
2. The biology influence of ionization radiation. The human body is made up of many organs, and each organ of the body is made up of specialized cells. Ionizing radiation can potentially affect the normal operation of these cells. Biological effect begins with the ionization of atoms. The mechanism by which radiation causes damage to human tissue, or any other material, is by ionization of atoms in the material. Ionizing radiation absorbed by human tissue has enough energy to remove electrons from the atoms that make up molecules of the tissue. When the electron that was shared by the two atoms to form a molecular bond is dislodged by ionizing radiation, the bond is broken and thus, the molecule falls apart. This is a basic model for understanding radiation damage. When ionizing radiation interacts with cells, it may or may not strike a critical part of the cell. We consider the chromosomes to be the most critical part of the cell since they contain the genetic information and instructions required for the cell to perform its function and to make copies of itself for reproduction purposes. Also, there are very effective repair mechanisms at work constantly which repair cellular damage - including chromosome damage. Possible effects of radiation on cells: 1.Cells are undamaged by the dose
Ionization may form chemically active substances which in some cases alter the structure of the cells. These alterations may be the same as those changes that occur naturally in the cell and may have no negative effect. 2.Cells are damaged, repair the damage and operate normally. Some ionizing events produce substances not normally found in the cell. These can lead to a breakdown of the cell structure and its components. Cells can repair the damage if it is limited. Even damage to the chromosomes is usually repaired. Many thousands of chromosome aberrations (changes) occur constantly in our bodies. We have effective mechanisms to repair these changes. 3.Cells are damaged, repair the damage and operate abnormally. If a damaged cell needs to perform a function before it has had time to repair itself, it will either be unable to perform the repair function or perform the function incorrectly or incompletely. The result may be cells that cannot perform their normal functions or that now are damaging to other cells. These altered cells may be unable to reproduce themselves or may reproduce at an uncontrolled rate. Such cells can be the underlying causes of cancers. 4.Cells die as a result of the damage. If a cell is extensively damaged by radiation, or damaged in such a way that reproduction is affected, the cell may die. Radiation damage to cells may depend on how sensitive the cells are to radiation. 5.All cells are not equally sensitive to radiation damage. In general, cells which divide rapidly and/or are relatively non-specialized tend to show effects at lower doses of radiation then those which are less rapidly dividing and more specialized. Examples of the more sensitive cells are those which produce blood. This system (called the hemopoietic system) is the most sensitive biological indicator of radiation exposure.
11. Nuclear reactions. In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be the process in which two nuclei, or else a nucleus of an atom and a subatomic particle (such as a proton, neutron, or high energy electron) from outside the atom, collide to produce one or more nuclides that are different from the nuclide(s) that began the process. Thus, a nuclear reaction must cause a transformation of at least one nuclide to another. If a nucleus interacts with another nucleus or particle and they then separate without changing the nature of any nuclide, the process is simply referred to as a type of nuclear scattering, rather than a nuclear reaction.
In principle, a reaction can involve more than two particles colliding, but because the probability of three or more nuclei to meet at the same time at the same place is much less than for two nuclei, such an event is exceptionally rare. "Nuclear reaction" is a term implying an induced change in a nuclide, and thus it does not apply to any type of radioactive decay (which by definition is a spontaneous process). Natural nuclear reactions occur in the interaction between cosmic rays and matter, and nuclear reactions can be employed artificially to obtain nuclear energy, at an adjustable rate, on demand. Perhaps the most notable nuclear reactions are the nuclear chain reactions in fissionable materials that produces induced nuclear fission, and the various nuclear fusion reactions of light elements that power the energy production of the Sun and stars. Both of these types of reactions are employed in nuclear weapons.
38. Elastic scattering of neutrons. "Fast neutrons" have a kinetic energy far above 1 eV. Their scattering by condensed matter (with nuclei having kinetic energies far below 1 eV) is in a good approximation an elastic collision with a particle at rest. At each collision the fast neutron transfers a significant part of its kinetic energy to the scattering nucleus; the more so the lighter the nucleus. In this way the neutron is slowed down until it reaches thermal equilibrium with the material in which it is scattered.
Neutron moderators are used to produce "thermal neutrons" that have kinetic energies below 1 eV (T < 500K).[1] Thermal neutrons are used to maintain a nuclear chain reaction in a nuclear reactor, and as a research tool in neutron science comprising scattering experiments and other applications.
Neutron diffraction (elastic scattering) is used for determining structures; Inelastic neutron scattering is used for the study of atomic vibrations and other excitations.
47. Scintillating method. When a charged particle strikes the scintillator, its atoms are excited and photons are emitted. These are directed at the photomultiplier tube's photocathode, which emits electrons by the photoelectric effect. These electrons are electrostatically accelerated and focused by an electrical potential so that they strike the first dynode of the tube. The impact of a single electron on the dynode releases a number of secondary electrons which are in turn accelerated to strike the second dynode. Each subsequent dynode impact releases further electrons, and so there is a current amplifying effect at each dynode stage. Each stage is at a higher potential than the previous to provide the accelerating field. The resultant output signal at the anode is in the form of a measurable pulse for each photon detected at the photocathode, and is passed to the processing electronics. The pulse carries information about the energy of the original incident radiation on the scintillator. Thus both intensity and energy of the radiation can be measured.
The scintillator must be in complete darkness so that visible light photons do not swamp the individual photon events caused by incident ionising radiation. To achieve this a thin opaque foil, such as aluminised mylar, is often used, though it must have a low enough mass to prevent undue attenuation of the incident radiation being measured.
56. Calculations of beta radiation protection. You cannot tell if you are being exposed to beta radiation. You cannot see, or feel radiation hitting your body. Specialized equipment is required to determine if you are near a beta radiation source. However, you should be familiar with the radiation warning symbols such as the trefoil , which indicate that radioactivity is present.
You can protect yourself by avoiding devices with this symbol, and not entering areas where this symbol or others are posted.
Distance. The farther away people are from a radiation source, the less their exposure. How close to a source of radiation can you be without getting a high exposure? It depends on the energy of the radiation and the size (or activity) of the source. Distance is a prime concern when dealing with gamma rays, because they can travel long distances. Alpha and beta particles don't have enough energy to travel very far.
Time. The amount of radiation exposure increases and decreases with the time people spend near the source of radiation. In general, we think of the exposure time as how long a person is near radioactive material. It's easy to understand how to minimize the time for external (direct) exposure. Gamma and x-rays are the primary concern for external exposure. However, if radioactive material gets inside your body, you can't move away from it. You have to wait until it decays or until your body can eliminate it. When this happens, the biological half-life of the radionuclide controls the time of exposure. Biological half-life is the amount of time it takes the body to eliminate one half of the radionuclide initially present. Alpha and beta particles are the main concern for internal exposure.
Shielding. The greater the shielding around a radiation source, the smaller the exposure. Shielding simply means having something that will absorb radiation between you and the source of the radiation (but using another person to absorb the radiation doesn't count as shielding). The amount of shielding required to protect against different kinds of radiation depends on how much energy they have.
Additional covering, for example heavy clothing, is necessary to protect against beta-emitters. Some beta particles can penetrate and burn the skin.
1. The basic definition of interaction of ionizing radiation with matter. Types of radiation are direct ionizing radiation and indirect ionizing radiation. The flows of charged particles, such as alpha particles, beta particles, electrons, are phenomena of direct ionizing radiation, because though coulomb interaction with matter it directly causes ionization and excitation of atoms. Indirect ionizing radiation (neutrons, γ-quantums) is radiation of particles or photons, which have no charge and during interaction with matter can transfer energy to charged particles, nuclei and atom electrons due to electromagnetic or nuclear interaction. Alpha particles are comparatively heavy and have a charge, they react strongly with matter, producing large numbers of ions per unit length of their path. Alpha particles can interact with either nuclei or orbital electrons in any absorbing medium such as air, water, tissue or metal. The most probable process involved in the absorption of alphas, however, are ionization and excitation of orbital electrons. Ionization occurs whenever the alpha particle is sufficiently close to electron to pull it out from orbit though coulomb attraction. Each time this occurs, the alpha loses kinetic energy and is thus slowed. The alpha also loses kinetic energy by exciting orbital electrons with interactions that are insufficient to cause ionization. Beta particles can interact with electrons as well as nuclei in the medium through which they are travelling. Beta particles passing near nucleus will be deflected by the coulomb forces and losses of the beta particles kinetic energy may or may not (Rutherford scattering) occur. The interactions of beta particles with orbital electrons are most important. Coulomb repulsion between beta particles and electrons frequently results in ionization. In the ionization process, the beta particles lose an amount of energy equal to the kinetic energy of the electron plus the energy used to free it from the atom. A beta particle may produce 50 to 150 ion pairs per centimeter of air before its kinetic energy is completely dissipated. The interaction of photons (γ-quantums) with matter involves several distinct processes. The relative importance and efficiency of each process is strongly dependent upon the energy of the photons and upon the density and atomic number of the absorbing medium. We shall first consider the general case of photon attenuation and then discuss some of the important processes separately.We will now consider the passage of each type of radiation through matter with most attention given to gamma-rays because they are the most common type used in nuclear medicine. One of the main effects that you will notice irrespective of the type of radiation is that ions are produced when radiation interacts with matter. It is for this reason that it is called ionizing radiation.
10. Multiple scattering of the recoil of heavy charge particle. Multiple scattering . Charged particle moving in a substance undergoes a large number of collisions , leading to changes in the direction of its movement. This process is called multiple Coulomb scattering. Elementary scattering model can estimate the scattering angle θ particle with momentum p, velocity v and charge Ze on a fixed nucleus of charge ze
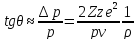
Hence, for the mean square angle of multiple scattering on the path x in matter with density n nuclei can obtain an expression

If you choose to assess as ρmax and ρmin size of the atom and the nucleus , this formula takes the form
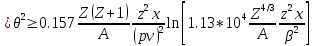
where A - the atomic mass of the substance, pv - in MeV , x – in cm. The logarithm is a slowly varying function , so that the main role played by the factor standing in front of him.
A charged particle traversing a medium is deflected by many small angle scatterings. These scattering are due to the coulomb field of atoms and are assumed to be elastic. In each scattering the energy of the particle is constant but the particle direction changes. In the simplest model of multiple scattering we ignore large angle scatters. In this approximation, the distribution of scattering angle qplane after traveling a distance x through a material with radiation length =Lr is approximately gaussian:
 with
with
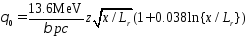
In
the above equation b=v/c, and p=momentum of incident particle. The
space angle q= qplane
The average scattering angle <qplane>=0, but the RMS scattering angle <q2plane>1/2= q0
37. Classification of neutrons. Neutrons are classified according to their energy. The following is a listing of the terms used to describe the various classifications, followed by the basis for each term, and then the range of neutron energies that defines the class: Thermal:
Neutrons in thermal equilibrium with their surroundings
Most probable energy at 20 degrees (C) - 0.025 eV; Maxwellian distribution of 20 degrees (C) extends to about 0.2 eV… Epithermal:
Neutrons of energy greater than thermal
Greater than 0.2 eV…Cadmium:
Neutrons which are strongly absorbed by cadmium
Less than 0.4 eV…Epicadmium:
Neutrons which are not strongly absorbed by cadmium
Greater than 0.6 eV…Slow:
Neutrons of energy slightly greater than thermal
Less than 1 to 10 eV (sometimes up to 1 keV)…Resonance:
In pile neutron physics, usually refers to neutrons which are strongly captured in the resonance of U-238, and of a few commonly-used detectors (e.g., Indium, Gold, etc.)
1 eV to 300 eV…Intermediate:
Neutrons that are between slow and fast
Few hundred eV to 0.5 MeV…Fast:
Greater than 0.5 MeV…Utrafast:
Relativistic
Greater than 20 MeV…Pile:
Neutrons of all energies present in nuclear reactors
0.001 eV to 15 MeV… Fission:
Neutrons formed during fission
100 keV to 15 MeV (Most probable - 0.8 Mev; Average - 2.0 MeV)
46. Ionizing method. Electron ionization (EI, formerly known as electron impact) is an ionizationmethod in which energeticelectronsinteract with gas phase atoms or molecules to produceions. This technique is widely used in mass spectrometry, particularly for gases and volatileorganic molecules. The followinggas phase reaction describes the electron ionization process:
![]()
where M is the analyte molecule being ionized, e− is the electron and M+• is the resultingion. In an EIion source, electrons are produced throughthermionic emissionby heating a wire filament that haselectric currentrunning through it. The electrons are accelerated to 70 eV in the region between the filament and the entrance to the ion source block. The accelerated electrons are then concentrated into a beam by being attracted to the trap electrode. The sample under investigation which contains the neutral molecules is introduced to the ion source in a perpendicular direction to the electron beam. Close passage of highly energetic electrons, referred to as a hard ionization source, causes large fluctuations in the electric field around the neutral molecules and induces ionization and fragmentation. The radical cationproducts are then directed towards the mass analyzer by a repeller electrode. The ionization process often follows predictable cleavage reactions that give rise to fragment ions which, following detection and signal processing, convey structural information about the analyte.
The ionization efficiencyand production of fragment ions depends strongly on the chemistry of the analyte and the energy of the electrons. At low energies (around 20eV), the interactions between the electrons and the analyte molecules do not transfer enough energy to cause ionization. At around 70 eV, the de Broglie wavelengthof the electrons matches the length of typical bonds in organic molecules (about 0.14nm) and energy transfer to organic analyte molecules is maximized, leading to the strongest possible ionization and fragmentation. Under these conditions, about 1 in 1000 analyte molecules in the source are ionized. At higher energies, the de Broglie wavelength of the electrons becomes smaller than the bond lengths in typical analytics; the molecules then become "transparent" to the electrons and ionization efficiency decreases.
55. Calculation alpha radiation protection. The larger the nucleus of an atom becomes, the more the repulsive coulomb force between the positively charged protons begins to overcome the attractive nuclear force that binds the nucleus together. In order to regain stability, the nucleus radiates an alpha particle, which contains two protons and two neutrons. This process continues until the nuclear force and the Coulomb force once again "balance".
Alpha particles are considered "large" and relatively slow moving particles in the nuclear world. They also carry 2 positive charges. This strong charge will interact with the electrons of whatever substance it enters, pulling them away from their atoms, thereby causing ions - the negative electrons and the now positively charged atoms. Because the alpha particle carries a large charge and moves slowly, it causes many ionizations along a very short path as it gives up its energy (tens of thousands per centimeter in air). The alpha particle quickly gives up all its energy and is stopped in a short distance.
The range of the alpha particle is so small, that this highly ionizing particle cannot penetrate the dead layer of human skin. It is only a hazard when it can come in contact with live tissue, as is the case when it is ingested or inhaled. Protection from alpha emitting radiation is based on prevention of radioactive dust, gas, or smoke, or if these exist or may exist, in using proper respiratory protection, anti-contamination clothing, and engineering controls to prevent inhalation or ingestion.
One important note before moving on to the other radiations. Just because a radionuclide is listed as an alpha emitter it doesn't mean it only emits alphas. Many alpha emitters also give off beta, gamma, and/or x-ray radiation as the nucleus seeks stability. It is important to know all the radioactive emissions in order to accurately evaluate the hazards.
The most common alpha source at Ames is Am-241 - both in sealed sources and in smoke detectors. The greatest concentration of this isotope is located in the Motor Mount of Building N218 - mainly because this is where we collect the discarded industrial smoke detectors.
These alpha sources are a very low hazard and would only be a concern in a major fire. Emergency measures for a fire involving large quantities of Am-241 would be respiratory protection, protective clothing, plume control/evaluation, and control of the water run-off if possible.
13. Elastic scattering of electron. When an alpha particle is an incident particle and it is diffracted in the Coulomb potential of atoms and molecules, the elastic scattering process is called Rutherford scattering. In many electron diffraction techniques like reflection high energy electron diffraction (RHEED), transmission electron diffraction (TED), and gas electron diffraction (GED), where the incident electrons have sufficiently high energy (>10 keV), the elastic electron scattering becomes the main component of the scattering process and the scattering intensity is expressed as a function of the momentum transfer defined as the difference between the momentum vector of the incident electron and that of the scattered electron.
Inelastic scattering of electron. When an electron is the incident particle, the probability of inelastic scattering, depending on the energy of the incident electron, is usually smaller than that of elastic scattering. Thus in the case of gas electron diffraction, reflection high-energy electron diffraction (RHEED), and transmission electron diffraction, because the energy of the incident electron is high, the contribution of inelastic electron scattering can be ignored. Deep inelastic scattering of electrons from protons provided the first direct evidence for the existence of quarks.
31.Interaction
of gamma rays with matter.
When a gamma ray passes through matter, the probability for
absorption is proportional to the thickness of the layer, the density
of the material, and the absorption cross section of the material.
The total absorption shows an exponential
decrease of
intensity with distance from the incident surface:
![]() where
x is the distance from the incident surface,μ
= nσ
is the absorption coefficient, measured in cm−1, n the
number of atoms per cm3of
the material (atomic density) and σ
the absorption cross
section in
cm2.
As it passes through matter, gamma radiation ionizes via three
processes: the photoelectric
effect, Compton
scattering,
and pair
production.
Photoelectric
effect:
This describes the case in which a gamma photon interacts
with and transfers its energy to an atomic electron, causing the
ejection of that electron from the atom. The kinetic energy of the
resulting photoelectron is
equal to the energy of the incident gamma photon minus the energy
that originally bound the electron to the atom (binding energy).
Compton
scattering:
This is an interaction in which an incident gamma photon loses enough
energy to an atomic electron to cause its ejection, with the
remainder of the original photon's energy emitted as a new, lower
energy gamma photon whose emission direction is different from that
of the incident gamma photon, hence the term "scattering".
Pair
production:
This becomes possible with gamma energies exceeding 1.02 MeV, and
becomes important as an absorption mechanism at energies over 5 MeV
(see illustration at right, for lead). By interaction with
the electric
field of
a nucleus, the energy of the incident photon is converted into the
mass of an electron-positron pair.
where
x is the distance from the incident surface,μ
= nσ
is the absorption coefficient, measured in cm−1, n the
number of atoms per cm3of
the material (atomic density) and σ
the absorption cross
section in
cm2.
As it passes through matter, gamma radiation ionizes via three
processes: the photoelectric
effect, Compton
scattering,
and pair
production.
Photoelectric
effect:
This describes the case in which a gamma photon interacts
with and transfers its energy to an atomic electron, causing the
ejection of that electron from the atom. The kinetic energy of the
resulting photoelectron is
equal to the energy of the incident gamma photon minus the energy
that originally bound the electron to the atom (binding energy).
Compton
scattering:
This is an interaction in which an incident gamma photon loses enough
energy to an atomic electron to cause its ejection, with the
remainder of the original photon's energy emitted as a new, lower
energy gamma photon whose emission direction is different from that
of the incident gamma photon, hence the term "scattering".
Pair
production:
This becomes possible with gamma energies exceeding 1.02 MeV, and
becomes important as an absorption mechanism at energies over 5 MeV
(see illustration at right, for lead). By interaction with
the electric
field of
a nucleus, the energy of the incident photon is converted into the
mass of an electron-positron pair.
9. Elastic and inelastic scattering of heavy charge particle. Heavy charged particles travel in essentially straight lines in matter, while electrons travel in tortuous paths. Frequent multiple elastic Coulomb scattering by atomic nuclei is often cited as the reason for this electron behavior. Heavy charged particles also undergo multiple Coulomb scattering. However, because they are massive, significant deflections occur only in rare, close encounters with nuclei. In contrast to heavy particles, the inelastic interaction of an electron with an atomic electron represents a collision with a particle of equal mass. In principle, therefore, repeated inelastic scattering of an electron can also produce large-angle deflections and thus contribute to the tortuous nature of an electron's track. To investigate the relative importance of elastic and inelastic scattering on determining the appearance of electron tracks, detailed Monte Carlo transport computations have been carried out for monoenergetic pencil beams of electrons normally incident on a water slab with initial energies from 1 keV to 1 MeV. The calculations have been performed with deflections due to (1) inelastic scattering only, (2) elastic scattering only, and (3) both types of scattering. Results are presented to show the spreading of the pencil beams with depth in the slab, the transmission through slabs of different thicknesses, and back-scattering from the slab. The results show that elastic nuclear scattering is indeed the principal physical process that causes electron paths to be tortuous; however, the smaller effect of inelastic electronic scattering is far from negligible. Scattering is called elastic if the total kinetic energy of the particles does not change, does not change the internal state of the particle or particles in the transformation of some others. Otherwise called inelastic scattering , and the kinetic energy is converted into other forms of energy to change the collective (eg , deformation) or microscopic (eg , excitation of the nucleus ) degrees of freedom of the incident particles or target. Usually, the experimental target composed of many particles. If the target is thin , then the particle can be scattered only once. This scattering is called single scattering . When thick target should take into account the multiple scattering of particles.
45.
Equivalent
dose. The equivalent
absorbed radiation dose,
usually shortened to equivalent
dose,
or formerly dose
equivalent,
is a computed average measure of the radiation absorbed
by a fixed mass of biological tissue, that attempts to account for
the different biological damage potential of different types
of ionizing
radiation.
It is therefore a less fundamental quantity than the total radiation
energy absorbed per mass (the absorbed
dose),
but is a more significant quantity for assessing the health risk of
radiation exposure. The
equivalent dose is calculated by multiplying the absorbed energy,
averaged by mass over an organ or tissue of interest, by a radiation
weighting factor appropriate to the type and energy of radiation. To
obtain the equivalent dose for a mix of radiation types and energies,
a sum is taken over all types of radiation energy dose.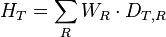 where.HT is
the equivalent dose absorbed by tissue TDT,R is
the absorbed dose in tissue T by radiation type RWR is
the radiation weighting factor defined by regulation
where.HT is
the equivalent dose absorbed by tissue TDT,R is
the absorbed dose in tissue T by radiation type RWR is
the radiation weighting factor defined by regulation
36. The total coefficient of gamma radiation attenuation the total linear attenuation coefficients μ(cm-1 ) were calculated and studied for particulate reinforced polymer-based composites. Unsaturated polyester (UP) resin was used as a matrix filled with different concentrations of Al, Fe, and Pb metal powders as reinforcements. The effect of the metal powders addition at different weight percentages in the range of (10,20,30,40,50)wt % and gamma energy on attenuation coefficients was studied. The results show, as the metallic particulates content increase, the attenuation coefficients will increase too, while it, were exhibited a decrease in their values when the gamma energy increase.The total linear attenuation coefficients of gamma ray for 15 composites have been calculated using the XCOM program (version 3.1) in the energy range of 0.1-20 MeV. In shielding calculations, materials made of homogeneous mixture of elements are frequently encountered. For a mixture of known composition, the total mass attenuation coefficient μ/ρ (cm2 g -1) can be determined from basic data by relationships
Where
μ:
the total linear attenuation coefficient, cm-1.Ni: number of atoms,
cm-iσ:
Microscopic cross section, cm2.iρ:
Density of the ith
constituent, g.cm-3.iw:
Proportion by weight of ith
constituentog
54. Calculation of gamma rays protection
The amount of radiation an individual accumulates will depend on how long the individual stays in the radiation field Dose = Dose Rate x Time
mrem = mrem/hr x hr .Therefore, to limit a persons dose, one can restrict the time spent in the area. How long a person can stay in an area without exceeding a prescribed limit is called the "stay time" and is calculated from the simple relationship:
Stay Time = Limit (mrem) Dose Rate (mrem/hr) Example: How long can a radiation worker stay in a 1.5 rem/hr radiation field if we wish to limit a dose to 100 mrem? Stay Time = 100 mrem = 0.0667 hr = 4 minutes
1500 mrem/hr
6. The unit of measurement is a definite magnitude of a physical quantity, defined and adopted by convention or by law, that is used as a standard for measurement of the same physical quantity. Any other value of the physical quantity can be expressed as a simple multiple of the unit of measurement. For example, length is a physical quantity. The metre is a unit of length that represents a definite predetermined length. When we say 10 metres (or 10 m), we actually mean 10 times the definite predetermined length called "metre". The definition, agreement, and practical use of units of measurement have played a crucial role in human endeavour from early ages up to this day. Different systems of units used to be very common. Now there is a global standard, the International System of Units (SI), the modern form of the metric system.
In trade, weights and measures is often a subject of governmental regulation, to ensure fairness and transparency. The International Bureau of Weights and Measures (BIPM) is tasked with ensuring worldwide uniformity of measurements and their traceability to the International System of Units (SI). Metrology is the science for developing nationally and internationally accepted units of weights and measures. In physics and metrology, units are standards for measurement of physical quantities that need clear definitions to be useful. Reproducibility of experimental results is central to the scientific method. A standard system of units facilitates this. Scientific systems of units are a refinement of the concept of weights and measures developed long ago for commercial purposes.
Science,
medicine,
and engineering
often use larger and smaller units of measurement than those used in
everyday life and indicate them more precisely. The judicious
selection of the units of measurement can aid researchers in problem
solving
(see, for example, dimensional
analysis).
In the social
sciences,
there are no standard units of measurement and the theory and
practice of measurement is studied in psychometrics
and the theory
of conjoint measurement.
Any value of a physical
quantity
is expressed as a comparison to a unit of that quantity. For example,
the value of a physical quantity Z
is expressed as the product of a unit [Z] and a numerical factor:
![]() The multiplication sign is usually left out, just as it is left out
between variables in scientific notation of formulas. The conventions
used to express quantities is referred to asquantity
calculus.
In formulas the unit [Z] can be treated as if it were a specific
magnitude of a kind of physical dimension:
see dimensional
analysis
for more on this treatment. Units can only be added or subtracted if
they are the same type; however units can always be multiplied or
divided, as George
Gamow
used to explain: "2 candlesticks" times "3 cabdrivers"
= 6 [candlestick][cabdriver]. A distinction should be made between
units and standards. A unit is fixed by its definition, and is
independent of physical conditions such as temperature. By contrast,
a standard is a physical realization of a unit, and realizes that
unit only under certain physical conditions. For example, the metre
is a unit, while a metal bar is a standard. One metre is the same
length regardless of temperature, but a metal bar will be exactly one
metre long only at a certain temperature.
The multiplication sign is usually left out, just as it is left out
between variables in scientific notation of formulas. The conventions
used to express quantities is referred to asquantity
calculus.
In formulas the unit [Z] can be treated as if it were a specific
magnitude of a kind of physical dimension:
see dimensional
analysis
for more on this treatment. Units can only be added or subtracted if
they are the same type; however units can always be multiplied or
divided, as George
Gamow
used to explain: "2 candlesticks" times "3 cabdrivers"
= 6 [candlestick][cabdriver]. A distinction should be made between
units and standards. A unit is fixed by its definition, and is
independent of physical conditions such as temperature. By contrast,
a standard is a physical realization of a unit, and realizes that
unit only under certain physical conditions. For example, the metre
is a unit, while a metal bar is a standard. One metre is the same
length regardless of temperature, but a metal bar will be exactly one
metre long only at a certain temperature.
15.
The law of gamma radiation. Gamma
radiation,
also known as gamma
rays,
and denoted by the Greek letter γ,
refers to electromagnetic
radiation
of extremely high frequency and therefore high energy per photon.
Gamma rays are ionizing
radiation,
and are thus biologically hazardous. They are classically produced by
the decay from high energy states of atomic
nuclei (gamma
decay),
but are also created by other processes. Paul
Villard,
a French chemist and physicist, discovered gamma radiation in 1900,
while studying radiation emitted from radium.
Villard's radiation was named "gamma rays" by Ernest
Rutherford
in 1903. Natural sources of gamma rays on Earth include gamma decay
from naturally occurring radioisotopes,
and secondary radiation from atmospheric interactions with cosmic
ray
particles. Rare terrestrial natural sources produce gamma rays that
are not of a nuclear origin, such as lightning
strikes
and terrestrial
gamma-ray flashes.
Gamma rays are produced by a number of astronomical processes in
which very high-energy electrons are produced, that in turn cause
secondary gamma rays by the mechanisms of bremsstrahlung,
inverse Compton
scattering
and synchrotron
radiation.
A large fraction of such astronomical gamma rays are screened by
Earth's atmosphere and can only be detected by spacecraft. Gamma rays
typically have frequencies above 10 exahertz
(or >1019
Hz), and therefore have energies above 100 keV
and wavelengths less than 10 picometers
(less than the diameter of an atom).
However, this is not a hard and fast definition, but rather only a
rule-of-thumb description for natural processes. Gamma rays from
radioactive
decay
are defined as gamma rays no matter what their energy, so that there
is no lower
limit to gamma energy derived from radioactive decay. Gamma decay
commonly produces energies of a few hundred keV,
and almost always less than 10 MeV.
In astronomy, gamma rays are defined by their energy, and no
production process need be specified. The energies of gamma rays from
astronomical sources range over 10 TeV, at a level far too large to
result from radioactive decay. [1]
A notable example is extremely powerful bursts of high-energy
radiation normally referred to as long duration gamma-ray
bursts,
which produce gamma rays by a mechanism not compatible with
radioactive decay. These bursts of gamma rays, thought to be due to
the collapse of stars called hypernovae,
are the most powerful events so far discovered in the cosmos.
When a gamma ray passes through matter, the probability for
absorption is proportional to the thickness of the layer, the density
of the material, and the absorption cross section of the material.
The total absorption shows an exponential
decrease
of intensity with distance from the incident surface:
![]() where x is the distance from the incident surface,μ
= nσ
is the absorption coefficient, measured in cm−1,
n
the number of atoms per cm3
of the material (atomic density) and σ
the absorption cross
section
in cm2.
As it passes through matter, gamma radiation ionizes via three
processes: the photoelectric
effect,
Compton
scattering,
and pair
production.
Photoelectric
effect:
This describes the case in which a gamma photon
interacts with and transfers its energy to an atomic electron,
causing the ejection of that electron from the atom. The kinetic
energy of the resulting photoelectron
is equal to the energy of the incident gamma photon minus the energy
that originally bound the electron to the atom (binding energy). The
photoelectric effect is the dominant energy transfer mechanism for
X-ray and gamma ray photons with energies below 50 keV (thousand
electron
volts),
but it is much less important at higher energies. Compton
scattering:
This is an interaction in which an incident gamma photon loses enough
energy to an atomic electron to cause its ejection, with the
remainder of the original photon's energy emitted as a new, lower
energy gamma photon whose emission direction is different from that
of the incident gamma photon, hence the term "scattering".
The probability of Compton scattering decreases with increasing
photon energy. Compton scattering is thought to be the principal
absorption mechanism for gamma rays in the intermediate energy range
100 keV
to 10 MeV. Compton scattering is relatively independent of the atomic
number
of the absorbing material, which is why very dense materials like
lead are only modestly better shields, on a per
weight
basis, than are less dense materials. Pair
production:
This becomes possible with gamma energies exceeding 1.02 MeV, and
becomes important as an absorption mechanism at energies over 5 MeV
(see illustration at right, for lead). By interaction with the
electric
field
of a nucleus, the energy of the incident photon is converted into the
mass of an electron-positron
pair. Any gamma energy in excess of the equivalent rest mass of the
two particles (totaling at least 1.02 MeV) appears as the kinetic
energy of the pair and in the recoil of the emitting nucleus. At the
end of the positron's range,
it combines with a free electron, and the two annihilate, and the
entire mass of these two is then converted into two gamma photons of
at least 0.51 MeV energy each (or higher according to the kinetic
energy of the annihilated particles). The secondary electrons (and/or
positrons) produced in any of these three processes frequently have
enough energy to produce much ionization
themselves. Additionally, gamma rays, particularly high energy ones,
can interact with atomic nuclei resulting in ejection of particles in
photodisintegration,
or in some cases, even nuclear fission (photofission).
where x is the distance from the incident surface,μ
= nσ
is the absorption coefficient, measured in cm−1,
n
the number of atoms per cm3
of the material (atomic density) and σ
the absorption cross
section
in cm2.
As it passes through matter, gamma radiation ionizes via three
processes: the photoelectric
effect,
Compton
scattering,
and pair
production.
Photoelectric
effect:
This describes the case in which a gamma photon
interacts with and transfers its energy to an atomic electron,
causing the ejection of that electron from the atom. The kinetic
energy of the resulting photoelectron
is equal to the energy of the incident gamma photon minus the energy
that originally bound the electron to the atom (binding energy). The
photoelectric effect is the dominant energy transfer mechanism for
X-ray and gamma ray photons with energies below 50 keV (thousand
electron
volts),
but it is much less important at higher energies. Compton
scattering:
This is an interaction in which an incident gamma photon loses enough
energy to an atomic electron to cause its ejection, with the
remainder of the original photon's energy emitted as a new, lower
energy gamma photon whose emission direction is different from that
of the incident gamma photon, hence the term "scattering".
The probability of Compton scattering decreases with increasing
photon energy. Compton scattering is thought to be the principal
absorption mechanism for gamma rays in the intermediate energy range
100 keV
to 10 MeV. Compton scattering is relatively independent of the atomic
number
of the absorbing material, which is why very dense materials like
lead are only modestly better shields, on a per
weight
basis, than are less dense materials. Pair
production:
This becomes possible with gamma energies exceeding 1.02 MeV, and
becomes important as an absorption mechanism at energies over 5 MeV
(see illustration at right, for lead). By interaction with the
electric
field
of a nucleus, the energy of the incident photon is converted into the
mass of an electron-positron
pair. Any gamma energy in excess of the equivalent rest mass of the
two particles (totaling at least 1.02 MeV) appears as the kinetic
energy of the pair and in the recoil of the emitting nucleus. At the
end of the positron's range,
it combines with a free electron, and the two annihilate, and the
entire mass of these two is then converted into two gamma photons of
at least 0.51 MeV energy each (or higher according to the kinetic
energy of the annihilated particles). The secondary electrons (and/or
positrons) produced in any of these three processes frequently have
enough energy to produce much ionization
themselves. Additionally, gamma rays, particularly high energy ones,
can interact with atomic nuclei resulting in ejection of particles in
photodisintegration,
or in some cases, even nuclear fission (photofission).
33. Thomson scattering is the elastic scattering of electromagnetic radiation by a free charged particle, as described by classical electromagnetism. It is just the low-energy limit of Compton scattering: the particle kinetic energy and photon frequency are the same before and after the scattering. This limit is valid as long as the photon energy is much less than the mass energy of the particle:
![]() In
the low-energy limit, the electric
field of the incident wave (photon) accelerates the charged particle,
causing it, in turn, to emit radiation
at the same frequency as the incident wave, and thus the wave is
scattered. Thomson scattering is an important phenomenon in plasma
physics
and was first explained by the physicist J.J.
Thomson.
As long as the motion of the particle is non-relativistic
(i.e. its speed is much less than the speed of light), the main cause
of the acceleration of the particle will be due to the electric field
component of the incident wave, and the magnetic field can be
neglected. The particle will move in the direction of the oscillating
electric field, resulting in electromagnetic
dipole radiation.
The moving particle radiates most strongly in a direction
perpendicular to its motion and that radiation will be polarized
along the direction of its motion. Therefore, depending on where an
observer is located, the light scattered from a small volume element
may appear to be more or less polarized. he scattering is best
described by an emission
coefficient
which is defined as ε
where ε
dt dV dΩ
dλ
is the energy scattered by a volume element dV in time dt into solid
angle dΩ
between wavelengths λ
and λ+dλ.
From the point of view of an observer, there are two emission
coefficients, εr
corresponding to radially polarized light and εt
corresponding to tangentially polarized light. For
unpolarized incident light, these are given by:
In
the low-energy limit, the electric
field of the incident wave (photon) accelerates the charged particle,
causing it, in turn, to emit radiation
at the same frequency as the incident wave, and thus the wave is
scattered. Thomson scattering is an important phenomenon in plasma
physics
and was first explained by the physicist J.J.
Thomson.
As long as the motion of the particle is non-relativistic
(i.e. its speed is much less than the speed of light), the main cause
of the acceleration of the particle will be due to the electric field
component of the incident wave, and the magnetic field can be
neglected. The particle will move in the direction of the oscillating
electric field, resulting in electromagnetic
dipole radiation.
The moving particle radiates most strongly in a direction
perpendicular to its motion and that radiation will be polarized
along the direction of its motion. Therefore, depending on where an
observer is located, the light scattered from a small volume element
may appear to be more or less polarized. he scattering is best
described by an emission
coefficient
which is defined as ε
where ε
dt dV dΩ
dλ
is the energy scattered by a volume element dV in time dt into solid
angle dΩ
between wavelengths λ
and λ+dλ.
From the point of view of an observer, there are two emission
coefficients, εr
corresponding to radially polarized light and εt
corresponding to tangentially polarized light. For
unpolarized incident light, these are given by:
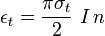
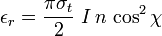
The Thomson differential cross section, related to the sum of the emissivity coefficients, is given by
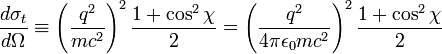
where the first expression is in cgs units, the second in SI units; q is the charge per particle, m the mass of particle, and E0 constant, the permittivity of free space. Integrating over the solid angle, we obtain the Thomson cross section (in cgs and SI units):
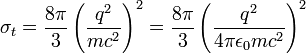
For an electron, the Thomson cross-section is numerically given by:
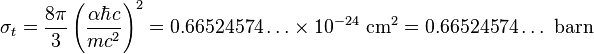
42. Classification of particles and antiparticles. Antiparticle. Corresponding to most kinds of particles, there is an associated antiparticle with the samemassand oppositecharge(includingelectric charge). For example, the antiparticle of theelectronis the positively charged electron, orpositron, which is produced naturally in certain types ofradioactive decay. The laws of nature are very nearly symmetrical with respect to particles and antiparticles. For example, an antiprotonand a positron can form anantihydrogenatom, which has almost exactly the same properties as ahydrogenatom. This leads to the question of why theformation of matter after the Big Bangresulted in a universe consisting almost entirely of matter, rather than being a half-and-half mixture of matter andantimatter. The discovery ofCP violation("CP" denotes "Charge Parity") helped to shed light on this problem by showing that this symmetry, originally thought to be perfect, was only approximate. Particle-antiparticle pairs canannihilateeach other, producingphotons; since the charges of the particle and antiparticle are opposite, total charge is conserved. For example, the positrons produced in natural radioactive decay quickly annihilate themselves with electrons, producing pairs ofgamma rays, a process exploited inpositron emission tomography. Antiparticles are produced naturally inbeta decay, and in the interaction ofcosmic raysin the Earth's atmosphere. Because charge is conserved, it is not possible to create an antiparticle without either destroying a particle of the same charge (as in beta decay) or creating a particle of the opposite charge. The latter is seen in many processes in which both a particle and its antiparticle are created simultaneously, as inparticle accelerators. This is the inverse of the particle-antiparticle annihilation process. Although particles and their antiparticles have opposite charges, electrically neutral particles need not be identical to their antiparticles. The neutron, for example, is made out ofquarks, theantineutronfromantiquarks, and they are distinguishable from one another because neutrons and antineutrons annihilate each other upon contact. However, other neutral particles are their own antiparticles, such asphotons, the hypotheticalgravitons, and someWIMPs. If a particle and antiparticle are in the appropriate quantum states, then they can annihilate each other and produce other particles. Reactions such ase− + e+ → γ + γ (the two-photon annihilation of an electron-positron pair) are an example. The single-photon annihilation of an electron-positron pair, e− + e+ → γ, cannot occur in free space because it is impossible to conserve energy and momentum together in this process. However, in the Coulomb field of a nucleus the translational invarianceis broken and single-photon annihilation may occur.[4] The reverse reaction (in free space, without an atomic nucleus) is also impossible for this reason. In quantum field theory, this process is allowed only as an intermediate quantum state for times short enough that the violation of energy conservation can be accommodated by the uncertainty principle. This opens the way for virtual pair production or annihilation in which a one particle quantum state may fluctuate into a two particle state and back. These processes are important in the vacuum stateandrenormalizationof a quantum field theory. It also opens the way for neutral particle mixing through processes such as the one pictured here, which is a complicated example ofmass renormalization.
Particles. The four fundamental interactions or forces that govern the behavior of elementary particles are listed below.
The strong force (It holds the nucleus together.)
The electromagnetic force (It causes interactions between charges.)
The weak force (It causes beta decay.)
The gravitational force (It causes interaction between states with energy.) A given particle may not necessarily be subject to all four interactions. Neutrinos, for example, experience only the weak and gravitational interaction. The fundamental particles may be classified into groups in several ways. First, all particles are classified into fermions, which obey Fermi-Dirac statistics and bosons, which obey Bose-Einstein statistics. Fermions have half-integer spin, while bosons have integer spin. All the fundamental fermions have spin 1/2. Electrons and nucleons are fermions with spin 1/2. The fundamental bosons have mostly spin 1. This includes the photon. The pion has spin 0, while the graviton has spin 2. There are also three particles, the W+, W− and Z0 bosons, which are spin 1. They are the carriers of the weak interactions. We can also classify the particles according to their interactions. The most basic way of classifying particles is by their mass. Each elementary particle is associated with an antiparticle with the same mass and opposite charge. Some particles, such as the photon, are identical to their antiparticle. Such particles must be neutral, but not all neutral particles are identical to their antiparticle. Particle-antiparticle pairs can annihilate each other if they are in appropriate quantum states, releasing an amount of energy equal to twice the rest energy of the particle. They can also be produced in various processes, if enough energy is available. The minimum amount of energy needed is twice the rest energy of the particle, if momentum conservation allows the particle-antiparticle pair to be produced at rest. Most often the antiparticle is denoted by the same symbol as the particle, but with a line over the symbol. For example, the antiparticle of the proton p, is denoted by p.
Protons and neutrons are made of still smaller particles called quarks. At this time it appears that the two basic constituents of matter are the leptons and the quarks. There are believed to be six types of each. Each quark type is called a flavor, there are six quark flavors. Each type of lepton and quark also has a corresponding antiparticle, a particle that has the same mass but opposite electrical charge and magnetic moment. An isolated quark has never been found, quarks appear to almost always be found in pairs or triplets with other quarks and antiquarks. The resulting particles are the hadrons, more than 200 of which have been identified. Baryons are made up of 3 quarks, and mesons are made up of a quark and an anti-quark. Baryons are fermions and mesons are bosons. Two theoretically predicted five-quark particles, called pentaquarks, have been produced in the laboratory. Four- and six-quark particles are also predicted but have not been found. The six quarks have been named up, down, charm, strange, top, and bottom. The top quark, which has a mass greater than an entire atom of gold, is about 35 times more massive than the next biggest quark and may be the heaviest particle nature has ever created. The quarks found in ordinary matter are the up and down quarks, from which protons and neutrons are made. A proton consists of two up quarks and a down quark, and a neutron consists of two down quarks and an up quark. The pentaquark consists of two up quarks, two down quarks, and the strange antiquark. Quarks have fractional charges of one third or two thirds of the basic charge of the electron or proton. Particles made from quarks always have integer charge.
Hadrons are the heaviest particles. This group is then spilt up into baryons and mesons. Baryons are the heaviest particles of all, followed by mesons.
Leptons are the lightest particles.
