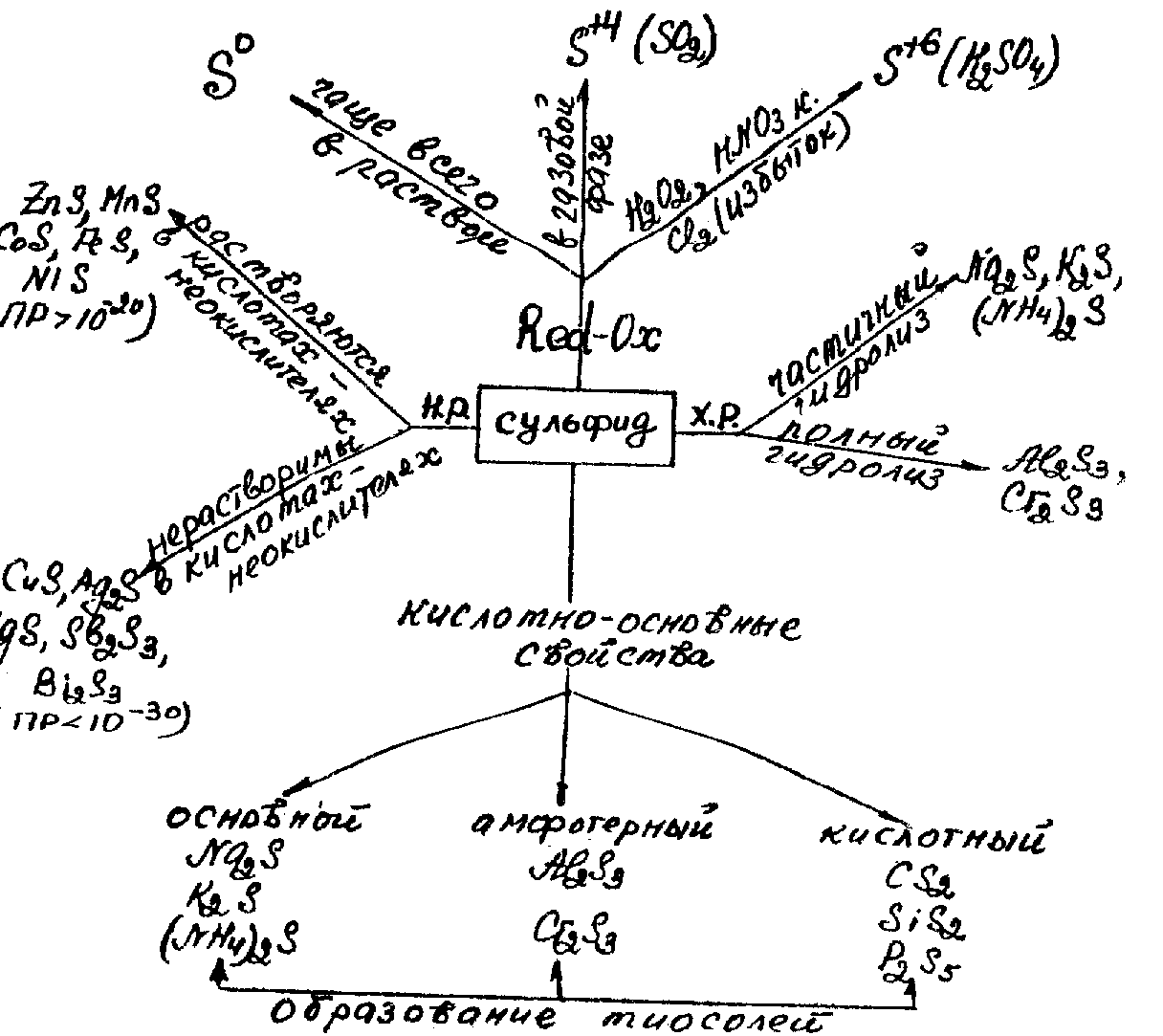
The neutral salts of H2S are sulfides, acidic salts – hydrogen sulfides.
A great majority of sulfides are insoluble in water. The soluble include only the sulfides of alkali metals and NH4+, and also hydrogen sulfides. Most of them exist only in solution.
Preparation of sulfides. Sulfides can be obtained in a number of different ways:
1. Reactions between simple substances (sulfur reacts with metals vigorously at to).
2. Action of H2S on soluble salts of metals. This method sometimes is unsuitable. The reason is a huge difference in solubility products of sulfides, e.g.: SPHgS = 4∙10-53, SPFeS = 3.7∙10-19.
Such reactions are reversible in general, since one of initial substances - H2S is a weak acid, and among products of reaction is MS - sparingly soluble salt.
The displacement of equilibrium of the double replacement reaction:
M2+ + H2S MS + 2H+
depends on which product is the weakest electrolyte: H2S (KH2S = K1∙K2 = 8.7∙10–22) or the sparingly soluble sulfide, MS.
Two variants are possible:
1). In case of virtually insoluble sulfides (SPMS << KH2S) reactions proceed to the end, for example:
Cu2+ + H2S = CuS + 2H+ (SPCuS = 4∙10-38)
Hg2+ + H2S = HgS + 2H+ (SPHgS = 4∙10-53)
These sulfides are insoluble in water and acids, their preparation is possible by H2S action on the solutions of salts of such metals.
2. In case of SPMS << KH2S, formation of sulfides does not occur (the equilibrium of reaction is displaced to the left). Such sulfides (FeS, MnS, BaS) are easily dissolved in acids, for example:
FeS + 2HCl = FeCl2 + H2S
Sulfides of both types are insoluble in water and their preparation using soluble sulfides instead of weak electrolyte H2S is a better alternative, for example:
FeSO4 + Na2S = FeS + Na2SO4
Under such conditions, the deepest proceeding of direct reaction is realised up to concentration of ions defined by SPMS.
Different solubility of metal sulfides in water and in acidic solutions make up a basis of qualitative analysis of cations in analytical chemistry. Some of them (Na+, K+, NH4+ and etc.) form soluble sulfides; the second group contains insoluble in water metal sulfides (Fe2+, Mn2+, Zn2+and etc.) but well soluble in acids; the third group metal sulfides of Cu2+, Pb2+, Hg2+ and etc. are insoluble neither in water nor in diluted acids.
3. Reduction of sulfates by carbon (for the sulfides of active metals)
Na2SO4
+ 4C
![]() Na2S
+ 4CO
Na2S
+ 4CO
Hydrolysis. In the solutions sulfides, as the salts of a weak acid, H2S, demonstrate strong hydrolysis by anіon. The hydrolysis of soluble sulfides proceeds step by step and reversibly:
S2- + HOH HS- + OH-
HS- + HOH H2S + OH-
As at hydrolysis forms a surplus of OH- ions, solutions of sulfides have an alkaline reaction (pH > 7).
The hydrolysis of sulfides of multicharged ions which are salts of very weak bases and acids proceeds completely to the end:
Al2S3 + 6H2O = 2Al(OH)3 + 3H2S
Cr2S3 + 6H2O = 2Cr(OH)3 + 3H2S
Therefore, their preparation by any exchange reaction is impossible since the product hydrolyses in aqueous solutions:
2AlCl3 + 3Na2S + 6H2O = 2Al(OH)3 + 3H2S + 6NaCl
Cr2(SO4)3 + 3Na2S + 6H2O = 2Cr(OH)3 + 3H2S + 3Na2SO4
Sulfides, as well as oxides, have basic, amphoteric or acid character. Interaction between them gives salts of thioacids, therefore the sulfides of non-metals are called thioanhydrides of corresponding acids, for example:
Na2S + CS2 = Na2CS3
Basic acid thiocarbonate is a thioanhydride of thiocarbonic acid (Н2CS3).
Acids which correspond to thiosalts are most frequently unstable and readily decompose:
2Na3AsS4 + 6HCl = As2S5 + 3H2S + 6NaCl
H2S and sulfides have the lowest oxidation state of sulfur (-2) and demonstrate only reduction properties, they belong to strong reductants.
At ignition H2S burns in air:
2H2S-2 + 3О20 = 2S+4O2-2 + 2H2O-2
In solution it is slowly oxidized by the oxygen of air to elementary sulfur:
H2S-2 + O20 = 2S0 + 2H2O-2
In aqueous solutions, H2S and sulfides are readily oxidized by halogens, KМnO4, K2Cr2O7 and other oxidants, for example:
Na2S-2 + I20 = 2S0 + 2NaI-1
H2S-2 + 4Br20 + 4H2O = H2SO4 + 8HBr
3H2S-2 + K2Cr2O7 + 4H2SO4 = 3S0 + Cr2+3(SO4)3 + K2SO4 + 2H2O
5H2S-2 + 2KMnO4 + 3H2SO4 = 5S0 + MnSO4 + K2SO4 + 8H2O
H2S-2 + 2Fe+3 = S0 + 2Fe+2 + 2H+
Depending on conditions and oxidants the products of oxidation can be sulfur, SO2 or H2SO4 . It can be represented by the chart:
TESTS FOR HYDROGEN SULFIDE
1. It’s unpleasant smell of rotten eggs.
2. The blackening of filter paper moistened with a soluble lead(II)salt (e.g. nitrate) due to the formation of lead(II) sulfide.
PolysulFides.
The sulfur ability for chains formation is observed in numerous polysulfides. They are prepared by interaction of sulfides of alkaline metals or NH4+ with sulfur in concentrated solutions (water solutions of sulfides simply dissolve sulfur):
Na2S-2 + S0 = Na2S2-1
Na2S + (n-1)S = Na2Sn
These reactions can be considered as an oxidation of sulfides by sulfur. They usually form the mixtures of polysulfides: n = 2-8 (n=9 only in ((NH4)2S9), more frequently n=2). At n growth the colour will change from yellow through orange to red. The richest in sulfur (NH4)2S9 has intensive red colour.
Under the action of strong acids, polysulfides form sulfanes Н2Sn:
Н2Sn + 2НСl = Н2Sn + 2NaCl
A heavy yellow oil-like liquid prepared is a mixture of sulfanes Н2Sn. In the individual state sulfanes up to Н2S8 are isolated: Н2S2 (m.p. = -880, b.p. = 750), Н2S3 (m.p. = -520), Н2S4 (m.p. = -850), Н2S5 (m.p. = -500). They are relatively stable only in a strongly acidic medium, under other conditions they decompose with elimination of sulfur.
Polysulfide and sulfane molecules are zig-zag chains made by atoms of sulfur, valence orbitals of which are in the state of sp3-hybridization:
![]()
The ends of these chains are the atoms of Н (H2Sn) or atoms of alkali metals in polysulfides. The dissociation energy of S—S bonds is equal to 226 kJ/mol and is similar enough for all sulfanes.
Sulfanes are weak acids in water solution. However, they considerably exceed H2S strength. Their strength grows at the increase of sulfur atoms number in the molecule, for example:
Н2S Н2S4 Н2S5
K1 = 8.7∙10-8 2∙10-4 3∙10-4
K2 = 1∙10-14 5∙10-7 2∙10-6
At the increase of sulfur atoms number H—S bonds become more polarized, therefore, its dissociation is facilitated under the action of polar water molecules. In accordance with the increase of acid strength, hydrolysis of their salts appropriately diminishes:
n in Н2Sn 2 3 4 5
Degree of hydrolysis, % 64.6 37.6 11.8 5.7
Like peroxides, polysulfides display reduction properties:
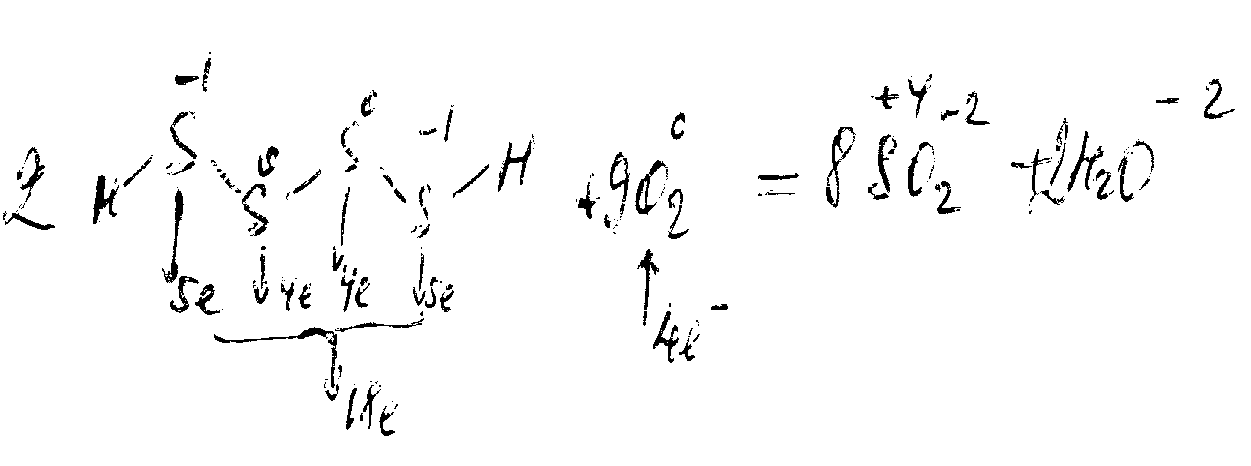
They can display oxidizing properties under certain conditions:
Sn+2S + Na2S2-1 = Sn+4S2 + Na2S-2.
oxidant
when heated, they disproportionate:
Na2S2-1 = Na2S-2 + S.
selenium, telurium, and polonium
Compounds of elements (-2)
Apart from the simple hydrogen chalcogenides of Se and Te (which are more acidic and thermally less stable than the corresponding sulfur analogs) the most stable inorganic compounds of selenium (and its higher homologs) are:
1. Chalcogenides and polychalcogenides with strongly electropositive ions such as alkali or alkaline-earth metals, and those that are more covalently bonded with transition metals;
2. Large variety of Se(II), Se(IV), and Se(VI) compounds with electronegative elements O, F, Cl, and Br;
3. Homologs belonging to the interesting class of sulfur–nitrogen compounds;
4. Organoselenium compounds.
When heated, selenium and tellurium react with metals forming selenides and tellurides:
2K + E = K2E-2; 2Al + 3E = Al2E3-2
Thus active metals form salt-like selenides and stoichiometric tellurides, which dissolve in water readily.
Reactions of Se and Те with transitional metals give selenides and tellurides of various types МЕ, М2Е3, М3Е4, МЕ2 and etc., with many of them having a varying composition. At low content of non-metallic elements they have mainly metallic nature, at high content of chalcogens — semiconductors.
Hydrogen compounds. Along with H2S hydrogen selenide, H2Se, and hydrogen telluride, H2Тe, are known. Hydrogen polonide, H2Ро, is extraordinarily unstable. Radioactivity of a gas formed after HCl treatment of polonium coated magnesium can serve as a proof. Polonides (Na2Po) are also known.
Preparation. The action of diluted HCl or Н2О on selenides and tellurides is used in a laboratory:
Al2E3 +6HCl = 3H2E + 2AlCl3
Al2E3 +6H2O = 3H2E + 2Al(OH)3
Н2Se can be prepared if gaseous Н2 passes over heated to 400C elemental selenium.
Properties. Under ordinary conditions H2Se and H2Тe are colourless toxic gases with a very unpleasant smell. Their structure and properties resemble those of H2S:
|
H2О |
H2S |
H2Se |
H2Te |
m.p., С |
0 |
-85.6 |
-65.7 |
-51 |
b.p., С |
100 |
-60.4 |
-41.4 |
-2 |
Bond length, nm |
0.096 |
0.133 |
0.146 |
0.169 |
Bond energy, J/mol |
463 |
347 |
276 |
238 |
Valence angle H-E-H |
104.5 |
92.2 |
91.0 |
90 |
Нf,298, kJ/mol |
-285.8 |
-20.6 |
33 |
99.7 |
Gf,298, kJ/mol |
-237.2 |
-33.8 |
19.7 |
85.2 |
K1(aqueous solution) |
1.8•10-16 |
1•10-7 |
1.9•10-4 |
2.3•10-3 |
Covalent radii of chalkogens grow in the series H2О—H2S—H2Se—H2Te diminishing Н-Е bonds energy and H2Е molecules stability. That is why unlike H2О and H2S, selenium and tellurium compounds with hydrogen are endothermic compounds (Нf>0), they readily decompose when heated: thermal decomposition of H2Se takes place with a noticeable rate at to > 300, and H2Тe is gradually decomposed already under ordinary conditions.
Similarly to molecule H2S the molecule of H2Se and H2Te have bent shape with valence angles 91 and 90, which signifies that hybridization of selenium and tellurium orbitals is absent.
Solubility of H2Se and H2Te is better as compared to H2S. In aqueous solutions they display properties of weak hydroselenic and hydrotelluric acids. The strength of these hydracids gradually grows in the series H2О-H2S-H2Se-H2Te. It is explained by diminishing of Н — Е bond energy and growth of their ability to polarization. Dissociation of compounds is hereupon facilitated under the action of polar molecules of water:
Н2Е Н+ + НЕ-
HЕ- Н+ + Е2-
H2Se can form two types of salts: neutral and acidic. H2Te has no reactions when acidic salts are the products. Exposed to air oxygen their solutions quickly acquire a red color owing to the formation of polyselenides and polytellurides of metals, similar to polysulfides:
12K2Te + 5O2 + 10H2O = 2K2Te6 + 20KOH
Neverthless, as a result of large size and low electronegativity of elements, a reaction between selenides of different chemical nature, as well as between tellurides with the formation of analogues of thiosalts can not be realised.
In the series H2S-H2Se-H2Te the reducing activity grows appropriately. So, sulfur is able to oxidize H2Se аnd H2Te:
H2Se-2 + S = Se + H2S-2,
and oxygen oxidizes hydrogen compounds and their derivatives of other elements of the subgroup:
2H2Е + O2 = 2E + 2H2O (in solution)
2H2Е + 3O2 = 2EО2 + 2H2O (burning)
Oxide of sulfur (IV). Preparation.
In industry:
1. Oxidative combustion of sulfides of metals (pyrites)
4FeS2 + 11О2 = 2Fe2О3 + 8SО2
2. Incineration of native sulfur (in countries with rich deposits of elementary sulfur):
S + О2 = SО2 ; Н0298 = -296.8 kJ/mol
3. Thermal decomposition or reduction of gypsum СaSо4.2h2o
2СaSО4
![]() 2СаО + 2SО2
+ О2
2СаО + 2SО2
+ О2
СaSО4
+ С
![]() СаО + SО2
+ СО2
(in
cylinder furnaces)
СаО + SО2
+ СО2
(in
cylinder furnaces)
In laboratory: 1. Using sulfites:
Na2SО3 + 2H2SО4 = 2NaHSО4 + SО2 + H2O,
2. Reduction of concentrated h2sо4 by low active metals (e.G. With copper):
Cu + 2H2SО4(conc.) = CuSО4 + SО2 + 2H2O
Structure. SО2 has a bent structure (S–O bond length 143.2 pm, O–S–O valence angle 119.5◦ in the gas phase),

The state of hybridization of sulfur orbitals in SО2 corresponds to the structure draft:
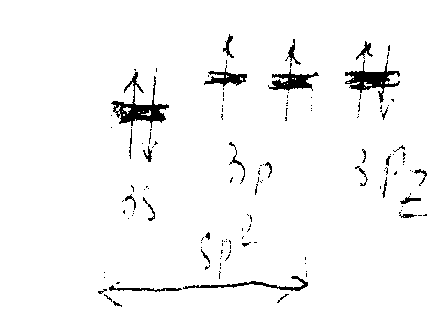
The formation of -bonds in SО2 molecule is a result of overlap of two sulfur monoelectronic hybrid orbitals with 2рх monoelectronic orbitals of two oxygen atoms. To achieve an octet electronic configuration 2рz-orbital of the oxygen atom accepts one electron of unhybridized 3рz-orbital of sulfur (electron transition is shown on the chart below with an arrow):
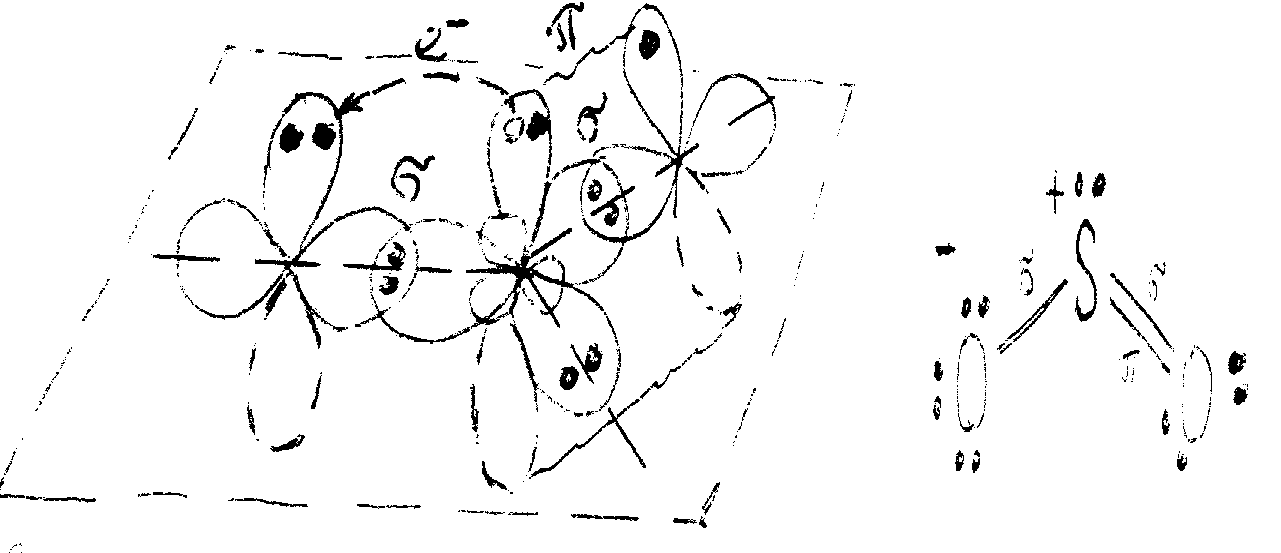
This arrangement results in the formation of a -bond between sulfur and the second atom of oxygen. The resonance structure of this overlapping state of atomic orbitals is shown near the chart. However, this structure has no similarity to actual SО2 structure since S-О bonds are identical in the real molecule. The same is the description of validity of the opposite resonance structure of overlapping orbitals.
A ccording
to the
resonance
theory
a
real molecule is an intermediate state between the limiting cases of
resonance structures, and its structure is the result of imposition
(superposition)
of all variants of resonance structures. Therefore, the real
structure of SО2
corresponds
to the first structure chart, where together with -bonds
delocalised three-centred -bond
is present.
ccording
to the
resonance
theory
a
real molecule is an intermediate state between the limiting cases of
resonance structures, and its structure is the result of imposition
(superposition)
of all variants of resonance structures. Therefore, the real
structure of SО2
corresponds
to the first structure chart, where together with -bonds
delocalised three-centred -bond
is present.
Properties. SO2 is a poisonous, colourless gas with a sharp smell; it condenses (−10 ◦C) to give a colourless liquid and ultimately (−76 ◦C) white crystals. SO2 is an acid oxide, its molecule is polar ( = 1.59 D = 0.53•10-29 C•m).
SO2 demonstrates reducing properties (that are more characteristic):
SO2 + Br2 + 2H2O = H2SO4 + 2HBr,
and oxidizing properties:
SO2 + 2H2S = 3S + 2H2O.
It is well soluble in water (36 volumes SO2 in one volume of water at 20 oC) with the formation of hydrates of variable composition SO2∙nH2O. Some dissolved molecules react with water and form sulfurous acid, Н2SO3.
SULFURUOUS ACID, H2SO3.
SO2 + H2O Н2SO3
Н2SO3 exists only in diluted solutions as a result of reversibility of this reaction. Its equilibrium is significantly shifted to the left hand side. On being heated, the solubility of SO2 diminishes and the equilibrium is even more displaced toward reagents. A reaction of acids with salts of sulfurous acid causes evolving SO2 because of Н2SO3 instability; therefore, Н2SO3 cannot be isolated in the free state.
Structure of sulfite-ion, SO32-. The structure is pyramidal with the atom of sulfur on the top.
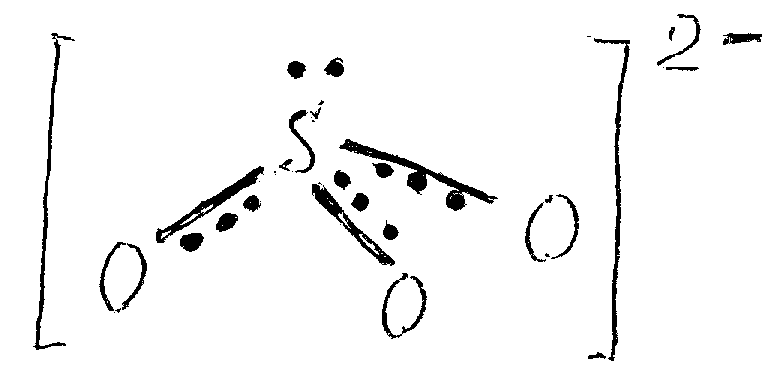
The value of valence angles ( 1050) is the evidence of sp3-hybridization. All bonds here are equivalent, and their length (151 pm) is somewhat longer of SO2 bonds (143 pm). At bonds formation, every atom surrounds electron octet that provides SO32- ion stability. The identity of S—O bonds suggests equivalent distribution of two negative charges between three atoms of oxygen.
Diminishment of valence angle in the sulfite ion (105o) in comparison with tetrahedral (109o28') is caused by the electrostatic repulsion between the unshared electron pair and the bond electrons.
Since the unshared electron pair of sulfur is located on spatially directed hybrid orbitals, the sulfite ion is an active donor of electron pair and its transformations into tetrahedral ions НSO3- and SO42- can be realised easily.
Properties. Sulfurous acid is a dibasic acid of intermediate strength:
Н2SO3 Н+ + НSO3-; K1 = 2∙10-2;
Н2SO3- Н+ + НSO32-; K2 = 6∙10-8.
It forms two types of salts: sulfites and hydrogen sulfites:
NaOH + SO2 = Na2SO3
Na2SO3 + SO2(excess) + H2O = 2NaНSO3
The hydrogen sulfite-ion, НSO3-, has two tautomeric forms:
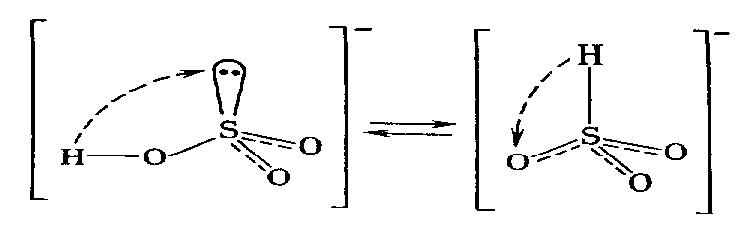
The first structure corresponds to generally neutral salts, the second structure is close to the salts of some low active metals.
Only sulfites of alkali metals and hydrogen sulfites are well soluble. Only solid sulfites can be prepared. Solid hydrogen sulfites are known for alkali metals only.
Salts of Н2SO3 are partially hydrolysed by anion, their solutions have alkaline reaction.
The sulfite and bisulfite anions are both moderately strong reducing agents.
Н2SO3 and its salts are gradually oxidized even by air oxygen in aqueous solutions:
2Na2SO3 + O2 = 2Na2SO4
Strong oxidants oxidize SO32- practically instantly:
Н2S+4O3 + Hal20 + Н2О = Н2S+6O4 + 2НHal1- (Hal2 = Cl2, Br2, I2)
5Na2SO3 + 2KMnO4 + 3H2SO4= 5Na2SO4 + 2MnSO4 + K2SO4 + 3H2O
Na2SO3 + H2O2 = Na2SO4 + H2O
Н2SO3 demonstrates oxidizing properties at the attack of strong reductants:
Н2SO3 + 2H2S = 3S + 3H2O
Being heated, sulfites disproportionate:
4Nа2S+4O3 = Na2S-2 + 3Na2S+6O4 .
POLYSULFURUOUS ACIDS. Zinc dithionite is formed at SO2 reduction with zinc dust in water:
Zn + 2SO2 = ZnS2O4.
Dithionate is also obtained by the reaction:
2NаHSO3 + Zn + H2SO3 = Na2S2O4 + ZnSO3 + 2H2O.
Dithionites are salts of dibasic dithionic acid, Н2S2O4. It is unstable and simultaneously strong acid (K1 = 5∙10-1, K2 = 4∙10-3) in aqueous solutions. It has a symmetric structure and forms tautomers:
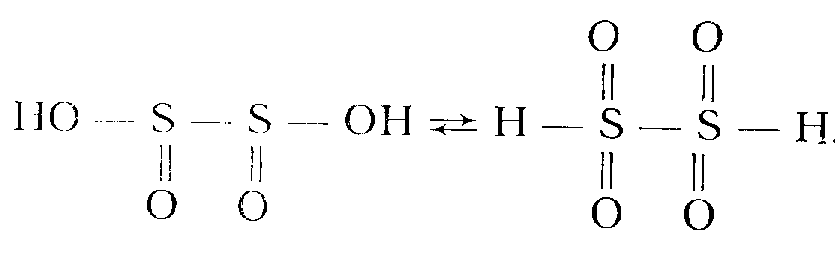
Н2S2O4 is one of the strongest reductants. In alkaline medium, it reduces even perchlorates. Salts are quickly converted into hydrogen sulfites in the presence of air oxygen:
2Na2S2O4 + O2 + 2H2O = 4NаHSO3.
Hydrogen sulfites lose water and transform into disulfite (pyrosulfite) which are salts of the disulfurous (pyrosulfurous) acid Н2S2О5 at moderate heating, for example, during crystallisation from solutions, unknown in a free state:
2NaHSO3 = Na2S2О5 + Н2O .
A more convenient method to prepare disulfites is presented below:
2SO2 + 2NaHCO3 = Na2S2О5 + 2CO2 + H2O.
This acid has the following structure:
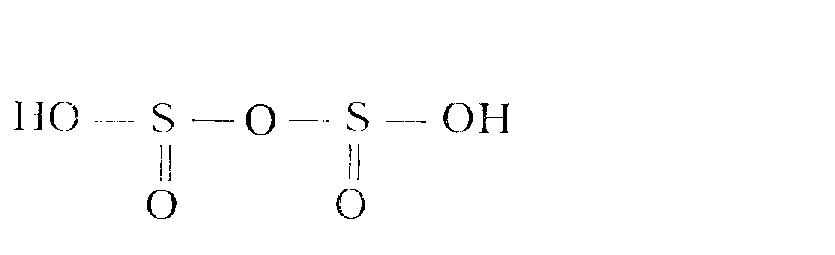
Dissolution of disulfites in water results in reverse reaction. Na2S2О5 salt is a conservator of moist corn and vine.
Oxygen - element(IV) compounds. EO2 oxides can be prepared from simple substances at combustion in air or oxygen atmosphere:
E + O2 = EO2.
Unlike SO2, EO2 oxides are polymer solids at STP: SeO2 (tsubl. = 315 ), TeO2 (m.p. = 733 ), PoO2 (m.p. = 885 ). Actually, SeO2 has a chain structure:
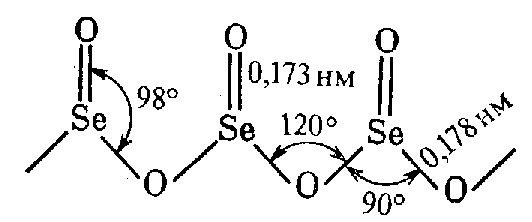
This is a tetrahedron where Se is in the state of sp3-hybridisation.
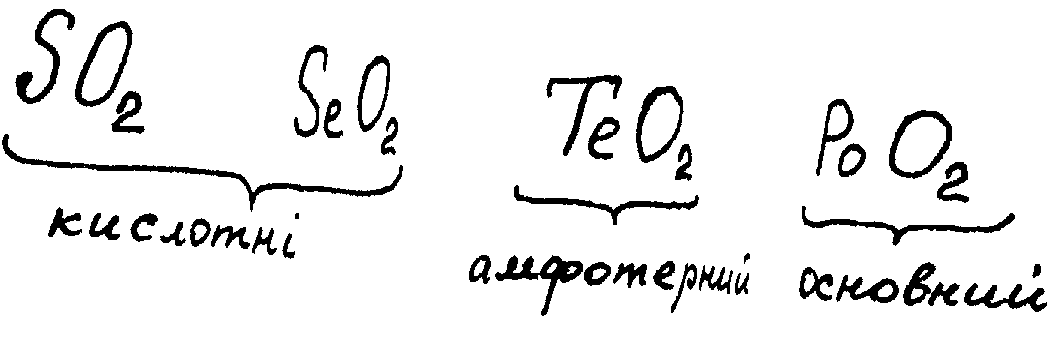 In
the series SeO2—TeO2—PoO2
acid properties are weakened and basic properties are strengthened.
The reason for this phenomenon is the growth of metallic properties
of elements and also increase of ionic mechanism contribution in E—O
bond. Certainly, SeO2,
like
SO2,
is dissolved in water and alkalis easily with the formation of
selenous acid, H2SeO3,
which is similar to sulfurous acid:
In
the series SeO2—TeO2—PoO2
acid properties are weakened and basic properties are strengthened.
The reason for this phenomenon is the growth of metallic properties
of elements and also increase of ionic mechanism contribution in E—O
bond. Certainly, SeO2,
like
SO2,
is dissolved in water and alkalis easily with the formation of
selenous acid, H2SeO3,
which is similar to sulfurous acid:
SeO2 +H2O H2SeO3
SeO2 + 2NaON = Na2SeO3 +H2O
TeO2 already shows amphoteric properties. It is not soluble in water, but like SeO2, is soluble in alkali solutions, forming a colourless telluride:
TeO2 + 2NaON = Na2TeO3 +H2O (TeO2 has acidic properties),
and in solutions of strong acids to form salts:
TeO2 + HI = TeI4 + 2H2O (TeO2 has basic behaviour),
TeI4 + 2HI H2[TeI6].
PoO2 is a basic oxide. That is why PoO2 has no interaction with solutions of alkali (the reaction takes place only with solid alkalis in the molten state), although gives salts with acids:
PoO2 + 2H2SO4 = Po(SO4) 2 + 2H2O.
Due to the action of strong acids on selenites and tellurites selenous and tellurious acids are formed. Unlike H2SO3, they can be obtained in the free state. H2SeO3 is a white hygroscopic solid, which easily loses water:
H2SeO3
![]() SeO2
+ H2O
SeO2
+ H2O
H2TeO3 has the ability to form polymers (its composition is TeO2∙hH2O). Low soluble TeO2∙hH2O can be deposited from concentrated solutions.
H2SeO3 and H2TeO3 are easily obtained during elemental Se and Te oxidation by concentrated HNO3.
In the series of acids H2TeO3 — H2SeO3 — H2SO3 the acid strength increases (K1 = 3.10-6, 2.10-3, 2.10-2). First of all, this behaviour is defined by the growth of chalcogen’s EN in this direction that leads to increased displacement of electrons in OH-groups to the oxygen atom and increases its polarity:
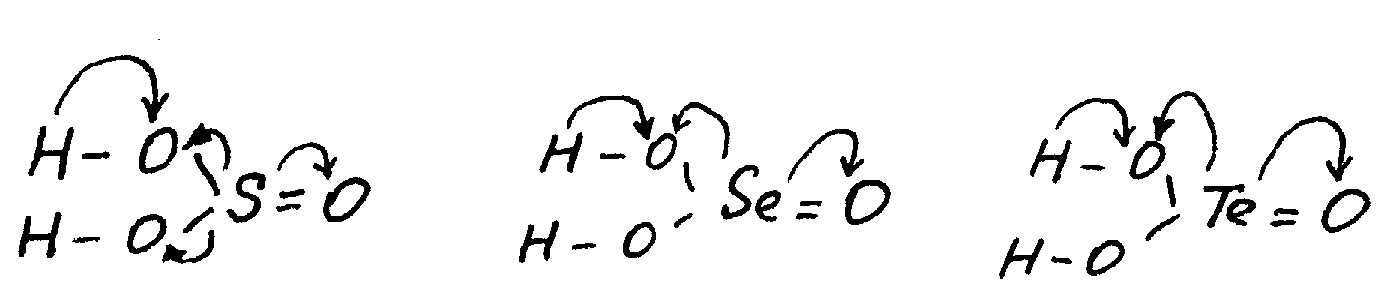
The stronger polarisation of H—O bond facilitates dissociation of acids under the influence of polar water molecules.
Elements display intermediate oxidation state in compounds Se4+ and Te+4, and these substances can be reductants and also oxidants in redox reactions, but unlike S4+ derivatives, oxidising properties are more typical of them, as evidenced by comparing Eo values:
H2SO3 + 4H+ + 4e- = S + 3H2O; E = 0.45 V
H2SeO3 + 4H+ + 4e- = Se + 3H2O; E = 0.74 V (a consequence of secondary periodicity)
H2TeO3 + 4H+ + 4e- = Te + 3H2O; E = 0.59 V
Therefore,
H2SO3 + HI no reaction
H2SeO3 + 4HI = 2I2 + Se + 3H2O
but as mentioned above: TeO2 + 4HI = TeI4 + 2H2O
Selenous acid oxidises SO2:
H2SeO3 + 2SO2 + H2O = Se + 2H2SO4
These acids can also have reducing properties (with very strong oxidants):
if H2SO3 + Br2 + H2O = H2SO4 + 2HBr
then
5H2SeO3 + 2HClO3 = 5H2SeO4 + Cl2 + H2O
Na2SeO3 + Cl2 + NaOH = Na2SeO4 + NaCl + H2O
5H2SeO3 + 2KMnO4 + 3H2SO4 = 5H2SeO4 + 2MnSO4 + K2SO4 + 3H2O
and
TeO2 + H2O2 + 2H2O = H6TeO6 ortho-telluric acid
Sulfur (VI) oxide
 Production
in industry.
Catalytic oxidation of SO2
by
air oxygen takes place in the presence of catalyst, V2O5
(440
oC):
Production
in industry.
Catalytic oxidation of SO2
by
air oxygen takes place in the presence of catalyst, V2O5
(440
oC):
2SO2 + O2 2SO3; Н0298 =-184.4 kJ/mol
In the laboratory it is commonly prepared by the reaction between sulfur dioxide and oxygen in the presence of platinum catalyst and high temperature:
2SO2 + O20 2SO3
Initially, sulfur trioxide was prepared by heating iron(III) sulfate:
Fe2(SO4)3 = Fe2O3 + 3SO3
It is also obtained by the dehydration of concentrated sulfuric acid with phosphorus(V) oxide:
2H2SO4 + P4O10 = 4HPO3 + 2SO3
Structure. SO3 is a nonpolar molecule that has a plane structure with the valence angle 120o (sp2-hybridisation of valence orbitals of sulfur) and equivalent bonds due to delocalised fourcentred -bond (like SO2):
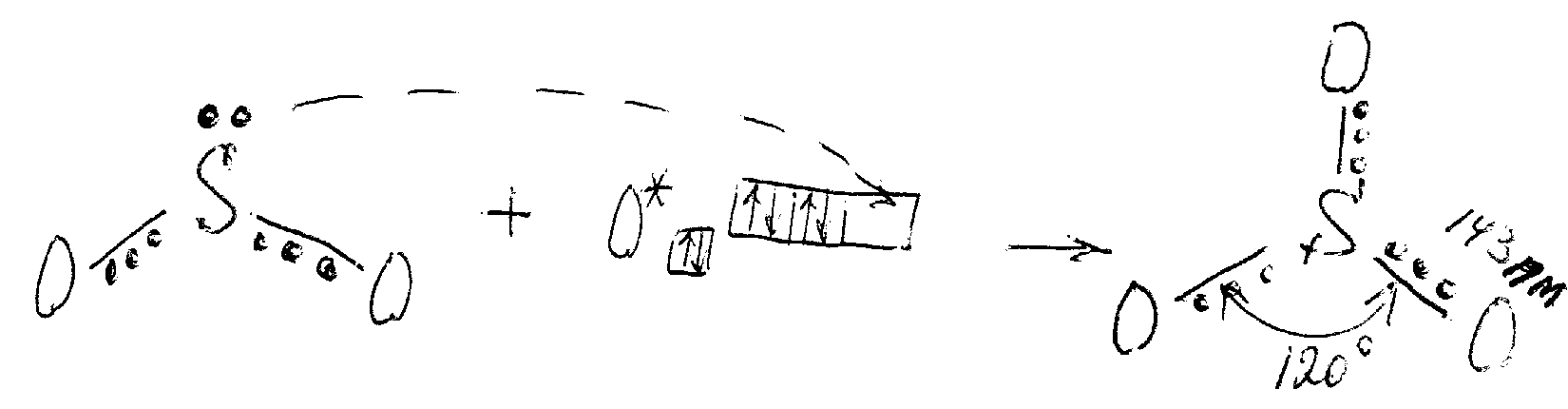
Polymorphic modifications of SO3. Molecules SO3 exist only in the gaseous phase. In the solid state, it is a polymer with (SO3)n chains:
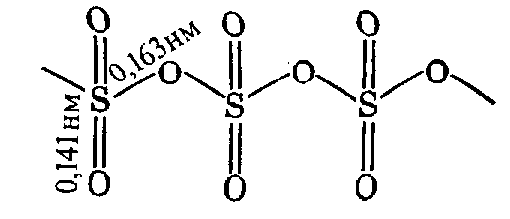
This solid polymorph is referred to as -SO3. It looks like transparent mass similar to ice; m.p. = 16.9 oC.
- SO3. It forms when - SO3 absorbs water. This polymer has lower molar mass than -SO3. It does not melt, but sublime at 50oC. Externally, it is similar to asbestos.
- SO3 is the product of condensation of SO3 vapours consisting of cyclic trimer (SO3)3 where S has sp3-hybridisation state:

Properties. This acid oxide reacts with basic oxides, and hydroxides. Moreover, what is most important: SO3 is an anhydride of sulfuric acid. It reacts with water rapidly:
SO3 + Н2O = Н2SO4; Н0298 = -89.2 kJ/mol
