
3 курс / Фармакология / Синтез_и_изучение_свойств_новых_материалов_с_противоопухолевой
.pdf
|
|
|
|
|
211 |
|
|
|
|
|
|
|
2.0 |
|
|
|
|
|
|
|
|
|
|
|
|
0 mM |
|
|
|
|
|
|
|
|
|
|
1.5 |
1 mM |
|
|
|
|
|
|
|
|
|
|
5 mM |
|
|
|
|
|
|
|
|
||
/ mgrad |
|
|
|
|
|
|
|
|
|
||
|
10 mM |
|
|
|
|
|
|
|
|
||
|
25 mM |
|
|
|
|
|
|
|
|
||
1.0 |
50 mM |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
dichroism |
0.5 |
|
|
|
|
|
|
|
|
|
|
0.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Circular |
-0.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-1.0 |
|
|
|
|
|
|
|
|
|
|
|
-1.5 |
|
|
|
|
|
|
|
|
|
|
|
220 |
230 |
240 |
250 |
260 |
270 |
280 |
290 |
300 |
310 |
320 |
|
|
|
|
|
|
l/nm |
|
|
|
|
|
Fig. 3.24. Circular dichroism spectra of DNA (C = 6.1 μM) as a function of compound concentration 1.57 (C = 1–100 µM).
An increase in temperature has a strong effect on the stability of the doublestranded DNA structure, causing breakage to a single-stranded structure, the so-called
‘melting’ of the nucleic acid. The temperature at which 50 % of the duplex DNA remains in the double-stranded state is referred to as the DNA melting point (Tm). The melting temperature of DNA (Tm) directly depends on the stability of its double helix. The interaction of DNA with small molecules can stabilise the structure of the nucleic acid, causing conformational changes, which usually leads to an increase in the Tm value.
The study of DNA melting by UV spectroscopy in the presence of compound
1.57 showed that the melting temperature of DNA remains practically unchanged, although the transition width increases (Fig. 3.25), which indicates the presence of an interaction. A similar shape of the melting curve is observed in the interaction of cisplatin with DNA at similar ratios. Thus, it can be assumed that the binding in both cases is carried out with DNA bases.

212
1.25 |
DNA |
|
|
|
|
|
|
0.006 |
|
|
|
|
|
|
|
||
|
DNA + compound 1.57 |
|
|
|
|
|
||
1.20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.004 |
1.15 |
|
|
|
|
|
|
|
Ad |
|
|
|
|
|
|
|
|
|
А |
|
|
|
|
|
|
|
td / |
1.10 |
|
|
|
|
|
|
|
0.002 |
1.05 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.000 |
1.00 |
|
|
|
64.4 |
65.9 |
|
|
|
|
|
|
|
|
|
|
|
|
20 |
30 |
40 |
50 |
60 |
70 |
80 |
90 |
100 |
|
|
|
|
t° / °C |
|
|
|
|
Fig. 3.25. Melting curves of DNA (6.1 μM) in the absence and presence of compound
1.57 in physiological saline (0.9 % NaCl) at a stoichiometric ratio [compound 1.57] / [DNA] = 1.5.
Assuming an equilibrium nature of binding, we calculated the Kbin values for DNA binding 1.57 using the Wolf–Shimmer equation [105]:
|
[DNA] |
= |
[DNA] |
+ |
1 |
|
(3.18) |
|
|
Kbin ( b |
- f ) |
||||
|
a - f |
b - f |
|
||||
where Kbin is a binding constant; [DNA] is the concentration of DNA, εa, εf and εb are the extinction coefficients of the complex of the ligand (compound 1.57) with DNA, the ligand and the ligand bound to DNA in a stoichiometric amount, respectively.
As shown in Fig. 3.26, the dependence of [DNA] / (εa − εf) on [DNA] is linear. Based on the Wolf–Shimmer equation, the binding constants obtained for compound
1.57 are 3.44·107 M−1, indicating that compound 1.57 has a high ability to form bonds with DNA, presumably by a covalent mechanism. It should be noted that the values of the binding constants obtained from the data of calorimetric titration and the spectrophotometric method are in good agreement.
Рекомендовано к изучению сайтом МедУнивер - https://meduniver.com/

|
|
|
|
|
213 |
|
|
|
|
|
|
|
0.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
Чcm |
|
y = 0,12x + 0,504 |
|
|
|
|
|
|
|
|
|
|
R² = 0,9827 |
|
|
|
|
|
|
0.6 |
|
|
|
2 |
2,5 |
|
|
|
|
|
|
|
|
|
M |
|
|
|
|
|
||
|
|
|
|
, |
|
|
|
|
|
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
|
|
10 |
2 |
|
|
|
|
|
|
|
|
|
|
f)}Ч |
|
|
|
|
|
|
|
|
|
|
|
− |
1,5 |
|
|
|
|
|
|
0.5 |
|
|
|
a |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
{[DNA]/( |
|
|
|
|
|
|
|
Absorbance |
|
|
|
|
1 |
|
|
|
|
|
|
|
|
|
|
0,5 |
|
|
|
|
|
||
0.4 |
|
|
|
1 |
6 |
1 1 |
16 |
21 |
|
||
|
|
|
|
|
[DNA] / mM |
|
|
|
|||
0.3 |
|
|
|
|
|
|
|
|
|
||
|
0.2 |
|
|
|
|
|
|
|
|
|
|
|
0.1 |
|
|
|
|
|
|
|
|
|
|
|
0.0 |
|
|
|
|
|
|
|
|
|
|
|
200 |
220 |
240 |
260 |
280 |
300 |
320 |
340 |
360 |
380 |
400 |
|
|
|
|
|
l / nm |
|
|
|
|
|
|
Fig. 3.26. Absorption spectra of DNA solutions (C = 3.6–24.4 μM) at a constant concentration of compound 1.57 (C = 5.4 μM), as well as the dependence in coordinates [DNA] / (εa − εf) on [DNA] (R2 = 0.982) at [DNA] = 3.6, 5.4, 8.2, 11.1,
13.5, 1.89 and 24.4 µM.
The value of G was −43.03 kJ/mol.
3.5.3.Antioxidant activity
3.5.3.1.Study of antiradical activity
To quantify the reaction rate between compound 1.57 and DPPH, we used a pseudo-first-order kinetic model of the reaction described by the Eq. 3.20:
ln |
A - At |
= -kt |
(3.20) |
||
A - A |
|||||
|
|
|
|||
|
|
0 |
|
|
|
where A∞, At and A0 are the optical densities of the solution after ‘infinity’ (six days after the start of the experiment), at time t and at the initial time, respectively.
From the tangents of the slopes of the curves in the coordinates ln A - At vs t
A - A0
(Fig. 3.27), the values of the apparent rate constants at various temperatures were obtained (Table 3.9).

214
|
0.00 |
|
|
|
|
|
|
)] |
-0.02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0 |
|
|
|
|
|
|
|
− A |
-0.04 |
|
|
|
|
|
|
∞ |
|
|
|
|
|
|
|
(A |
|
|
|
|
|
|
|
) / |
-0.06 |
|
|
|
|
|
|
t |
|
|
|
|
|
|
|
− A |
|
|
|
|
|
|
|
∞ |
-0.08 |
|
|
|
|
|
|
ln[(A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-0.10 |
|
|
|
|
|
|
|
-0.12 |
|
|
|
|
|
|
|
0 |
5 |
10 |
15 |
20 |
25 |
30 |
|
|
|
|
t / min |
|
|
|
Fig. 3.27. Kinetic dependence of DPPH reduction by compound 1.57 at 303.15 K (■), 308.15 K (●), 313.15 K (▲), 318.15 K (▼).
Table 3.9. The values of the rate constants for the reduction of DPPH by compound
1.57.
T / K |
k·10−3 / min−1 |
|
|
303.15 |
2.43 ± 0.06 |
308.15 |
3.14 ± 0.05 |
313.15 |
3.40 ± 0.03 |
318.15 |
4.30 ± 0.03 |
|
|
Next, dependences in the lnk coordinates on T−1 were plotted (Fig. 3.28) and the values of the activation energy (Ea = 28.74 ± 0.38 kJ mol−1) and frequency factor (lnA
= 5.40 ± 1.46) according to the Arrhenius equation:
ln k = ln A - |
Ea |
(3.21) |
i |
RT |
|
Рекомендовано к изучению сайтом МедУнивер - https://meduniver.com/

|
|
215 |
|
|
|
-5.4 |
|
|
|
|
-5.5 |
|
|
|
|
-5.6 |
|
|
|
] |
|
|
|
|
−1 |
|
|
|
|
/ ln[min |
-5.7 |
|
|
|
-5.8 |
|
|
|
|
lnk |
|
|
|
|
|
-5.9 |
|
|
|
|
-6.0 |
|
|
|
|
-6.1 |
|
|
|
|
0.00315 |
0.00320 |
0.00325 |
0.00330 |
|
|
|
T−1/ K−1 |
|
Fig. 3.28. Temperature dependence of the logarithm of the rate constant of the reaction of the interaction of compound 1.57 with DPPH. T is the absolute temperature, k is the pseudo first order rate constant.
3.5.3.2. Study of photodynamic properties
The study of photobleaching of Radachlorin in the presence of compound 1.57 makes it possible to evaluate the antioxidant properties of the drug upon irradiation with visible light. The practical significance of the study lies in the fact that the data obtained make it possible to evaluate the possibility of using substances for the implementation of photodynamic therapy, which is actively used in oncology [106,107] and the treatment of non-tumour diseases [108]. Carrying out photodynamic therapy requires the selection of effective photosensitisers that are selective with respect to the target and have a sufficient quantum yield of singlet oxygen upon irradiation. These requirements are met by preparations of chlorin e6, which have proven themselves in clinical practice. In this work, we used Radachlorin, a second-generation photosensitiser intended for fluorescent diagnostics and photodynamic therapy of malignant tumours. [109,110]. The method of photodynamic therapy is based on the ability of Radachlorin to selectively accumulate in a tumour upon its administration and generate singlet oxygen, which has a toxic effect on tumour cells and destroys their plasma membranes when
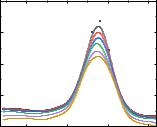
216
exposed to light with a wavelength corresponding to the maximum absorption of Radachlorin. Undoubtedly, the formation of reactive oxygen species can be enhanced by pro-oxidants and weakened by antioxidants. Thus, prooxidants accelerate the photobleaching of the preparation, while antioxidants inhibit it.
The photosensitiser degradation was assessed by measuring the photodegradation rate constants (kdeg), the values of which were determined from the slope of the kinetic dependences in the ln(At /A0) – t coordinates. A decrease in kdeg indicates that the compound under study has the properties of a singlet oxygen quencher. In turn, an increase in kdeg indicates that the test compound has the properties of a singlet oxygen inducer. The absorption spectra of Radachlorin were measured in the absence and in the presence of various concentrations of compound 1.57 (Fig. 3.29), the kinetic dependence of Radachlorin photofading is shown in Fig. 3.30. In this work, sodium azide solution (C = 500 μM) was used as a control. From the presented results, it can be concluded that the pronounced antioxidant properties of compound 1.57 upon irradiation.
|
0.4 |
|
|
|
|
0.3 |
|
|
|
A |
0.2 |
|
|
|
|
0.1 |
|
|
|
|
0.0 |
|
|
|
|
620 |
645 |
670 |
695 |
|
|
l / nm |
|
|
Fig. 3.29. Decrease in the optical density of Radachlorin in the presence of compound
1.57(C = 5–100 μM) upon irradiation: ▬ without irradiation; ▬ 10 s, ▬ 20 s,
▬30 s, ▬ 40 s, ▬ 50 s.
Рекомендовано к изучению сайтом МедУнивер - https://meduniver.com/

|
|
|
217 |
|
|
|
1.8 |
|
|
|
|
|
1.6 |
|
|
|
|
) |
1.4 |
|
|
|
|
t |
|
|
|
|
|
/ A |
|
|
|
|
|
0 |
|
|
|
|
|
ln(A |
1.2 |
|
|
|
|
|
|
|
|
|
|
|
1.0 |
|
|
|
|
|
0.8 |
|
|
|
|
|
10 |
20 |
30 |
40 |
50 |
|
|
|
t / s |
|
|
Fig. 3.30. Kinetic dependence of photobleaching of Radachlorin (►) in the presence of the compound 1.57 (▲ 5 µM, ▼ 10 µM, ● 25 µM, 50 µM, ▼75 µM, ■ 100 µM) compared to sodium azide (● 500 µM). A0 and At are optical densities of Radachlorin solutions at a wavelength of 664.9 nm before and after irradiation.
3.5.3.3. Photoinduced haemolysis
It is known that erythrocyte lysis is initiated by exposure to ultraviolet or visible light in the presence of photosensitisers, the most effective of which are porphyrins and their derivatives [109], in particular Radachlorin. It has been established that the photodynamic effect is primarily associated with the generation of singlet oxygen, and then other reactive oxygen species. Binding of porphyrins to cell membranes leads to a decrease in membrane photostability [110].
Fig. 3.31 shows the concentration dependence of the degree of photoinduced haemolysis in the presence of compound 1.57. As can be seen from the results obtained, compound 1.57 suppresses haemolysis induced by Radachlorin, which manifests itself in an increase in the time of haemolysis. From the obtained results, it can be concluded that compound 1.57 exhibits dose-dependent antioxidant activity.

218
1.57 control TC50 / TC50
1.6
1.5
1.4
1.3
1.2
1.1
1.0
0 |
50 |
100 |
150 |
200 |
C / mg∙l−1
Fig. 3.31. Dependence of the degree of photoinduced haemolysis in the presence of compound 1.57. C is the molar concentration of compound 1.57, T1.5750 is the time of photoinduced haemolysis of 50 % erythrocytes in the presence of compound 1.57, Tcontrol50 is the time of photoinduced haemolysis of 50 % in the presence of saline.
3.5.3.4. Antiradical activity of compound 1.57 with respect to the NO-radical
An imbalance between prooxidant and antioxidant systems can often lead to oxidative stress in the body, so it is important to consider the effect of biologically active substances on these processes, which can be used as medicines in the future.
Fig. 3.32 shows the dependence of the degree of binding of NO-radicals to compound 1.57 in the concentration range 1–200 μM. Sodium azide was used as a reference substance [111]. It can be seen from the obtained data that, in contrast to sodium azide, compound 1.57 practically does not interact with NO-radicals.
Рекомендовано к изучению сайтом МедУнивер - https://meduniver.com/

219
0.5 |
|
|
|
|
|
|
0.4 |
|
|
|
|
|
|
0.3 |
|
|
|
|
|
|
A |
|
|
|
|
|
|
0.2 |
|
|
|
|
|
|
0.1 |
|
|
|
|
|
|
0.0 |
|
|
|
|
|
|
0 |
10 |
25 |
50 |
75 |
100 |
200 |
|
|
|
C / mM |
|
|
|
Fig. 3.32. The efficiency of NO-radical capture by compound 1.57 (dark grey) compared to sodium azide (light grey). C is the molar concentration of compound
1.57.
3.5.4. Genotoxicity of compound 1.57
The mean % comet tail DNA content, tail length, and comet tail momentum observed from human PBMCs incubated in the presence of H2O2 (positive control), PBS (negative control), and compound 1.57 are presented in Table 3.10. The amount of DNA damage in the presence of H2O2 is significantly higher than in control cells. As an example, Fig. 3.33 shows micrographs of DNA comets in the presence of H2O2
(C = 100 μM), PBS, and compound 1.57 in the concentration range 1–200 μM. Analysis of Table 3.10 shows that incubation of human PBMC with compound 1.57 causes dosedependent damage to DNA integrity.

220
(a) |
(b) |
(c) |
(d) |
(e) |
(f) |
(g) |
(h) |
(i) |
(j) |
Fig. 3.33. DNA comets after cell electrophoresis in microgel. (a) positive control (H2O2), (b) negative control (PBS), (c–j) compound 1.57 (C = 1, 5, 10, 25, 50, 75, 100, and 200 μM).
Рекомендовано к изучению сайтом МедУнивер - https://meduniver.com/
