
THE ESSENTIAL OF IMMUNOLOGY
.pdfМинистерство здравоохранения и социального развития Российской Федерации Казанский государственный медицинский университет Кафедра клинической иммунологии и аллергологии
ИЗБРАННЫЕ ВОПРОСЫ ОБЩЕЙ ИММУНОЛОГИИ Методическое пособие для студентов
THE ESSENTIAL OF IMMUNOLOGY
Materials for the practical classes
INNATE (NON-SPECIFIC) IMMUNITY
TEACHING OBJECTIVES.
I. Recognize the significance of the immune system in combating infection and disease. 2.Distinguish between non-specific (innate) and specific (adaptive) immune systems. 3.Understand the mechanisms of combating infection/disease (killing pathogens). 4.Know the humoral and cellular components of the non-specific immunity.
5.Comprehend the mechanism of action of the humoral and cellular components of non-specific immunity.
Mechanisms of protection against infection and disease are diverse. Primarily they can be divided into two major categories: non-specific (innate) and specific (adaptive), which differ as follows:
Non-specific Immunity |
Specific Immunity |
Its response is antigen-independent. There is |
Its response is antigen-dependent |
immediate response. It is not antigen-specific. |
There is a lag time between exposure and |
Exposure does not result in induction of memory |
maximal response. |
cells. |
It is antigen-specific. |
Some of its cellular components or their |
Exposure results in induction |
products |
of memory cells. |
may aid specific immunity. |
Some of its products may help |
|
non-specific immunity |
The elements of the innate immune system include anatomical barriers, secretory molecules and cellular components (Table 1).
Anatomical barriers:
Skin, intestinal movement, oscillation of broncho-pulmonary cilia, etc. prevent pathogens from entering and/or getting a foothold in the body.
Secretory molecules:
These include
organic acids in skin secretions,
lysozyme in oro-naso-pharyngeal and lacrimal secretions,
thiocyanate in saliva,
low molecular weight fatty acids in the lower bowel:
bile acids and low molecular weight fatty acids in lower Gl tract,
transferrin, lactoferrin. lysozyme. interferons. fibronectin. complement, acute phase proteins, etc. in serum;
Interferons and tumor necrosis factor (TNF) at the site of inflammation.
Transferrin and lactoferrin deprive organisms of iron.
Interferons inhibit viral replication and activate other cells which kill pathogens.
Lysozyme, in scrum and tears, breaks down the bacterial cell wall (peptidoglycan)
fibronectin coats (opsonizes) bacteria and promotes their rapid phagocytosis.
Complement components and their products cause destruction of microorganism directly or with the help of phagocslic cells.
Acute phase proteins (such as CRP) interact with the complement system proteins to combat infections.
TNF-a suppresses viral replication and activates phagocytes (nitric oxide pathway).
Cellular Components:
Neutrdphils (PMN) and macrophages and monocytes are the most important cellular components of the non-specific immune system.
Neutrophils (polymorphonuclear: PMN) are most important cellular components in bacterial destruction. They are relatively large and most abundant white blood cells with 10bed nucleus and cytoplasmic granules (lysosomes). They are identified by their characteristic morphology. In addition, monoclonal antibodies against characteristic cell surface protein, cluster differentiation marker. CD66 can be used to identify these cells,
PMN granules are of two kinds: primary (azurophilic) and secondary (specific).
Primary azurophilic granules are characteristic of immature and very young roteins. defensins (small molecular weight proteins), proteases (elastase. cathepsin G. etc.), lysozyme and myeloperoxidase (characteristic of primary granules).
Secondary granules are more characteristic of (specific for) mature neutrophils They contain lysozyme and ADPH oxidase cofactors. lactoferrin and B-12-binding protein, the_last two are characteristic for these granules.
Mononuclcar phagocytes are the other population of phagocytic cells and include monocytes in circulation. histiocyles in tissues, microglilal cells in the brain. Kupffer cells in the liver and macrophages in serous cavities and lymphoid organs. They also have granules similar to those in neutrophils. although not as abundant. They are recognized by their morphology, ability to adhere on glass/plastic surface, and CD 14 marker.
2

All phagocytic cells have receptors for a variety of molecules (Figure I).
Figure 1: Adherence of bacteria via receptors
Scavenger R, IgG FcR, CR (complement R), Toll-like R (PRR)
Most pertinent to nonspecific immunity are scavenger and TOLL-like receptors and receptors for the Fc part of IgG, complement, interferons. TNF and certain bacterial components. Interaction of organisms with these receptors promotes phagocytosis and phagocyte activation for a more efficient killing of pathogens.
Phagocytes response to infection Chemotaxis:
Bacteria produce N-formyl-methionine-containing peptides which are powerful attractants (chemotactic) for phagoc\1ic cells. Many bacteria also act on proteins of the complement and clotting systems to produce peptides that cause vasodialation. vascular permeability and expression of adherence molecules on vascular endothelial cells. They also induce, on phagocytic cells, expression of proteins (e.g., integrins) that promote binding to endothelial cells. Phagocytic cells respond to chemotactic peptides of bacterial and host origin and migrate across the capillary wall (diapedesis) to the site of infection/inflammation.
Once at the site of infection, phagocytes can attach to bacteria via a number of receptors for bacterial components (scavenger receptor, LPS receptor, mannose receptor. TOLL-like receptors, etc.) or host proteins (opsonins) that coat (opsonize) bacteria (e.g., fibronectin. complement. IgG antibody, etc.). This attachment triggers the activation of respiratory burst (hexose monophosphate shunt), internalization of the organisms (phagosome formation), phagosome lysosome fusion (phagolysosome) etc.
Respiratory burst and phagocytosis :
The process of attachment and phagosome formation is accompanied by the activation of the respiratory burst (Figure 3), These molecules are microbicidal and cause killing of organisms in the phagosome.
Phagosome-lysosome fusion: Phagosome. soon after its formation, fuses \vith granules (lysosomes) to form a phago-lysosotne. As mentioned earlier, lysosomes contain a variety of anti-microbial substances (e.g., lysozyme. defensins. proteases, lactoferrin, transferrin. etc.) and phago-lysosome fusion results in the exposure of microorganisms to these substances and their destruction. Also, fusion of phagosome with primary granules (in newly recruited phagocytes) that contain myeloperoxidase results in the production of OC1". (or other halides: I and Br) and halogenation of bacterial proteins. This ultimately leads to bacterial killing (Figure 4).
3

Figure 3. Respiratory burst
Figure 4
Three modes of intracellular killing:
It should be apparent from the preceding discussion that there are three pathways of killing by phagocytes (Figure 3-4 and Table 2):
(1)by lysosomal antibacterial substances (lactoferrin, cationic proteins, lysozvme, defensins, proteases, etc.) without the requirement of respiratory burst (oxygen-independent killing: Table 2);
(2)by products of respiratory burst (super-oxide, singlet oxygen, hydroxyl radical, hydrogen peroxide, etc.) without the need for myelojieroxidase (oxygen-dependent, myeloperoxidaseindependent killing: table 3a): and
(3)by halogenation of bacterial proteins catalyzed by meyloperoxidase (oxygen-dependent, myeloperoxidase-dependent killing
These processes occur simultaneously and act synergistically. A defect in any of these pathways, for example, a deficiency of NADPH oxidase (cytochrome b558) components (p91, p22, 947 p6lphox), myeloperoxidase, etc. would impair the killing activity of phagocytes and render the host more susceptible to pyogenic infections. NADPH oxidase is the most serious of all these defects.
4
Neutrophils also contain catalase and glutathion (GS) which detoxify excess H2O2. GS. in its reduced form (GSH). also recycles NADP to NADPH. Interaction of phagocytic cells with certain humoral factors (e.g. interferons. TNF, C5a. IL-2, etc.) can increase their phagocytic function, respiratory burst and intra-cellular killing. Certain proteins secreted by various cells (cytokines) can also induce phagocytic cells, particularly macrophages. to produce nitric oxide (NO) that is toxic to microorganism and malignant cells (Figure 5). Thus certain bacterial products can cause macrophages to produce TNF that can. in turn, activate NO production. Likewise, interferon-y produced by NK (natural killer) cells (and T cells) can also activate the NO pathway of killing pathogens.
Other cells:
A number of other cells are also involved in nonspecific resistance: they include NK). antibody dependent cytotoxic cells (ADCC) also referred to as K-cells and lymphokine (proteins secreted by lymphocytes) activated killer (LAK) cells and eosinophils.
NK cells are important in defense against viral infections and malignancies. They resemble lymphocytes in morphology but are larger and granular, hence also known as large granular lymphocytes (LGL). The granules contain cytolytic proteins such as perform. NK cells recognize the difference between normal and malignant or virus-infected cells in a nonspecific manner via sugar-lectin interaction and kill them following intimate contact. They also have low affinity Fc (y III) receptor (CD 16) by which they can interact with antibody-coated cells and cause their death. NK cells also have receptors for interlcukin-2 (IL-2) and interferonand interaction with these cytokines leads to their activation (Figure 6). The presence of CD56 & CD 16 and absence of CD3 is currently used as characteristic markers for NK cells.
Interaction with IL-2 (or interferon-y) causes activation of NK cells. These activated cells are referred to as LAK (lymphokine-aclivated killer) cells. LAK cells function the same way as NK cells except that they are more potent killers and are capable of killing not only malignant but also transformed cells.
K-cells are morphologically undefined cells attach to target cells coated with IgG antibody \'ia the Fc receptor (Fc-yR) and cause their lysis; thus, they require interaction of specific antibody with target. Macrophages can also function as K cells since they also have Fc receptors.
Macrophages, when activated by a variety of cytokines. can kill malignant cells without the aid of antibody.
Eosinophils have Fc receptor for IgE (Fc-eR) and cause cytotoxicity to large multicellular parasites coated with specific IgE antibody, analogous to K cells.
5
There is no memory or specificity, in the components of non-specific immunity. However, cells of the non-specific immune system become functionally more efficient following exposure to to a pathogen because of interaction with products of the specific immune system (e.g.. antibodies and cvtokines).
At this time you should know the following:
1.Differences between the non-specific and specific immune functions.
2.Humoral components of the non-specific immune system and their action.
3.Cellular components of the non-specific immune function and their action.
4.Pathways of intracellular killing of bacteria by phagocytes and their characteristic features.
5.Effect of humoral components such as interferon, TNF. IL-2, complement etc. on cellular components of the non-specific immune system.
COMPLEMENT
OBJECTIVES:
1. Understand different pathways of complement (C )activation. 2 Know the enzymatic and nonenzymatic mechanisms of C activation.
3.Know the biological properties of C activation products.
4.Know the significance of C system in host resistance, inflammation and damage to self.
5.Understand the mechanisms of regulating C activation and it products.
Complement refers, historically, to fresh serum capable of Using antibody (Ab)-coated cells. This activity is destroyed (inactivated) by heating serum at 56EC for 30 minutes.
Definitions:
C-activation: Alteration of a complement component (protein) in such a way-thai it can proceed to interact with the next component in the pathway (cascade).
C-fixation: Utilization of complement components by the antigen-antibody complex.
6
Hemolytic units: The dilution of a serum sample which can lyse a predetermined proportion of a sheep erythrocyte (SRBC) suspension coated with anti-SRBC antibody. The SRBC concentration is usually 5% and the lysis is set at 50% and the unit is recorded as CH50.
C-inactivation: Denaturation (usually by heat) of one of the early components in C-activation pathway resulting in the destruction of C-hemoly1ic activity. Convertase/esterase: Activated (altered/cleaved) C-component which acts as a proteolytic enzyme specific for subsequent components.
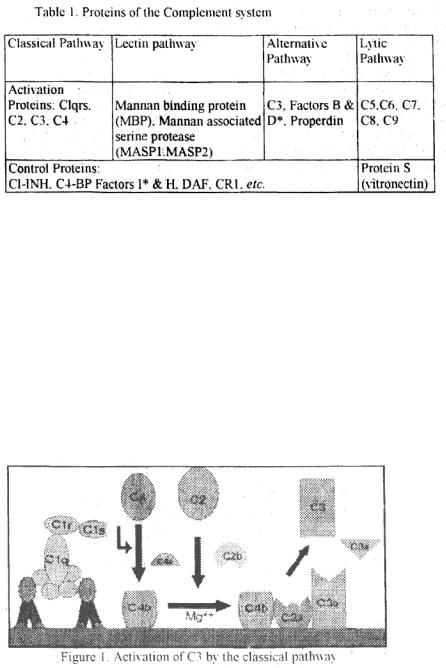
Proteins of the Complement System
Complement system is composed of more than 25 different proteins (Table I) produced by different tissues and cells including hepatocytes. macrophages and gut epithelial cells.
Components underlined acquire enzymatic activity when activated. Components marked with have enzymatic activity in native form.
These proteins are activated by a variety of agents and their activation proceeds via different pathways and if the activated products bind to a cellular target, their deposition leads to cell lysis. Since the complement components are activated in a cascade fashion, the absence W one of the components in the pathway can disrupt the cascade and terminate the reaction.
Pathways of complement activation:
The complement activation can occur by three pathways, classical, lectio (mannose binding protein) and alternative pathway, all leading to the activation of C5 that follows the activation of the membrane attack (lytic) pathway.
Classical pathway. Classical pathway (Figure 1) normally requires a suitable antibody (Ab) bound to an antigen (Ag). complement components 1,4,2 and 3 and Ca and Mg cations.
CI activation: Binding of Cl (Clq. Clr & Cls: a Ca— dependent complex), present in normal serum, to Ag-Ab complexes results in the autocatalysis of Clr. The altered Clr cleaves Cls and this cleaved Cls functions as C4-C2 convertase capable of cleaving both C4 and C2.
7
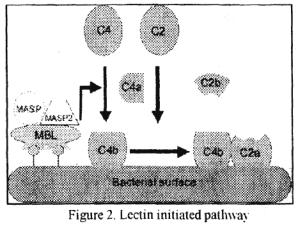
C4 and C2 activation (generation of C3 convertase): Activated Cls enzymalically cleaves C4 into C4a and C4b. C4b binds to the Ag-bearing particle or cell membrane while C4a remains a biologically active pcptide at the reaction site. C4b binds C2 that becomes susceptible to Cls and is cleaved into C2a and C2b. C2a remains complexed with C4b whereas C2b is released in the microemironment. C4b2a complex is known as C3 convertase in which C2a is the enzymatic moiety.
C3 activation (generation of C5 convertase): C3 convertase. in the presence of Mg. cleaves C3 into C3a and C3b. C3b binds to the membrane to form C4b2a3b complex; C3a is released in the micro-environment. 4b2a3b complex functions as C5 convertase that cleaves C5 into C5a and C5b. Generation of C5 convertase marks the end of the classical pathway. Clqrs can also bind to a number of agents including some retroviruses. mycoplasma. poly-inosinic acid and aggregated IgG. and initiate the classical pathway.
Lectin pathway:
C4 activation can be achieved without antibody and C1 participation via the lectin pathway (Figure 2).
This pathway is initiated by three proteins, a mannan-binding lectin (MBL). also known as mannan-binding protein (MBP) and two
mannan-binding lectin-associated serine proteases (MASP-1 and MASP-2). all present in normal serum. MBL binds to certain mannose residues on many bacteria and subsequently interacts with MASP and MASP2. The MBL-MASP-1-MASP-2 complex is analogous to Ab-Clqrs comlex and leads to antibody-independent activation of C4. C2 and C3. Thus, the lectin pathway provides a means of non-specific protection against certain pathogens before any antibody response can be mounted.
Alternative Pathway:
Alternative pathway begins with the activation of C3 and requires Factors B and D and Mg' cation, all present in normal serum.
Spontaneous activation of C3: A metastable C3b like molecule (C3i) is generated by slow hydrolysis of native C3. C3i binds factor B that is cleaved by Factor D to produce C3iBb. C3iBb acts as C3-convertase and cleaves native C3 into C3a and C3b (Figure 3). C3b. if not inactivated and disposed of, binds factor B, which is again cleaved by Factor D to produce C3bBb complex (C3 convertase). This C3 convertase (or the one generated by the classical pathway, i.e., C4b2a), if not inactivated, will continue to act on C3 and cause its exhaustion.
Normal regulation of C3 convertase:
C3b. in fluid phase, is very short lived unless it finds a suitable stabilizing membrane or molecule (C3 activator; see later) present on many pathogens. In the absence of such a molecule, it binds quickly to autologous red cells via the C3b receptor. CR1 at a site close to decay accelerating factor (DAF) that prevents the binding of Factor B. Binding to CR1 also makes C3b susceptible to
8
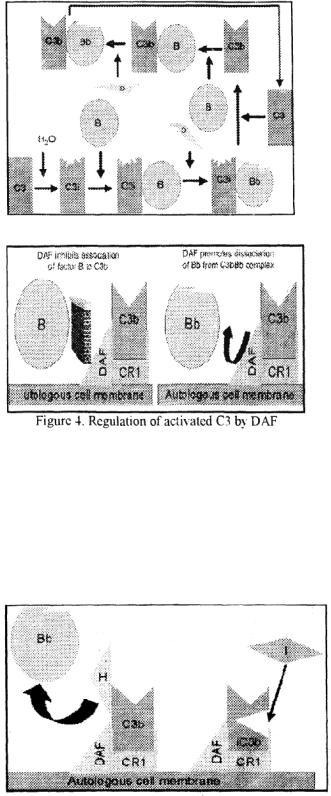
Factor I (Figure 4) that cleaves it into many fragments (iC3b. C3d. C3e. etc.: Figure 5). C4b, generated in the classical pathway, is also regulated by DAF. CR1 and Factor I. A defect in or deficiency of DAF can lead to red cell lysis and anemia, as in its absence, further activation of C will proceed and lead to the lytic pathway (see below).
Figure 3. Spontaneous activation of C3 (C3-tickover)
Another serum protein, factor H, can displace factor B and bind to C3b. Binding of factor H also makes C3b more susceptible to factor I (see figure 5). DAF. Crl and Factor 1 also regulate, in a similar manner. C3 convertase generated by the classical pathway. The only difference is that C4b-binding protein (C4b-BP. not factor H) makes it susceptible to Factor 1 A genetic jleficjency of factor 1 (or factor Ff) leads to uncontrolled C3 activation and exhaustion. A genetic defect in factor I or factor H and is a significant cause of inherited C3 deficiency and increased susceptibility to certain infections.
Figure 5. Regulation of activated C3 by Factor H
9

Stabilization of C3 convertase: Certain bacteria or their products (peptidoglycan. polv saccharides. etc.). provide a protected (activator) surface for C3b. Thus. C3b bound to such a surface becomes relatively resistant to the action of factor 1 (Figure 6). Even membrane bound C3bBb dissociates fairly rapidly. However, binding of another protein, propcrdin, further stabilizes this complex Consequently, the altcrnativ c pathway is also referred to as the properdin pathway.
Generation of C5 convertase: Stabilized C3 convertase cleaves more C3 and produces C3bBbC3b complex the C5 convertase of the alternative pathway (analogous to C4b2a3b of the classical pathway), which cleaves C5 into C5a and C5b. C5b initiates the membrane attack pathway that leads to cell lysis. Thus. C3 can be activated by several pathways that are analogous to each other and they all can lead to membrane lysis.
The alternative pathway can be activated by many Gram-negative (most significant!}. Neisseria meningitidis and V. gonorrhoea), some Gram-positive bacteria and certain viruses and parasites, that results in the lysis of these organisms. Thus, the alternative pathway of C activation provides another means of protection against certain pathogens before an antibody response is mounted. A deficiency of C3 results in an increased susceptibility to these organisms.
Lytic Pathway: |
|
|
|
|
The |
lytic |
pathway |
invokes the C5-C9 components. |
C5 convertase. generated by |
one of |
the pathways described above, cleaves C5 |
into C5a and C5b C5b |
||
instantaneously |
binds C6 |
and subsequently C7 to yield a |
hydrophobic C5bf7 complex |
|
that attaches quickly to plasma membrane (Figure 7). |
|
|||
Subsequently. C8 binds to this complex and causes the insertion of several C9 molecules The insertion of C8(9)n complex causes formation of a hole in the membrane and cell lysis. This lysis is nonenzymatic and is believed to be due to a physical change in the plasma membrane C5b67 can bind indiscriminately to any cell membrane leading to their lysis. However, such an indiscriminate damage to by-standing cells is prevented by protein S (vitroncctin) that binds to C5b67 complex and blocks its
10
