
Химия на английском. 2
.pdfМинистерство образования и науки Российской Федерации Федеральное агентство по образованию Южно-Уральский государственный университет Химический факультет
Ш143.21-9 Д182
Е.И.Данилина
ХИМИЯ НА АНГЛИЙСКОМ ЯЗЫКЕ
Модуль 2 ОБЩАЯ И НЕОРГАНИЧЕСКАЯ ХИМИЯ
Учебное пособие
Челябинск Издательский центр ЮУрГУ
2009
ББК Ш143.21-923 УДК 54(075.8)
Одобрено учебно-методической комиссией химического факультета
Рецензенты:
Балыкин В.П., д-р хим. наук, профессор кафедры аналитической и физической химии Челябинского государственного университета,
Толчев А.В., д-р хим. наук, профессор, зав. кафедрой общетехнических дисциплин Челябинского государственного педагогического университета
Д182 Данилина, Е.И.
Химия на английском языке. Модуль 2. Общая и неорганическая химия: учебное пособие. – Челябинск: Издательский центр ЮУрГУ, 2009. – 48 с.
Учебное пособие составлено на английском языке по материалам британских, канадских и американских учебников по химии для колледжей и университетов и предназначено для практических занятий и самостоятельной работы студентов. В учебном пособии предложены качественные вопросы и расчетные задачи, охватывающие основные темы курса общей и неорганической химии. В приложениях приведены необходимые справочные материалы для численного решения задач и их устного чтения: Периодическая таблица, таблицы констант ионизации, произведений растворимости и стандартных электродных потенциалов, а также транскрипция названий элементов и соединений по правилам ИЮПАК.
Пособие предназначено для студентов 2 курса химического факультета.
УДК 54(075.8)
© Издательский центр ЮУрГУ
2
|
CONTENTS |
|
1. |
The Structure of the Atom................................................................................ |
4 |
2. |
The Periodic Table and Periodic Law............................................................... |
8 |
3. |
Energy and Chemical Change........................................................................... |
12 |
4. |
Rates of Chemical Reactions............................................................................. |
18 |
5. |
Dynamic Chemical Equilibrium........................................................................ |
21 |
6. |
Ionic Equilibria.................................................................................................. |
28 |
7. |
Electrochemistry................................................................................................ |
35 |
Appendix 1. Periodic Table of Chemical Elements............................................... |
38 |
|
Appendix 2. Elements and Electronegative Components...................................... |
40 |
|
Appendix 3. Acids and Anions.............................................................................. |
43 |
|
Appendix 4. Ionization Constants for Acids.......................................................... |
44 |
|
Appendix 5. Ionization Constants for Nitrogen Bases........................................... |
45 |
|
Appendix 6. Solubility Product Constants in Water at 25 oC................................. |
45 |
|
Appendix 7. Standard Reduction Potentials........................................................... |
46 |
|
References.............................................................................................................. |
48 |
|
3
1.THE STRUCTURE OF THE ATOM
1.1.How many protons and electrons are in each of the following atoms?
a) boron; |
c) platinum; |
b) radon; |
d) magnesium. |
1.2.An atom of an element contains 66 electrons. What element is it?
1.3.An atom of an element contains 14 protons. What element is it?
1.4.How many protons and electrons are contained in an atom of element 44?
1.5.For each of the following chemical symbols, determine the element name and the number of protons and electrons an atom contains.
a) V; |
e) Zn; |
i) Mo; |
b) Ir; |
f) Al; |
j) Sc; |
c) Mn; |
g) Cs; |
k) Bi; |
d) S; |
h) Br; |
l) Cu. |
1.6.A carbon atom has a mass number of 12 and an atomic number of 6. How many neutrons does it have?
1.7.An isotope of mercury has 80 protons and 120 neutrons. What is the mass number of this isotope?
1.8.An isotope of xenon has an atomic number of 54 and contains 77 neutrons. What is the xenon isotope’s mass number?
1.9.How many electrons, protons, and neutrons are contained in each of the following
atoms: 13255 Cs; 2759 Co; 16369Tm; 7030 Zn.
1.10.How many electrons, protons, and neutrons are contained in each of the following atoms: a) gallium-64; b) titanium-48; c) fluorine-23; d) helium-8.
1.11.Determine the number of protons, electrons, and neutrons for isotopes in the table. Name each isotope, and write its symbol.
Element |
Atomic number |
Mass number |
Neon |
10 |
22 |
Calcium |
20 |
46 |
Oxygen |
8 |
17 |
Iron |
26 |
57 |
Mercury |
80 |
204 |
4
1.12.Boron has two naturally occurring isotopes, namely: boron-10 (abundance = 19.8%, mass = 10.013 amu), boron-11 (abundance = 80.2%, mass = 11.009 amu). Calculate the atomic mass of boron.
1.13.Helium has two naturally occurring isotopes, helium-3 and helium-4. The atomic mass of helium is 4.003 amu. Which isotope is more abundant in nature?
1.14.Chlorine, which has an atomic mass of 35.453 amu, has two naturally occurring isotopes, Cl-35 and Cl-37. Which isotope occurs in greater abundance?
1.15.Calculate the atomic mass of magnesium. The three magnesium isotopes have atomic masses and relative abundances of 23.985 amu (78.99%), 24.986 amu (10.00%), and 25.982 amu (11.01%).
1.16. Silver has two isotopes, 10749 Ag has a mass of 106.905 amu (52.00%), and 10949 Ag has a mass of 108.905 amu (48.00%). What is the atomic mass of silver?
1.17.Calculate the atomic mass of titanium. The five titanium isotopes have atomic masses and relative abundances of 45.953 amu (8.00%), 46.952 amu (7.30%), 47.948 amu (73.80%), 48.948 amu (5.50%), and 49.945 amu (5.40%).
1.18.Complete the table below.
|
|
Composition of Various Isotopes |
|
|||
|
Isotope |
Atomic |
Mass |
Number of |
Number of |
Number of |
|
|
number |
number |
protons |
neutrons |
electrons |
|
|
|
32 |
16 |
|
|
|
|
|
|
|
24 |
20 |
|
Zn-64 |
|
|
|
|
|
|
|
9 |
|
|
10 |
|
|
|
11 |
23 |
|
|
|
1.19. Write ground-state electron configurations for the following elements. |
||||||
a) bromine (Br); |
d) rhenium (Re); |
|
|
|||
b) strontium (Sr); |
e) terbium (Tb); |
|
|
|||
c) antimony (Sb); |
f) titanium (Ti). |
|
|
|||
1.20.How many electrons are in orbitals related to the third energy level of a sulfur atom?
1.21.How many electrons occupy p orbitals in a chlorine atom?
1.22.Sketch the electron arrangements for the first 20 elements in the periodic table.
5

1.23. What element has the following ground-state electron configuration: [Kr]5s24d105p1?
1.24. What element has the following ground-state electron configuration: [Xe]6s2?
1.25. Write out the electron configuration and draw the orbital diagram for each of the following elements.
a) silicon; |
c) calcium; |
b) fluorine; |
d) krypton. |
1.26.What is a valence electron? Draw the electron-dot structures (Lewis structures) for the elements in the previous problem (1.25).
1.27.Identify the number of valence electrons in the outer energy levels of the following elements:
a) chlorine; |
f) lead; |
b) helium; |
g) antimony; |
c) indium; |
h) selenium; |
d) strontium; |
i) arsenic; |
e) rubidium; |
j) xenon. |
1.28. Draw electron-dot structures (Lewis structures) for the following elements:
a) magnesium; |
d) rubidium; |
b) sulfur; |
e) thallium; |
c) bromine; |
f) argon. |
1.29.Draw the first 20 elements in the periodic table using Lewis structures (electrondot structures).
1.30.Use the periodic table to draw Lewis (electron-dot) structures for the following elements: barium (Ba), gallium (Ga), tin (Sn), bismuth (Bi), iodine i), cesium (Cs), selenium (Se), neon (Ne).
1.31.Which element has the following orbital diagram?
↑↓ |
|
↑↓ |
|
↑ |
1s |
|
2s |
|
2p |
1.32. Write orbital notations and complete electron configurations for atoms of the following elements, compare them to electron-dot structures.
a) beryllium; |
c) nitrogen; |
b) aluminum; |
d) sodium. |
|
6 |
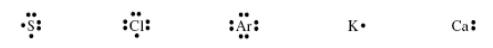
1.33. Use noble-gas notation to describe the electron configurations of the elements represented by the following symbols.
a) Mn; |
d) Zn; |
g) Pb; |
b) Kr; |
e) Zr; |
h) Ra; |
c) P; |
f) W; |
i) Sm. |
1.34. What elements are represented by each of the following electron configurations?
a) 1s22s22p5; |
d) [Kr]5s24d105p4; |
b) [Ar]4s2; |
e) [Rn]7s25f13; |
c) [Xe]6s24f4; |
f) 1s22s22p63s23p64s23d104p5. |
1.35. Draw electron configurations and electron-dot structures (Lewis structures) for atoms of each of the following elements:
a) carbon; |
d) potassium; |
b) arsenic; |
e) barium; |
c) polonium; |
f) indium. |
1.36.Answer the following questions for the atom of antimony:
a)How many electron-containing orbitals has the atom?
b)How many of the orbitals are completely filled?
c)How many of the orbitals are associated with the atom’s n = 5 principal energy
level?
1.37.Shown below are the Lewis structures (electron-dot structures) for five elements: sulfur (S), chlorine (Cl), argon (Ar), potassium k), and calcium (Ca). Answer the questions below about these structures.
a)Which of the above Lewis structures (electron-dot structures) is the same as the Lewis structure for the ion S2–? Explain your answer describing the process of electron gain/loss.
b)Which of the above Lewis structures (electron-dot structures) is the same as the Lewis structure for the ion Cl–? Explain your answer describing the process of electron gain/loss.
c)Which of the above Lewis structures (electron-dot structures) is the same as the Lewis structure for the ion K+? Explain your answer describing the process of electron gain/loss.
d)Name an ion of calcium that has electron-dot structure similar to that of argon. Explain your answer describing the process of electron gain/loss.
7
2.THE PERIODIC TABLE AND PERIODIC LAW
2.1.Identify the name and symbol of the elements in the following locations of the periodic table:
a)Group IVA, Period 2;
b)Group IB, Period 4;
c)Group VIIIA, Period 6;
d)Group IA, Period 1;
e)Group IIB, Period 5;
f)Group 2 IIA, Period 4;
g)Group VIIA, Period 5;
h)Group IIIA, Period 3.
2.2.Identify the element that is described by the following information. Refer to a periodic table as necessary.
a)It is a Group IVA element in the third period.
b)It is a Group VA element in the fifth period.
c)It is the other element in Group VA, with smaller total number of electrons.
d)It is a halogen that exists in the liquid state at room temperature.
2.3.Develop four more element descriptions like those in problem 2.2. Exchange them with a classmate and identify each other’s elements.
2.4.Without using the periodic table, determine the group, period, and block of an atom
with the following electron configurations. |
|
|
a) [Ne]3s2; |
b) [He]2s2; |
c) [Kr]5s24d105p5. |
2.5. Determine the group, period, and block in which each of the following elements is located on the periodic table.
a) [Kr]5s24d1; |
c) [He]2s22p6; |
b) [Ar]4s23d104p3; |
d) [Ne]3s23p1. |
2.6. Identify the elements with the following valence electron configurations:
a) 5s1; |
c) 3s2; |
b) 4s23d2; |
d) 4s24p3. |
2.7.Write the electron configuration of the element fitting each of the following descriptions.
a)The group 2A element in the fourth period.
b)The noble gas in the fifth period.
c)The group 2B element in the fourth period.
d)The group 6A element in the second period.
2.8.What are the symbols for the elements with the following valence electron
configurations? |
|
|
a) s2d1; |
b) s2p3; |
c) s2p6. |
2.9. Consider the following elements: H, Li, N, F, Co, Ag, Kr, I, Hg. 8
a)Sketch an outline of the periodic table, with these elements properly placed.
b)State the group number and period number each element belongs to.
c)Identify each element as a metal, metalloid, or non-metal.
d)Identify the state of each element at room temperature.
e)Draw the Lewis structure for each of these elements.
2.10. How many valence electrons are there in an atom of each of these elements?
a) strontium; |
f) tin; |
b) silicon; |
g) chlorine; |
c) bromine; |
h) magnesium; |
d) sulfur; |
i) helium; |
e) neon; |
j) sodium. |
Which of them have the same Lewis structures and which are different?
2.11.Draw Lewis structures for the elements: lithium, barium, boron, carbon, nitrogen.
2.12.Find the group and period of each of the following elements in the periodic table:
a) europium; |
f) mercury; |
b) neodymium; |
g) ytterbium; |
c) carbon; |
h) bromine; |
d) nitrogen; |
i) chromium; |
e) silicon; |
j) krypton. |
2.13.Which has the largest radius: helium (He), xenon (Xe), or argon (Ar)? Which has the smallest?
2.14.Which has the largest radius: magnesium (Mg), silicon (Si), sulfur (S), or sodium (Na)? The smallest?
2.15.Which has the largest atomic radius: nitrogen (N), antimony (Sb), or arsenic (As)? The smallest?
2.16.Using only their location in the periodic table, rank the atoms in each set by decreasing atomic size. Explain your answers.
a)Mg, Be, Ba;
b)Ca, Se, Ga;
c)Br, Rb, Kr;
d)Se, Br, Ca;
e)Ba, Sr, Cs;
f)Se, Br, Cl;
g)Mg, Ca, Li;
h)Sr, Te, Se;
i)In, Br, I;
j)S, Se, O.
2.17. Using only a periodic table, rank the atoms in each set in order of decreasing size.
Explain your ranking. |
|
|
a) Na, K, H; |
b) Mg, S, Si; |
c) Cl, K, Ar. |
9
2.18. Using only their location in a periodic table, rank each of the following sets of elements in order of increasing atomic size. Explain your answer in each case.
a) Mg, S, Cl; |
d) Rb, Xe, Te; |
b) Al, B, In; |
e) P, Na, F; |
c) Ne, Ar, Xe; |
f) O, S, N. |
2.19.Can you determine which of two unknown elements has the larger radius if the only known information is that the atomic number of one of the elements is 20 greater than the other?
2.20.Using only their location in a periodic table, rank each of the following sets of elements in order of decreasing ionization energy. Explain your answer in each case.
a)Cl, Br, I;
b)Ga, Ge, Se;
c)K, Ca, Kr;
d)Na, Li, Cs;
e)S, Cl, Br;
f)Cl, Ar, K.
2.21. Using only a periodic table, rank the elements in each set in order of increasing ionization energy. Explain your ranking.
a) B, N, F; b) F, Cl, Br; c) Na, Cs, K.
2.22. Using only a periodic table, rank the elements in each set by increasing ionization
energy. Explain your answers. |
|
a) Xe, He, Ar; |
d) Kr, Br, K; |
b) Sn, In, Sb; |
e) K, Ca, Rb; |
c) Sr, Ca, Ba; |
f) Kr, Br, Rb. |
2.23. Using only a periodic table, identify the atom in each of the following pairs with the lower first ionization energy.
a) B, O; |
d) F, N; |
b) B, In; |
e) Ca, K; |
c) I, F; |
f) B, Tl. |
2.24. Using only a periodic table, rank the elements in each set in order of increasing electron affinity. Explain your ranking.
a) Be, Ca, Mg; b) Kr, Se, Br; c) Na, Cs, K.
2.25. Which element in each of the following pairs will have the lower electron affinity? Explain your answer in each case.
a) K or Ca; |
c) S or Se; |
b) O or Li; |
d) Cs or F. |
2.26. Based only on their position in the periodic table, arrange the elements in each set in order of increasing attraction for electrons in a bond.
a) Li, Br, Zn, La, Si; b) P, Ga, Cl, Y, Cs.
10
